About two-thirds of patients with a depressive disorder respond to antidepressant drugs. This proportion was described in the 1950s at the time it was discovered that monoamine oxidase inhibitors (MAOIs) and imipramine had antidepressant properties (Reference HealyHealy, 1997). In the four decades since, there has been enormous progress in neuroscience. The pharmacology of the first antidepressants is now known in greater detail and we have seen increasing development of new antidepressants with specific, designed pharmacological properties. In spite of these advances, there has been no convincing demonstration that an antidepressant has any greater efficacy than the first serendipitously discovered drugs, although progress has been made in improving side-effects and safety. However, for over a decade it has been recognised that combinations of drugs may be more effective than a single drug: the best combination established is the augmentation of antidepressants with lithium (Reference Austin, Souza and GoodwinAustin et al, 1991). This suggests that it should be possible to design a drug with more than one pharmacological action, which would be more effective than the selective, single-action drugs. Clinical belief in the greater effectiveness of clomipramine, and recent claims that some drugs, such as venlafaxine (Clerc et al, 1994), may be more effective than the selective serotonin reuptake inhibitor (SSRI) fluoxetine, have raised the issue of whether a joint action in inhibiting the reuptake of both 5-hydroxytryptamine (serotonin, 5-HT) and noradrenaline may confer added benefit. This has also been suggested by open studies of combined treatment with an SSRI and a tricyclic antidepressant (TCA) (Reference Nelson, Mazure and BowersNelson et al, 1991). Systematic reviews, using different methodologies, seeking to find out whether some antidepressants may be more effective than SSRIs, have reached differing conclusions. One overview of the effectiveness of various antidepressant drugs found statistical heterogeneity (systematic differences between studies) in treatment effects estimated in different studies, but not significant benefit for any one agent compared with others (Reference Geddes, Freemantle and MasonGeddes et al, 2000). Other systematic reviews have suggested that SSRIs may be less effective than amitriptyline (Reference AndersonAnderson, 2000), TCAs (in in-patients) (Reference AndersonAnderson, 1998) and venlafaxine (Reference Rudolph, Entsuah and ChitraRudolph et al, 1998).
One way to address these discrepancies is to ask whether particular pharmacological properties or their combination might increase efficacy. We used an extension of traditional meta-analytic methods - meta-regression - which provides a robust new way of exploring the factors which could explain differences between treatments. In addition, other potentially confounding factors which may affect relative efficacy were investigated.
METHOD
Objective
Our primary objective was to examine the predictive value of different pharmacological action for antidepressant drugs, singly and in combination, on outcome. The factors studied were noradrenaline reuptake inhibition, serotonin (5-HT) reuptake inhibition and 5-HT2 receptor antagonism. They were chosen because they have all, independently, been associated with antidepressant activity in specific drugs.
The important structural factors examined were: treatment setting (inpatient v. out-patient or family practice); dose of comparator (high v. low dose, based on the British National Formulary (British Medical Association & Royal Pharmaceutical Society of Great Britain, 1997), with a daily dose of < 100 mg of most comparators defined as a low dose, apart from 75 mg for nortriptyline and venlafaxine, 45 mg for mianserin, 150 mg for trazodone, 200 mg for nefazodone); method of analysis (last observation carried forward v. end-point analysis); age of patients (defined as over 65 or of mixed age); measurement scale used (either Hamilton Rating Scale for Depression (Reference HamiltonHamilton, 1960) or alternative scale); sponsor of the trial (where not stated, taken as SSRIs in comparisons with TCAs and older antidepressants, and the comparator in studies against drugs marketed since SSRIs).
Data-set and included trials
We analysed all available double-blind randomised trials which compared treatment of depression with an SSRI and with an alternative antidepressant drug that had a primary effect on 5-HT and/or noradrenaline reuptake and/or 5-HT2 antagonism. This data-set was chosen because it provides a large group of studies of antidepressants with a well-defined single pharmacological action (5-HT reuptake inhibition). Eligible trials had to include adult or elderly patients with a major depressive episode for which relevant data were available. As SSRIs are a relatively homogeneous group in terms of pharmacological action, the planned comparisons enabled us to examine the relative efficacy of other antidepressants with different single and combined sites of action against a common standard. Given the increasing pre-eminence of SSRIs in first-line treatment of depressive illness, this is also relevant to current practice.
