That sexual dysfunction occurs in schizophrenia is not in doubt. Both the illness (Reference Aizenberg, Zemishlany and Dorfman-EtrogAizenberg et al, 1995) and antipsychotic medication (Reference Kotin, Wilbert and VerburgKotin et al, 1976) have been implicated. However, the few previously published studies which have measured its prevalence in schizophrenia have been weakened by the absence of a control group (Reference Ghadirian, Chouinard and AnnableGhadirian et al, 1982), a focus on one gender only (Reference RabochRaboch, 1984; Reference Aizenberg, Zemishlany and Dorfman-EtrogAizenberg et al, 1995), a failure to consider men and women separately (Reference Kockott and PfeifferKockott & Pfeiffer, 1996), a focus on married patients only (Reference Aizenberg, Zemishlany and Dorfman-EtrogAizenberg et al, 1995), a focus on long-stay in-patients only (Reference Lyketsos, Sakka and MailisLyketsos et al, 1983), or, more recently, a focus on patients taking conventional antipsychotic medication (Reference Smith, O'Keane and MurraySmith et al, 2002). In our study we report the prevalence of sexual dysfunction in a community of men and women with schizophrenia and compare them with members of the general population. We also examine the association between sexual dysfunction and mental state, types of antipsychotic medication and smoking.
METHOD
Setting
The study took place in Nithsdale, southwest Scotland (population 57 000).
Participants
Patients
The patients were recruited as part of a larger lifestyle survey (McCreadie on behalf of Scottish Comorbidity Study Group, 2002). The ‘key informant’ method was used to find cases (Reference McCreadieMcCreadie, 1982). Patients living in Nithsdale with a clinical ICD—10 diagnosis of schizophrenia (World Health Organization, 1992) were identified in April 1999. These included all current in-patients, day patients and out-patients at Crichton Royal Hospital, Dumfries, and patients supported by community psychiatric nurses. In addition, all general practitioners in Nithsdale were asked to notify us of any other patients with schizophrenia known to them. Finally, mental health officers (social workers) and voluntary agencies were asked to identify any others.
General population control group
Through the use of the Community Health Index (a national database that holds details for all patients registered with a Scottish general practitioner), a control group was identified. For each patient interviewed (excluding long-stay in-patients) a person of the same gender, age (within 1 year) and postcode area of residence (matched to five characters) was recruited as a control.
Assessments
A self-completed gender-specific questionnaire was devised for the study (by S.M.). Initial versions were piloted. The final version contains 11 questions for men and 10 for women (see Appendix). The questions cover four areas of sexual functioning: desire, arousal, performance and satisfaction. The participants were given the questionnaire by one of the researchers, and asked to complete it in the privacy of their home. The questionnaire used colloquial language for ‘erection’, ‘ejaculation’ and ‘orgasm’ to make questions more understandable.
Patients' mental state was rated by one of four psychiatrists (S.M., J.H., T.M., R.G.M.) using the Positive and Negative Syndrome Scale (PANSS) for schizophrenia (Reference Kay, Fishbein and OplerKay et al, 1987). This scale gives a total score and scores on positive symptom, negative symptom and general psychopathology sub-scales. Current medication was recorded. As part of the larger study, both patients and controls completed a smoking questionnaire recently used in a health and lifestyle survey of the general population in south-west Scotland (Reference Waldron, Chalmers and BoneWaldron et al, 1995).
Statistical analysis
Data were analysed as a case—control study. For comparisons between groups, chisquared tests, Fisher's exact tests and unpaired t-tests were used. The case—control comparison was unmatched for the following reasons. The answers to questions 2 and 3 (see Appendix) determined whether the participant had any sexual activity, either intercourse or masturbation. If the participant did not, then the response to subsequent questions (4-11 for men, 4-9 for women), namely ‘e’ (no sexual activity or masturbation), was ignored. The remaining number of people in each group who circled ‘a’ or ‘b’ in response to these questions was compared with the number circling ‘c’ or ‘d’.
Two-sided significance tests were used, and as there were many comparisons only differences at least at the 1% level are reported.
Ethical approval
The Dumfries and Galloway Research Ethics Committee approved the study. All patients gave written informed consent.