Classification of drugs
Pharmacological classification of drugs was undertaken using the best available evidence. There are considerable difficulties in doing this, including availability of data in humans (species differences may be important), extrapolation from binding or in vitro data to activity in vivo (including the threshold at which an action becomes important) and the effect of metabolites. The classification used is described in Table 1 and is based, as far as possible, on recently available human binding data. Some generally accepted assumptions appeared less than well founded, from the available data, and there was uncertainty about the classification of some drugs. With regard to 5-HT reuptake inhibition, some drugs traditionally regarded, on the basis of studies in rats, as having minimal activity (especially dothiepin, but also nortriptyline and desipramine) may in fact have a significant degree of affinity for the human 5-HT transporter (Reference Tatsumi, Groshan and BlakelyTatsumi et al, 1997). In the case of desipramine and nortriptyline, dynamic studies in transfected cells or human platelets found low activity (Reference LingjaerdeLingjaerde, 1985; Reference Barker, Blakely, Bloom and KupferBarker & Blakely, 1995), but uncertainty remains about dothiepin. Trazodone and nefazodone are sometimes described as 5-HT reuptake inhibitors, but both animal and human data suggest low affinity for, and activity at, the 5-HT transporter (Reference Richelson and PfenningRichelson & Pfenning, 1984; Reference LingjaerdeLingjaerde, 1985; Reference Tatsumi, Groshan and BlakelyTatsumi et al, 1997). With regard to noradrenaline reuptake inhibition, the main uncertainty centred on venlafaxine, marketed as having both 5-HT and noradrenaline activity. However, the most comprehensive animal and human data indicate that it has low affinity for the noradrenaline transporter (Reference Bolden-Watson and RichelsonBolden-Watson & Richelson, 1993; Reference Tatsumi, Groshan and BlakelyTatsumi et al, 1997) and human functional data suggest that inhibition of noradrenaline reuptake only occurs at higher doses (Reference Abdelmawla, Langley and SzabadiAbdelmawla et al, 1999). Concerning antagonism of human 5-HT2 receptors, there is some uncertainty about the activity of clomipramine, which shows relatively low binding in animal studies (Reference Pälvimäki, Roth and MajasuoPälvimäki et al, 1996), higher affinity in the human brain (Reference Wander, Nelson and OkazakiWander et al, 1986), but intermediate binding and activity in platelets (Reference Ohsuka, Mashiko and KanekoOhsuka et al, 1995), raising uncertainty as to its effect in vivo, particularly at lower doses. The implication of this uncertainty was assessed in each case through a sensitivity analysis in which the initial classification excluded borderline properties, but separate analyses were performed in which they were included.
Table 1 Selected pharmacological action of antidepressants in humans

| 5-HT reuptake | Noradrenaline reuptake | 5-HT2 antagonism | |
|---|---|---|---|
| Tricyclic antidepressants | |||
| Amitriptyline | + | + | + |
| Clomipramine | + | + | ? |
| Desipramine | - | + | - |
| Dothiepin | ? | + | - |
| Doxepin | - | + | + |
| Imipramine | + | + | - |
| Lofepramine | - | + | - |
| Nortriptyline | - | + | + |
| Selective serotonin reuptake inhibitors | |||
| Citalopram | + | - | - |
| Fluoxetine | + | - | - |
| Fluvoxamine | + | - | - |
| Paroxetine | + | - | - |
| Sertraline | + | - | - |
| Others | |||
| Amoxapine | - | + | + |
| Buproprion | - | - | - |
| Maprotiline | - | + | - |
| Mianserin | - | - | + |
| Nefazodone | - | - | + |
| Nomifensine | - | + | - |
| Trazodone | - | - | + |
| Venlafaxine | + | ? | - |
Search strategy
We undertook an optimally sensitive electronic search for randomised trials meeting our entry criteria. We searched Medline (1966-1997 via OVID) and EMBASE (1974-1997 via DIALOG) and reviewed the reference list of each identified study. Existing bibliographies and reviews for relevant studies were also examined.
Data abstraction
For each study located, data on main outcome were abstracted. The Hamilton Depression Rating Scale (Reference HamiltonHamilton, 1960) was the preferred outcome scale, but where this was not available the Montgomery-Åsberg Depression Rating Scale (Reference Montgomery and ÅsbergMontgomery & Åsberg, 1979), or the Clinical Global Impression Scale (Reference GuyGuy, 1976) were abstracted. Where data were not available in published reports, we routinely contacted the principal author and, where necessary, the sponsor of the study, to request data.