RESULTS
One hundred and thirty-five patients with schizophrenia and 114 members of the general population were approached. Ninety-eight (73%) patients and 81 (71%) control participants returned the questionnaire. Sixteen of the 179 (9%) questionnaires returned were incomplete. There was no difference in response rates between men and women, or between the patient and control groups.
Patients and controls
We report the results in cases where both the patient and the recruited control returned the questionnaire. There were 60 such patients and controls; 34 male and 26 female. More patients than control participants were single and were living alone (Table 1).
Table 1 Demographic data for patients and controls
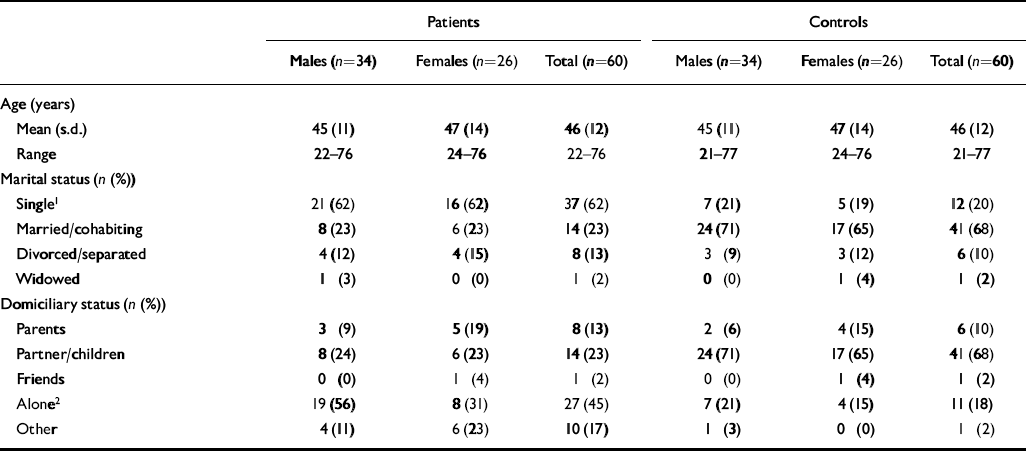
| Patients | Controls | |||||
|---|---|---|---|---|---|---|
| Males (n=34) | Females (n=26) | Total (n=60) | Males (n=34) | Females (n=26) | Total (n=60) | |
| Age (years) | ||||||
| Mean (s.d.) | 45 (11) | 47 (14) | 46 (12) | 45 (11) | 47 (14) | 46 (12) |
| Range | 22-76 | 24-76 | 22-76 | 21-77 | 24-76 | 21-77 |
| Marital status (n (%)) | ||||||
| Single1 | 21 (62) | 16 (62) | 37 (62) | 7 (21) | 5 (19) | 12 (20) |
| Married/cohabiting | 8 (23) | 6 (23) | 14 (23) | 24 (71) | 17 (65) | 41 (68) |
| Divorced/separated | 4 (12) | 4 (15) | 8 (13) | 3 (9) | 3 (12) | 6 (10) |
| Widowed | 1 (3) | 0 (0) | 1 (2) | 0 (0) | 1 (4) | 1 (2) |
| Domiciliary status (n (%)) | ||||||
| Parents | 3 (9) | 5 (19) | 8 (13) | 2 (6) | 4 (15) | 6 (10) |
| Partner/children | 8 (24) | 6 (23) | 14 (23) | 24 (71) | 17 (65) | 41 (68) |
| Friends | 0 (0) | 1 (4) | 1 (2) | 0 (0) | 1 (4) | 1 (2) |
| Alone2 | 19 (56) | 8 (31) | 27 (45) | 7 (21) | 4 (15) | 11 (18) |
| Other | 4 (11) | 6 (23) | 10 (17) | 1 (3) | 0 (0) | 1 (2) |
Men
The following statistically significant differences were found between male patients and their controls (Table 2). More patients than controls did not have sexual intercourse and did not masturbate: 9 (27%) v. 0 (0%); χ2=8.2, d.f.=1, P<0.002. More had at least one sexual dysfunction (answered ‘a’ or ‘b’ to questions 1, 4-11 for men; questions 1, 4-9 for women): 28 (82%) v. 13 (38%); χ2=12.04, d.f.=1, P=0.0005.
Table 2 Responses to questionnaire (see Appendix for content of questions and responses)
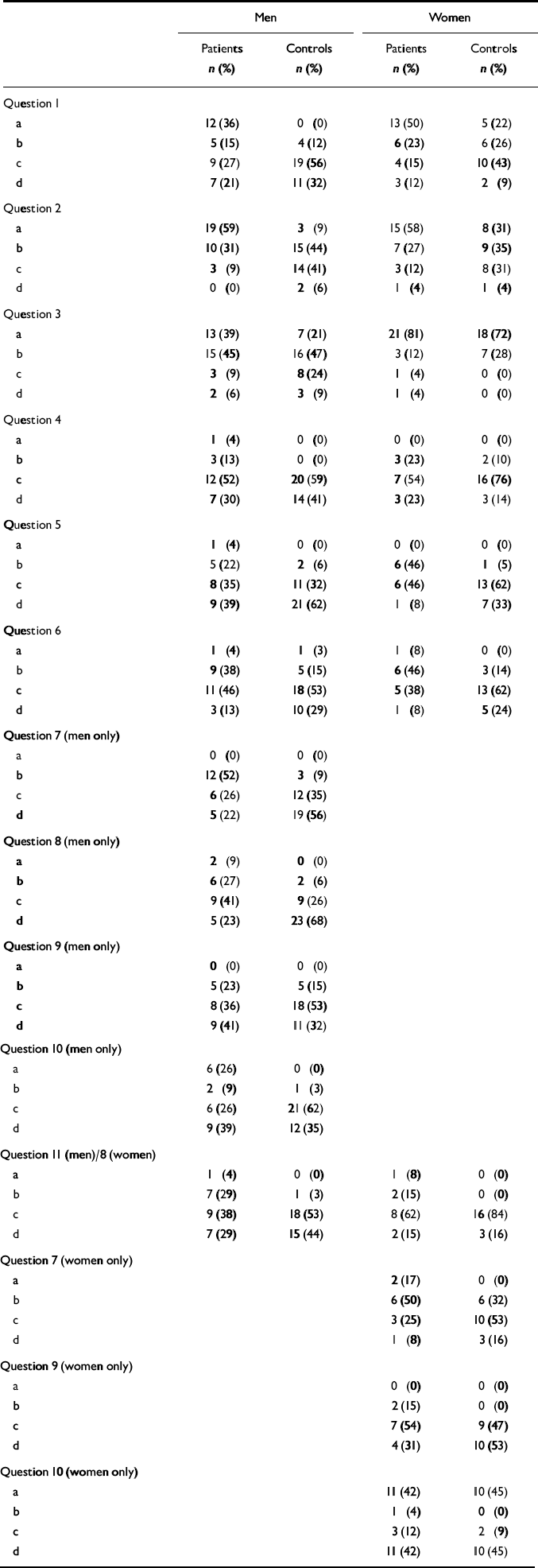
| Men | Women | |||
|---|---|---|---|---|
| Patients n (%) | Controls n (%) | Patients n (%) | Controls n (%) | |
| Question 1 | ||||
| a | 12 (36) | 0 (0) | 13 (50) | 5 (22) |
| b | 5 (15) | 4 (12) | 6 (23) | 6 (26) |
| c | 9 (27) | 19 (56) | 4 (15) | 10 (43) |
| d | 7 (21) | 11 (32) | 3 (12) | 2 (9) |
| Question 2 | ||||
| a | 19 (59) | 3 (9) | 15 (58) | 8 (31) |
| b | 10 (31) | 15 (44) | 7 (27) | 9 (35) |
| c | 3 (9) | 14 (41) | 3 (12) | 8 (31) |
| d | 0 (0) | 2 (6) | 1 (4) | 1 (4) |
| Question 3 | ||||
| a | 13 (39) | 7 (21) | 21 (81) | 18 (72) |
| b | 15 (45) | 16 (47) | 3 (12) | 7 (28) |
| c | 3 (9) | 8 (24) | 1 (4) | 0 (0) |
| d | 2 (6) | 3 (9) | 1 (4) | 0 (0) |
| Question 4 | ||||
| a | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| b | 3 (13) | 0 (0) | 3 (23) | 2 (10) |
| c | 12 (52) | 20 (59) | 7 (54) | 16 (76) |
| d | 7 (30) | 14 (41) | 3 (23) | 3 (14) |
| Question 5 | ||||
| a | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| b | 5 (22) | 2 (6) | 6 (46) | 1 (5) |
| c | 8 (35) | 11 (32) | 6 (46) | 13 (62) |
| d | 9 (39) | 21 (62) | 1 (8) | 7 (33) |
| Question 6 | ||||
| a | 1 (4) | 1 (3) | 1 (8) | 0 (0) |
| b | 9 (38) | 5 (15) | 6 (46) | 3 (14) |
| c | 11 (46) | 18 (53) | 5 (38) | 13 (62) |
| d | 3 (13) | 10 (29) | 1 (8) | 5 (24) |
| Question 7 (men only) | ||||
| a | 0 (0) | 0 (0) | ||
| b | 12 (52) | 3 (9) | ||
| c | 6 (26) | 12 (35) | ||
| d | 5 (22) | 19 (56) | ||
| Question 8 (men only) | ||||
| a | 2 (9) | 0 (0) | ||
| b | 6 (27) | 2 (6) | ||
| c | 9 (41) | 9 (26) | ||
| d | 5 (23) | 23 (68) | ||
| Question 9 (men only) | ||||
| a | 0 (0) | 0 (0) | ||
| b | 5 (23) | 5 (15) | ||
| c | 8 (36) | 18 (53) | ||