Data synthesis
Standardised effect sizes for each arm of included trials were estimated from the data, using the final rating scale score and the pooled estimate of study variance as described by Hedges & Olkin (Reference Hedges and Olkin1985). The use of an effect size has the advantage of standardising the scores from different studies, which may adopt differing approaches to assessing treatment effect, on a common and thus comparable scale.
We used a meta-regression technique to examine the extent to which the value of individual factors such as specific pharmacological properties predicted a positive outcome in the trials. We have taken a similar approach in other meta-regression analyses (Reference Davis, Thomson O'Brien and FreemantleDavis et al, 1999; Reference Freemantle, Cleland and YoungFreemantle et al, 1999). BUGS software, described by Smith et al (Reference Smith, Spiegelhalter and Thomas1995), was used to specify the statistical model that attempted to explain variation in the results of different studies on the basis of a range of potentially important factors. This approach is analogous to standard regression analysis, but takes into account the fact that study results are estimated with measurement error (described by the confidence intervals), rather than known. The covariate terms for each factor applied to the model are multipliers which describe the positive or negative impact of different factors on the observed results. Where the estimated effect of a factor is not significantly different from zero, it does not contribute to an understanding of the differences in observed results, and so is not considered further in the analysis.
The statistical methods applied in this analysis have been developed relatively recently and are the subject of considerable interest. Further details of the general approach are available in the excellent introductory text by Gilks et al (Reference Gilks, Richardson and Spiegelhalter1996) and details of the software are available from http://www.mrc-bsu.cam.ac.uk/bugs/.
RESULTS
In total, 105 trials comparing SSRIs with alternative antidepressant drugs were included. These trials looked at 11 537 patients - 5937 treated with an SSRI contrasted with 5600 treated with an alternative antidepressant drug. The most commonly used SSRI was fluoxetine, while the most commonly used alternative was amitriptyline. Trials of five SSRIs and 12 comparator drugs were identified. The major characteristics of each trial included are described in Table 2.
Table 2 Major characteristics of included trials (references are listed in the Appendix)

| Trial | Effect size | Number in comparator group | Number in treatment group | SSRI | Comparator | Setting | Age | Methods | Scale | Active treatment (weeks) | Dose of SSRI | Dose of comparator |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference Ahlfors, Elovaara and HarmaAhlfors et al, 1988 | 0.63 | 34 | 37 | Citalopram | Mianserin | Out-patients/family practice | Adult | End-point | MADRS | 4 | 38.6 | 61.1 |
| Reference Amin, Anath and ColemanAmin et al, 1984 | -0.11 | 106 | 105 | Fluvoxamine | Imipramine | Out-patients/family practice | Adult | LOCF | HAM-D | 6 | 155 | 156 |
| Arminen et al, 1982 | -0.30 | 29 | 21 | Paroxetine | Imipramine | In-patients | Adult | End-point | HAM-D | 12 | 20-40 | 100-200 |