| d | 9 (41) | 11 (32) | ||
| Question 10 (men only) | ||||
| a | 6 (26) | 0 (0) | ||
| b | 2 (9) | 1 (3) | ||
| c | 6 (26) | 21 (62) | ||
| d | 9 (39) | 12 (35) | ||
| Question II (men)/8 (women) | ||||
| a | 1 (4) | 0 (0) | 1 (8) | 0 (0) |
| b | 7 (29) | 1 (3) | 2 (15) | 0 (0) |
| c | 9 (38) | 18 (53) | 8 (62) | 16 (84) |
| d | 7 (29) | 15 (44) | 2 (15) | 3 (16) |
| Question 7 (women only) | ||||
| a | 2 (17) | 0 (0) | ||
| b | 6 (50) | 6 (32) | ||
| c | 3 (25) | 10 (53) | ||
| d | 1 (8) | 3 (16) | ||
| Question 9 (women only) | ||||
| a | 0 (0) | 0 (0) | ||
| b | 2 (15) | 0 (0) | ||
| c | 7 (54) | 9 (47) | ||
| d | 4 (31) | 10 (53) | ||
| Question 10 (women only) | ||||
| a | 11 (42) | 10 (45) | ||
| b | 1 (4) | 0 (0) | ||
| c | 3 (12) | 2 (9) | ||
| d | 11 (42) | 10 (45) | ||
Male patients had less desire for sexual intercourse: 17 (52%) v. 4 (12%); χ2=12.30, d.f.=1, P=0.001; were less likely to achieve an erection: 12 (52%) v. 3 (9%); χ2=11.58, d.f.=1, P=0.0007; were less likely to maintain an erection: 8 (36%) v. 2 (6%); χ2=6.51, d.f.=1, P=0.009; were more likely to ejaculate too quickly: 8 (35%) v. 1 (3%); χ2=8.20, d.f.=1, P=0.002; and were less satisfied with the intensity of their orgasms: 8 (33%) v. 1 (3%); χ2=7.73, d.f.=1, P=0.002.
Women
More female patients than controls had at least one sexual dysfunction: 23 (96%) v. 14 (58%); χ2=7.55, d.f.=1, P=0.006; and patients were less likely to enjoy sex: 6 (46%) v. 1 (5%); χ2=6.07, d.f.=1, P=0.007. Although 13 (50%) female patients did not have sexual intercourse and did not masturbate and 19 (73%) had little or no desire for sex, these numbers were not significantly higher, statistically speaking, than in the control group (n=5, 19% for sexual intercourse and masturbation; n=11, 46% for sexual desire).