| Reference Baker, Dorian and SandorBaker et al, 1997 | 0.01 | 19 | 20 | Fluoxetine | Doxepin | Out-patients | Adult | End-point | HAM-D | 6 | 97 | 169 |
| Reference Baldwin, Halwy and AbedBaldwin et al, 1996 | -0.08 | 100 | 95 | Paroxetine | Nefazodone | Out-patients | Adult | LOCF | HAM-D | 8 | 32.7 | 472 |
| Reference Battegay, Hager and RauchfleischBattegay et al, 1985 | 0.10 | 6 | 8 | Paroxetine | Amitriptyline | Out-patients | Adult | LOCF | HAM-D | 6 | 30 | 75 |
| Reference Beasley, Dornseif and PultzBeasley et al, 1991 | 0.11 | 57 | 63 | Fluoxetine | Trazodone | Out-patients | Adult | LOCF | HAM-D | 6 | 20.9 | 244.1 |
| Reference Beasley, Holman and PotvinBeasley et al, 1993a | 0.47 | 60 | 54 | Fluoxetine | Imipramine | In-patients | Adult | LOCF | HAM-D | 6 | 72 | 192 |
| Reference Beasley, Sayler and PotvinBeasley et al, 1993b | -0.08 | 71 | 65 | Fluoxetine | Amitriptyline | Out-patients | Adult | LOCF | HAM-D | 5 | 65.2 | 201.4 |
| Reference Berlanga, Arechavaleta and HeinzeBerlanga et al, 1997 | -0.17 | 36 | 37 | Fluoxetine | Nefazodone | Out-patients | Adult | LOCF | HAM-D | 8 | 24.0 | 400.0 |
| Reference Bersani, Rapisarda and CianiBersani et al, 1994 | 0.00 | 30 | 31 | Sertraline | Amitriptyline | Out-patients | Adult | End-point | HAM-D | 8 | 88 | 84 |
| Reference Besançon, Cousin, Guitton and LavergneBesançon et al, 1993 | 0.63 | 32 | 33 | Fluoxetine | Mianserin | Out-patients | Adult | End-point | MADRS | 8 | 26.7 | 72 |
| Reference Bouchard, Dalaunay and DelisleBouchard et al, 1987 | 0.01 | 34 | 39 | Citalopram | Maprotiline | In-patients | Adult | End-point | MADRS | 4 | 46 | 101 |
| Reference Bramanti, Ricci and RoncariBramanti et al, 1988 | 0.58 | 29 | 28 | Fluvoxamine | Imipramine | Not clear | Adult | LOCF | HAM-D | 4 | 100-120 | 100-135 |
| Reference BremnerBremner, 1984 | -1.32 | 19 | 16 | Fluoxetine | Imipramine | Out-patients | Adult | End-point | CGI | 5 | 60 | 175-200 |
| Reference Byerley, Reimherr and WoodByerley et al, 1988 | -0.11 | 24 | 20 | Fluoxetine | Imipramine | Out-patients | Adult | End-point | HAM-D | 6 | 40-80 | 150-300 |
| Reference Christiansen, Behnke and BlackChristiansen et al, 1996 | 0.20 | 57 | 56 | Paroxetine | Amitriptyline | Family practice | Adult | End-point | HAM-D | 8 | 28.1 | 112.7 |
| Reference Clerc, Ruimy and Verdeau-PaillèsClerc et al, 1994 | 0.58 | 33 | 34 | Fluoxetine | Venlafaxine | In-patients | Adult | LOCF | HAM-D | 6 | 40 | 200 |
| Reference Cohn and WilcoxCohn & Wilcox, 1984 | 0.02 | 31 | 35 | Fluoxetine | Imipramine | Out-patients | Adult | End-point | HAM-D | 6 | ||
| Reference Cohn, Shrivastava and MendelsCohn, C. K. et al, 1990 | -0.07 | 64 | 121 | Sertraline | Amitriptyline | Out-patients | Elderly | End-point | HAM-D | 8 | 116.2 | 88.3 |
| Reference Cohn, Crowder and WilcoxCohn, J. B. et al, 1990 | 0.24 | 31 | 35 | Paroxetine | Imipramine | Out-patients | Adult | End-point | HAM-D | 6 | 65 | 275 |
| Reference Corne and HallCorne & Hall, 1989 | 0.42 | 44 | 34 | Fluoxetine | Dothiepin | Family practice | Adult | End-pont | HAM-D | 8 | 40± | 75± |
| Reference Dalery, Rochat and PeyronDalery et al, 1992 | 0.09 | 68 | 73 | Fluoxetine | Amineptine | Out-patients | Adult | End-point | MADRS | 13 | 20 | 200 |
| Danish University, 1990 | 0.65 | 36 | 34 | Paroxetine | Clomipramine | In-patients | Adult | End-point | HAM-D | 6 | 30 | 150 |
| Reference de Jonghe, Ravelli and Tuynman-Quade Jonghe et al, 1991a | 0.33 | 34 | 28 | Fluoxetine | Maprotiline | In-patients | Adult | End-point | HAM-D | 6 | 40-80 | 50-150 |
| Reference de Jonghe, Swinkels and Tuynman-Quade Jonghe et al, 1991b | -0.01 | 21 | 21 | Fluvoxamine | Maprotiline | Out-patients | Adult | End-point | HAM-D | 6 | 100-300 | 50-150 |
| De Mendonça Lima, 1997 | -0.02 | 20 | 20 | Fluvoxamine | Maprotiline | In-patients | Adult | End-point | MADRS | 4 | 100 | 75 |
| Reference De Wilde, Mertens and WakelinDe Wilde et al, 1983 | -0.42 | 15 | 15 | Fluvoxamine | Clomipramine | Out-patients | Adult | End-point | HAM-D | 4 | 259 | 231 |
| Reference De Wilde, Mertens and OveroDe Wilde et al, 1985 | -0.44 | 29 | 29 | Citalopram | Mianserin | In-patients | Adult | LOCF | CGI | 6 | 53.1 | 94.1 |
| Reference Dick and FerroDick & Ferro, 1983 | 0.30 | 13 | 13 | Fluvoxamine | Clomipramine | In-patients | Adult | End-point | HAM-D | 4 | 130.9 | 132.8 |
| Reference Dominguez, Goldstein and JacobsenDominguez et al, 1985 | -0.26 | 19 | 16 | Fluvoxamine | Imipramine | Out-patients | Adult | End-point | CGI | 4 | 100-300 | 100-300 |
| Reference DormanDorman, 1992 | -0.68 | 25 | 24 | Paroxetine | Mianserin | Out-patients | Elderly | End-point | HAM-D | 6 | 15-30 | 30-60 |
| Fabre, 1996 | -0.62 | 48 | 46 | Fluvoxamine | Imipramine | Out-patients | Adult | LOCF | HAM-D | 6 | 117 | 180 |
| Reference Falk, Rosenbaum and OttoFalk et al, 1989 | -0.75 | 12 | 13 | Fluoxetine | Trazodone | Out-patients | Elderly | LOCF | HAM-D | 6 | 48 | 350 |
| Reference Feighner, Boyer and MeridethFeighner et al, 1989 | 0.18 | 45 | 52 | Fluoxetine | Imipramine | Out-patients | Adult | End-point | HAM-D | 6 | NA | NA |
| Reference Fudge, Perry and GarveyFudge et al, 1990 | 0.26 | 15 | 17 | Fluoxetine | Trazodone | Out-patients | Adult | End-point | HAM-D | 6 | 20-60 | 50-400 |
| Reference Geretsegger, Stuppaeck and MairGeretsegger et al, 1995 | -0.19 | 31 | 28 | Paroxetine | Amitriptyline | In-patients | Elderly | End-point | HAM-D | 6 | 22.7 | 109.6 |
| Reference GinestetGinestet, 1989 | 0.89 | 26 | 28 | Fluoxetine | Clomipramine | In-patients | Elderly | Not clear | HAM-D | 8 | 58 | 148 |
| Reference Gonella, Baignoli and EcariGonella et al, 1990 | -0.22 | 20 | 20 | Fluvoxamine | Imipramine | Out-patients | Adult | LOCF | HAM-D | 4 | 140 | 130 |
| Reference Gravem, Amthor and AstrupGravem et al, 1987 | -0.27 | 14 | 12 | Citalopram | Amitriptyline | Out-patients/Family practice | Adult | End-point | CGI | 6 | 36.1905 | 161.84211 |
| Reference Guelfi, Dreyfus and PichotGuelfi et al, 1983 | -0.24 | 68 | 59 | Fluvoxamine | Imipramine | In-patients | Not clear | End-point | HAM-D | 4 | 221 | 112 |
| Reference Guillibert, Pelicier and ArchambaultGuillibert et al, 1989 | 0.03 | 39 | 40 | Paroxetine | Clomipramine | Out-patients | Elderly | Not clear | HAM-D | 6 | 30 | 75 |
| Reference Harris, Szulecka and AnsteeHarris et al, 1991 | 0.63 | 26 | 24 | Fluvoxamine | Amitriptyline | Out-patients | Adult | Not Clear | HAM-D | 6 | 100-150 | 100-150 |
| Hutchinson, 1992 | 0.00 | 21 | 46 | Paroxetine | Amitriptyline | Family practice | Elderly | End-point | HAM-D | 6 | 30 | 100 |
| Reference Itil, Shrivastava and MukherjeeItil et al, 1983 | 0.31 | 14 | 9 | Fluvoxamine | Imipramine | Out-patients | Adult | End-point | HAM-D | 4 | 101 | 127 |