Factors associated with sexual dysfunction
Within the group of patients (n=98) we examined associations between sexual dysfunction and having or not having a partner, mental state, types of antipsychotic medication and smoking. The areas of sexual dysfunction examined were those in which we had found differences between patients and controls.
Partners
There was no difference in any area of sexual dysfunction between those who did and did not have a partner.
Mental state
There was no difference between men and women patients in mean total and sub-scale PANSS scores. In male patients there was no statistically significant association between any area of sexual dysfunction and PANSS total and sub-scale scores. In female patients, those reporting problems with enjoyment during sex had higher negative symptom scores (mean 16.2, s.d. 7.8 v. mean 10.6, s.d. 4.5; t=0.4, d.f.=22, P=0.01); general psychopathology scores (mean 31.3, s.d. 4.5 v. mean 23.6, s.d. 6.4; t=3.2, d.f.=22, P=0.001); and total scores (mean 62.0, s.d. 12.6 v. mean 47.4, s.d. 14.9; t=2.5, d.f.=22, P=0.01).
Medication
As only eight patients were not taking antipsychotic medication, numbers were too small to compare those taking and not taking such drugs. There was no statistically significant difference between men and women in the proportion of those taking typical and atypical antipsychotic drugs (Table 3). In both men and women patients there was no association between type of antipsychotic medication and sexual dysfunction, or between taking or not taking antidepressant medication and sexual dysfunction.
Table 3 Medication regimen of participants with schizophrenia
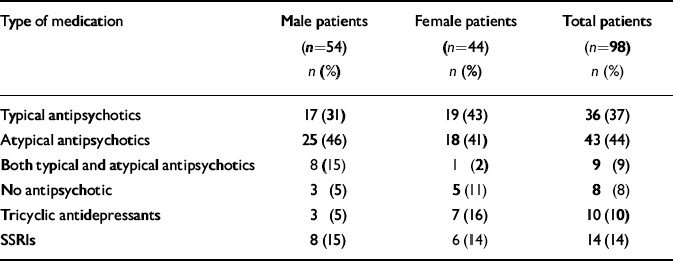
| Type of medication | Male patients (n=54) n (%) | Female patients (n=44) n (%) | Total patients (n=98) n (%) |
|---|---|---|---|
| Typical antipsychotics | 17 (31) | 19 (43) | 36 (37) |
| Atypical antipsychotics | 25 (46) | 18 (41) | 43 (44) |
| Both typical and atypical antipsychotics | 8 (15) | 1 (2) | 9 (9) |
| No antipsychotic | 3 (5) | 5 (11) | 8 (8) |
| Tricyclic antidepressants | 3 (5) | 7 (16) | 10 (10) |
| SSRIs | 8 (15) | 6 (14) | 14 (14) |
Smoking
Sixty-five (66%) patients were current smokers. There was no statistically significant difference in the number of men (41, 77%) and women (24, 55%) who smoked, nor in the mean number of cigarettes smoked per day (men 30, s.d. 10; women 22, s.d. 11). Among male patients, those who did not smoke had less desire for sexual intercourse: 10 (83%) v. 14 (36%); χ2=6.90, d.f.=1, P=0.01. In female patients there was no association between smoking and any area of sexual dysfunction.
DISCUSSION
Sexual dysfunction questionnaire
Other studies to assess sexual dysfunction in schizophrenia have used a variety of techniques: review of medical records (Reference Mullen, Brar and VagnucciMullen et al, 2001); open interview (Reference Kotin, Wilbert and VerburgKotin et al, 1976); semi-structured interview (Reference Teusch, Scherbaum and BöhmeTeusch et al, 1995; Reference Kockott and PfeifferKockott & Pfeiffer, 1996); self-rating questionnaires completed in the presence of an interviewer (Reference Ghadirian, Chouinard and AnnableGhadirian et al, 1982; Reference Aizenberg, Zemishlany and Dorfman-EtrogAizenberg et al, 1995; Reference Smith, O'Keane and MurraySmith et al, 2002); and structured interview and questionnaire (Reference RabochRaboch, 1984).