| Reference Judd, Moore and NormanJudd et al, 1993 | -0.33 | 23 | 23 | Fluoxetine | Amitriptyline | Out-patients/Family practice | Adult | End-point | HAM-D | 6 | 20 | 176 |
| Reference Kasper, Voll and VieiraKasper et al, 1990 | 0.05 | 20 | 21 | Fluvoxamine | Maprotiline | In-patients | Adult | LOCF | HAM-D | 4 | 229 | 236 |
| Reference Kasper, Möller and MontgomeryKasper et al, 1995 | -0.11 | 106 | 105 | Fluvoxamine | Imipramine | Out-patients/Family practice | Adult | End-point | HAM-D | 4 | 50-300 | 50-300 |
| Reference Kerkhofs, Rielaert and de MaertelaerKerkhofs et al, 1990 | -0.25 | 10 | 9 | Fluoxetine | Amitriptyline | In-patients | Adult | End-point | HAM-D | 6 | 60 | 150 |
| Reference Klok, Brouwer and Van PraagKlok et al, 1981 | 0.36 | 15 | 13 | Fluvoxamine | Clomipramine | In-patients | Adult | End-point | HAM-D | 4 | 150 | 150 |
| Reference Kuhs and RudolfKuhs & Rudolf, 1989 | 0.08 | 17 | 14 | Paroxetine | Amitriptyline | In-patients | Adult | End-point | HAM-D | 6 | 30 | 150 |
| Reference La Pia, Giorgio and CirielloLa Pia et al, 1992 | -0.31 | 16 | 19 | Fluoxetine | Mianserin | Out-patients/Family practice | Elderly | End-point | HAM-D | 6 | 20 | 40 |
| Laakmann et al, 1988 | 0.53 | 46 | 39 | Fluoxetine | Amitriptyline | Out-patients | Adult | End-point | HAM-D | 5 | 20-60 | 50-150 |
| Laakmann, 1991 | 0.02 | 62 | 62 | Fluoxetine | Amitriptyline | In-patients | Adult | End-point | HAM-D | 6 | 40 | 100 |
| Reference Lapierre, Browne and HornLapierre et al, 1987 | -1.17 | 2 | 7 | Fluvoxamine | Imipramine | In-patients | Adult | End-point | HAM-D | 6 | 207.1 | 191.7 |
| Reference Laursen, Mildcelson and RasmussenLaursen et al, 1985 | 0.07 | 14 | 16 | Paroxetine | Amitriptyline | In-patients | Adult | End-point | HAM-D | 6 | 38.75 | 160.71429 |
| Reference Lydiard, Laird and MortonLydiard et al, 1989 | 0.31 | 15 | 17 | Fluvoxamine | Imipramine | Out-patients | Adult | End-point | HAM-D | 6 | 240 | 180 |
| Reference Lydiard, Stahl and HertzmanLydiard et al, 1997 | 0.16 | 104 | 119 | Sertraline | Amitriptyline | Out-patients | Adult | LOCF | HAM-D | 8 | 90.8 | 91.3 |
| Reference Manna, Martucci and AgnoliManna et al, 1989 | -0.30 | 15 | 15 | Fluoxetine | Clomipramine | In-patients | Adult | LOCF | HAM-D | 6 | 20 | 75 |
| Reference Mertens and PintensMertens & Pintens, 1988 | -0.39 | 31 | 36 | Paroxetine | Mianserin | In-patients | Adult | LOCF | HAM-D | 6 | 30 | 60 |
| Reference Moller, Berzewski and EckmannMoller et al, 1993 | 0.30 | 68 | 72 | Paroxetine | Amitriptyline | In-patients | Not clear | End-point | HAM-D | 6 | 30 | 150 |
| Reference Muijen, Roy and SilverstoneMuijen et al, 1988 | -0.45 | 14 | 14 | Fluoxetine | Mianserin | Out-patients | Adult | End-point | HAM-D | 6 | 60-80 | 60-80 |
| Reference Mullin, Pandita-Gunawardena and WhiteheadMullin et al, 1988 | -0.04 | 24 | 26 | Fluvoxamine | Dothiepin | Out-patients | Adult | End-point | HAM-D | 6 | 100-300 | 75-225 |
| Reference Nathan, Perel and PollackNathan et al, 1990 | -0.08 | 18 | 17 | Fluvoxamine | Desipramine | In-patients | Adult | Not clear | HAM-D | 4 | 203 | 206 |
| Reference Nielsen, Morsing and PetersenNielsen et al, 1991 | 0.00 | 12 | 11 | Paroxetine | Imipramine | Not clear | Adult | End-point | HAM-D | 4 | 30 | 150 |
| Reference Noguera, Altuna and AlvarezNoguera et al, 1991 | -0.35 | 60 | 60 | Fluoxetine | Clomipramine | Out-patients | Adult | LOCF | HAM-D | 6 | 40 | 100 |
| Reference Norton, Sireling and BhatNorton et al, 1984 | 0.02 | 30 | 33 | Fluvoxamine | Imipramine | Out-patients | Adult | End-point | HAM-D | 4 | 132.8 | 153.3 |
| Reference Ohrberg, Christiansen and SeverinOhrberg et al, 1992 | -0.07 | 59 | 61 | Paroxetine | Imipramine | Out-patients | Adult | End-point | HAM-D | 6 | 32.2973 | 166.88312 |
| Reference OttevangerOttevanger, 1995 | 0.12 | 20 | 20 | Fluvoxamine | Clomipramine | In-patients | Adult | LOCF | HAM-D | 4 | 204 | 106 |
| Reference Pakesch and DossenbachPakesch & Dossenbach, 1991 | 0.01 | 48 | 91 | Fluoxetine | Clomipramine | Out-patients | Adult | LOCF | HAM-D | 4 | 30.1 | 50 |
| Reference Peters, Lenhard and MetzPeters et al, 1990 | 0.13 | 41 | 40 | Fluoxetine | Amitriptyline | Out-patients | Adult | End-point | HAM-D | 5 | 20 | 100 |
| Reference Phanjoo, Wonnacott and HodgsonPhanjoo et al, 1991 | 0.35 | 15 | 16 | Fluvoxamine | Mianserin | Out-patients/family practice | Elderly | End-point | MADRS | 6 | 170 | 60 |
| Reference Poelinger and HaberPoelinger & Haber, 1989 | -0.25 | 69 | 73 | Fluoxetine | Maprotiline | Out-patients/family practice | Adult | LOCF | HAM-D | 4 | NA | NA |
| Rahman et al, 1991 | 0.13 | 19 | 17 | Fluvoxamine | Dothiepin | In-patients | Elderly | End-point | MADRS | 6 | 157 | 159 |
| Reference Ravindran, Teehan and BakishRavindran et al, 1995 | 0.16 | 30 | 34 | Sertraline | Desipramine | Out-patients | Adult | End-point | HAM-D | 8 | 50-200 | 50-225 |
| Reference Ravindran, Judge and HunterRavindran et al, 1997 | -0.02 | 502 | 500 | Paroxetine | Clomipramine | Family practice | Adult | LOCF | MADRS | 8 | 28.2 | 99.75 |
| Reference Reimherr, Chouinard and CohnReimherr et al, 1990 | 0.13 | 144 | 142 | Sertraline | Amitriptyline | Out-patients | Adult | LOCF | HAM-D | 8 | 145 | 104 |
| Reference Remick, Claman and ReesalRemick et al, 1993 | 0.82 | 15 | 24 | Fluoxetine | Desipramine | Out-patients/family practice | Adult | End-point | HAM-D | 6 | ||
| Reference Remick, Reesal and OakanderRemick et al, 1994 | -0.10 | 17 | 16 | Fluvoxamine | Amitriptyline | Out-patients | Adult | LOCF | HAM-D | 7 | 175 | 135 |
| Reference Robertson, Abou-Saleh and HarrisonRobertson et al, 1994 | 0.13 | 77 | 76 | Fluoxetine | Lofepramine | Out-patients/family practice | Adult | LOCF | HAM-D | 6 | 20 | 140-210 |
| Reference RopertRopert, 1989 | -0.29 | 48 | 55 | Fluoxetine | Clomipramine | Out-patients | Adult | End-point | HAM-D | 6 | 20 | 75 |
| Reference Rosenberg, Damsbo and FuglumRosenberg et al, 1994 | -0.02 | 85 | 187 | Citalopram | Imipramine | Family practice | Adult | LOCF | HAM-D | 6 | 25 | 120 |
| Reference Rosenberg, Damsbo and FuglumRosenberg et al, 1994 | 0.00 | 85 | 193 | Citalopram | Imipramine | Family practice | Adult | LOCF | HAM-D | 6 | 48 | 120 |
| Reference Roth, Mattes and SheehanRoth et al, 1990 | -0.13 | 24 | 27 | Fluvoxamine | Desipramine | Out-patients | Adult | End-point | HAM-D | 6 | 218.2 | 224.6 |
| Reference Rush, Armitage and GillinRush et al, 1998 | 0.03 | 62 | 60 | Fluoxetine | Nefazodone | Out-patients | Adult | LOCF | HAM-D | 8 | 20 | 200 |
| Reference Schnyder and Koller-LeiserSchnyder & Koller-Leiser, 1996 | 0.09 | 34 | 37 | Paroxetine | Maprotiline | Out-patients/family practice | Adult | LOCF | HAM-D | 4 | 32.2 | 107.4 |