In our study, which had a response rate of 72%, we used a self-completed, gender-specific questionnaire. People were given the opportunity to complete it in privacy and in their own time. The advantages of a self-completed questionnaire include less embarrassment, which might encourage people to be more honest in their answers, and the absence of interviewer bias. A disadvantage of this method is that responders are not able to address any points they do not understand within the questionnaire. To overcome this problem we used colloquial explanations for such words as ‘erection’ and ‘orgasm’ and used the same question type throughout. Only 9% of the questionnaires were incomplete, which suggests that most people had understood the questions being asked.
We devised our own self-rating questionnaire because at the time we could find none that could be completed by both men and women, by those with and without a partner, and that covered the main areas of sexual functioning: namely, desire, arousal, performance and satisfaction. A recent paper (Reference Smith, O'Keane and MurraySmith et al, 2002) used a questionnaire somewhat similar to ours.
Reliability and validity
When this paper was first submitted for publication an assessor sought information about the reliability and validity of the scale. In only one scale used in the studies quoted in the first paragraph of this discussion was reliability assessed (Reference Smith, O'Keane and MurraySmith et al, 2002).
Although they cannot be measured, we believe that our scale has both content validity (the scale contains the number and content of questions appropriate to the attribute to be measured) and face validity (the scale appears to measure what it is supposed to measure). Interrater reliability is not relevant as it is only applicable to observer-rated scales. The scale does not lend itself to assessment of split-half reliability. However, we have some additional evidence that the scale, or at least part of it, may be both reliable and valid. As a result of this survey, carried out in 1999, we realised that erectile dysfunction was a major problem for men with schizophrenia. In 2002 we embarked on a randomised, placebo-controlled study of sildenafil in erectile dysfunction in men with schizophrenia. To enter the study, patients fulfilled the following criterion: ‘the patient for 6 months or longer has been unable to achieve or maintain an erection, sufficient for satisfactory sexual performance either with a partner or through masturbation’. Seven men who volunteered for the sildenafil study and fulfilled the entry criterion had been reviewed in 1999, and their psychotropic medication had remained unchanged over the subsequent 3 years. Of the seven, six (86%) in the 1999 survey had reported either ‘never’ or only ‘occasionally’ getting an erection. Therefore the patients' assessments of themselves had not changed over 3 years, a measure of test—retest reliability. Also, if patients did not have erectile dysfunction, it is unlikely that they would volunteer for such a study — a measure of predictive validity.
Patients and controls
The principal difference between the patient and control groups was that the majority of the people in the latter had partners. However, in our questionnaire responders were asked to answer each question with reference to either sexual intercourse or masturbation. A previous study (Reference Aizenberg, Zemishlany and Dorfman-EtrogAizenberg et al, 1995), which considered only those with a partner, found higher rates of sexual dysfunction in people with schizophrenia. One study found that having a partner was protective against sexual dysfunction (Reference RabochRaboch, 1984); another did not (Reference Kockott and PfeifferKockott & Pfeiffer, 1996). In our study the level of sexual dysfunction was the same in patients with and without partners.
Sexual dysfunction
Sexual dysfunction was common in patients, with 82% of men and 96% of women reporting at least one sexual dysfunction. Fewer male patients than controls reported any sexual activity, whether sexual intercourse or masturbation. Where sexual activity was reported, male patients reported a broader range of sexual dysfunction than controls, with desire, performance and satisfaction all affected. The most prominent problem was difficulty achieving an erection (52%). This percentage is a little higher than in previous studies, in which the percentages were 38% (Reference Ghadirian, Chouinard and AnnableGhadirian et al, 1982) and 47% (Reference Teusch, Scherbaum and BöhmeTeusch et al, 1995).
There were fewer differences between female patients and controls, largely because sexual dysfunction was also wide-spread in the control group. Differences were in enjoyment of sex, a finding reported in a previous study (Reference Miller and FinnertyMiller & Finnerty, 1996). In another study (Reference Teusch, Scherbaum and BöhmeTeusch et al, 1995) 60% of women lacked interest in sex and 92% had at least one sexual dysfunction. Ghadirian et al (Reference Ghadirian, Chouinard and Annable1982) reported that 30% had current difficulty in sexual functioning. Finally, Friedman & Harrison (Reference Friedman and Harrison1984) found that 60% of female patients had never had an orgasm, compared with 13% of controls.
Mental state
A previous study (Reference Kockott and PfeifferKockott & Pfeiffer, 1996) found an association in both male and female patients between being less well mentally and having sexual dysfunction. A study of long-stay patients found that the severely ill patients had less interest in sex (Reference Lyketsos, Sakka and MailisLyketsos et al, 1983). A study of patients receiving conventional antipsychotic medication found an association between depression and sexual dysfunction (Reference Smith, O'Keane and MurraySmith et al, 2002). In our study, there was an association in female but not in male patients between a poorer mental state and sexual dysfunction. Scores were higher in both the negative symptoms and general psychopathology sub-scales; perhaps symptoms of withdrawal, anxiety and depression contribute to sexual dysfunction in women patients.
Medication
Most classes of antipsychotic drugs are implicated in sexual dysfunction (Reference Sadock, Kaplan and SadockSadock, 1989). However, evaluating their effect on sexual dysfunction is complicated by the illness itself, compliance with treatment, and an incomplete understanding of all the variables involved in human sexual functioning.
In our study there were too few patients not receiving antipsychotic medication to compare those taking and not taking such drugs. One previous study (Reference Kockott and PfeifferKockott & Pfeiffer, 1996) found a trend only between receiving antipsychotic medication and sexual dysfunction. Another study (Reference Aizenberg, Zemishlany and Dorfman-EtrogAizenberg et al, 1995) found that the quality of coital erections was significantly more reduced in men with schizophrenia treated with antipsychotic medication than in a similar untreated group.
The newer atypical antipsychotic drugs have a greater affinity for serotonin (5-hydroxytryptamine) 5-HT2 receptors than for dopamine D2 receptors. This is believed to account for the reduced incidence of hyperprolactinaemia in patients receiving this class of antipsychotic. Studies suggest that these drugs may cause fewer sexual side-effects (Reference Meltzer, Goode and SchyveMeltzer et al, 1979; Reference Aizenberg, Modai and LandaAizenberg et al, 2001). However, in our study we found no difference in the reporting of sexual dysfunction between those taking typical and atypical antipsychotic drugs, as did a previous study (Reference Hummer, Kemmler and KurzHummer et al, 1999). This may reflect the impact that the schizophrenic illness itself has on sexual functioning. It may also be that dopamine receptor blockade and hyperprolactinaemia are only a small part of the complex relationship between illness, treatment and sexual functioning.
Smoking
In our study 67% of patients were current smokers. Non-smoking male patients had less desire for sex than did those who smoked. Lower blood levels of antipsychotic drugs in men who smoke may be one possible explanation of this finding; smoking increases the metabolism of antipsychotic drugs by inducing hepatic microsomal enzymes (Reference Salokangas, Saarijarvi and TaiminemSalokangas et al, 1997).
Treatment of sexual dysfunction
We conclude that sexual dysfunction is very common indeed in patients with schizophrenia. This is yet another aspect of the poor quality of life led by many people with schizophrenia that should be addressed. To this end, we have now embarked on the first double-blind, placebo-controlled, randomised trial of sildenafil in male patients with schizophrenia and erectile dysfunction.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ The majority of patients with schizophrenia have at least one form of sexual dysfunction.
-
▪ Erectile dysfunction was present in the majority of male patients.
-
▪ The findings highlight yet another aspect of the poor quality of life led by many people with schizophrenia.
LIMITATIONS
-
▪ Few of the patients but most members of the control group had a partner.
-
▪ We used a self-report questionnaire not previously validated in other studies.
-
▪ As only small numbers of patients were not receiving antipsychotic medication, it was not possible to separate the effects of illness and medication on sexual dysfunction.
APPENDIX
Sexual Behaviour Questionnaire
The participants were asked to complete the following, with the assurance that the answers would be completely confidential by circling the applicable letter in each question. A stamped addressed envelope was provided for the return of the completed questionnaire.
-
1. How frequently would you like to have sexual intercourse?
-
(a) I have no desire for sexual intercourse.
-
(b) I would like to have sexual intercourse less than once per week.
-
(c) I would like to have sexual intercourse 1-3 times per week.
-
(d) I would like to have sexual intercourse more than 3 times per week.
-
-
2. Do you have sexual intercourse?
-
(a) I do not have sexual intercourse.
-
(b) I have sexual intercourse less than once per week.
-
(c) I have sexual intercourse 1-3 times per week.
-
(d) I have sexual intercourse more than 3 times per week.
-
-
3. How often do you masturbate?
-
(a) I never masturbate.
-
(b) I masturbate less than once per week.
-
(c) I masturbate 1-3 times per week.
-
(d) I masturbate more than 3 times per week.
-
-
4. How easily are you excited during sex (or masturbation)
-
(a) I am never excited at all.
-
(b) I am excited with difficulty.
-
(c) I am excited moderately easily.
-
(d) I am excited very easily.
-
(e) I do not have sexual intercourse or masturbate.
-
-
5. How would you describe your ability to enjoy sex (either sex or masturbation)
-
(a) I never enjoy sex.
-
(b) I occasionally enjoy sex.
-
(c) I often enjoy sex.
-
(d) I always enjoy sex.
-
(e) I do not have sex or masturbate.
-
-
6. How satisfied are you with your sex life? (either sex or masturbation)
-
(a) I am never satisfied.
-
(b) I am occasionally satisfied.
-
(c) I am often satisfied.
-
(d) I am always satisfied.
-
(e) I do not have sex or masturbate.
For men:
-
-
7 Do you ever get an erection? (a hard-on) (either sex or masturbation)
-
(a) I never get an erection.
-
(b) I occasionally get an erection.
-
(c) I often get an erection.
-
(d) I always get an erection.
-
(e) I do not have sex or masturbate.
-
-
8. How often can you maintain an erection (a hard-on)? (either sex or masturbation)
-
(a) I am never able to maintain an erection.
-
(b) I am occasionally able to maintain an erection.
-
(c) I am often able to maintain an erection.
-
(d) I am always able to maintain an erection.
-
(e) I do not have sex or masturbate.
-
-
9 How often is ejaculation delayed (took a long time to come)? (either sex or masturbation)
-
(a) Ejaculation is always delayed.
-
(b) Ejaculation is often delayed.
-
(c) Ejaculation is occasionally delayed.
-
(d) Ejaculation is never delayed.
-
(e) I do not have sex or masturbate.
-
-
10. How often do you ejaculate too quickly (come too quickly)? (either sex or masturbation)
-
(a) I always ejaculate too quickly.
-
(b) I often ejaculate too quickly.
-
(c) I occasionally ejaculate too quickly.
-
(d) I never ejaculate too quickly.
-
(e) I do not have sex or masturbate.
-
-
11. How satisfied are you with the intensity of your orgasm (come)? (either sex or masturbation)
-
(a) I am not at all satisfied.
-
(b) I am slightly satisfied.
-
(c) I am moderately satisfied.
-
(d) I am highly satisfied.
-
(e) I do not have sex or masturbate.
For women:
-
-
7. How easily do you have an orgasm (come)? (either sex or masturbation)
-
(a) I never have an orgasm.
-
(b) I occasionally have an orgasm.
-
(c) I frequently have an orgasm.
-
(d) I always have an orgasm.
-
(e) I do not have sex or masturbate.
-
-
8. How satisfied are you with the intensity of your orgasm (come)? (either sex or masturbation)
-
(a) I am not at all satisfied.
-
(b) I am slightly satisfied.
-
(c) I am moderately satisfied.
-
(d) I am highly satisfied.
-
(e) I do not have sex or masturbate.
-
-
9. Do you experience pain during sexual intercourse?
-
(a) I always experience pain.
-
(b) I often experience pain.
-
(c) I occasionally experience pain.
-
(d) I never experience pain.
-
(e) I do not have sex or masturbate.
-
-
10. How regularly do you menstruate? (i.e. have a period)
-
(a) I no longer menstruate as I have reached the menopause.
-
(b) I have not menstruated for over 6 months.
-
(c) I do not menstruate regularly every month but I have menstruated within the last 6 months.
-
(d) I menstruate regularly every month.
-
Acknowledgements
We thank the patients and the control group for their cooperation, and Miss Heather Barrington for statistical advice.






eLetters
No eLetters have been published for this article.