| Reference Shaw, Thomas and BriscoeShaw et al, 1986 | -0.08 | 20 | 24 | Citalopram | Amitriptyline | Out-patients/family practice | Adult | LOCF | HAM-D | 6 | 46 | 148 |
| South Wales Antidepressant Drug Trial Group, 1988 | -0.06 | 21 | 16 | Fluoxetine | Dothiepin | Out-patients/family practice | Adult | End-point | HAM-D | 6 | 67 | 172 |
| Reference Staner, Kerkhofs and DetrouxStaner et al, 1995 | 0.72 | 19 | 21 | Paroxetine | Amitriptyline | In-patients | Adult | LOCF | HAM-D | 5 | 30 | 150 |
| Reference Stark and HardisonStark & Hardison, 1985 | 0.03 | 186 | 185 | Fluoxetine | Imipramine | Out-patients | Adult | LOCF | HAM-D | 6 | 69.2 | 219.1 |
| Reference Stott, Blagden and AitkenStott et al, 1993 | -0.01 | 262 | 243 | Paroxetine | Amitriptyline | Family practice | Adult | Not clear | MADRS | 8 | 20 | 75 |
| Reference Stratta, Bolino and CupillariStratta et al, 1991 | -0.04 | 9 | 14 | Fluoxetine | Imipramine | Not clear | Adult | End-point | HAM-D | 6 | 20 | NA |
| Reference Stuppaeck, Geretsegger and WhitworthStuppaeck et al, 1994 | -0.05 | 66 | 68 | Paroxetine | Amitriptyline | In-patients | Adult | End-point | HAM-D | 6 | 33.3 | 166 |
| Reference Szegedi, Wetzel and AngersbachSzegedi et al, 1997 | -0.01 | 260 | 257 | Paroxetine | Maprotiline | Out-patients | Adult | LOCF | HAM-D | 6 | 35.3 | 109.9 |
| Reference Timmerman, de Beurs and TanTimmerman et al, 1987 | 0.20 | 13 | 14 | Citalopram | Maprotiline | In-patients | Adult | End-point | HAM-D | 4 | 40-60 | 75-150 |
| Reference Tollefson, Greist and JeffersonTollefson et al, 1994 | -0.08 | 62 | 62 | Fluoxetine | Imipramine | Out-patients | Adult | LOCF | HAM-D | 8 | 43 | 165 |
| Reference Tylee, Beaumont and BowdenTylée et al, 1997 | -0.11 | 147 | 156 | Fluoxetine | Venlafaxine | Family practice | Adult | LOCF | HAM-D | 12 | 20 | 75 |
| Unpublished, 1998a 1 | 0.34 | 75 | 80 | Paroxetine | Venlafaxine | Out-patients | Adult | LOCF | HAM-D | 12 | 20 | 150 |
| Unpublished, 1998b 1 | 0.45 | 82 | 80 | Paroxetine | Venlafaxine | Out-patients | Adult | LOCF | HAM-D | 12 | 20 | 75 |
| Unpublished, 1998c 1 | 0.10 | 175 | 161 | Paroxetine | Venlafaxine | Family practice | Adult | LOCF | HAM-D | 12 | 20 | 75 |
| Unpublished, 1998d 1 | 0.20 | 44 | 52 | Paroxetine | Venlafaxine | Out-patients/family practice | Adult | LOCF | HAM-D | 6 | 36.3 | 269 |
| Unpublished, 1998e 1 | 0.02 | 196 | 186 | Fluoxetine | Venlafaxine | Out-patients | Adult | LOCF | HAM-D | 8 | 20-40 | 75-150 |
| Unpublished, 1998f 1 | 0.21 | 95 | 103 | Fluoxetine | Venlafaxine | Out-patients | Adult | LOCF | HAM-D | 8 | 20-40 | 75-150 |
| Unpublished, 1998g 1 | 0.06 | 122 | 119 | Fluoxetine | Venlafaxine | Out-patients | Adult | LOCF | HAM-D | 12 | 39.9 | 140.8 |
| Reference Young, Coleman and LaderYoung et al, 1987 | 0.11 | 25 | 25 | Fluoxetine | Amitriptyline | Out-patients | Adult | End-point | HAM-D | 6 | 73 | 122 |
The predictive value of each factor was assessed in turn. None of the factors achieved a statistically significant predictive effect upon outcome and thus all coefficients reflect the predictive value of a factor alone in the model. As expected, 5-HT reuptake inhibition on its own did not predict any difference in efficacy; the coefficient was - 0.003 (95% CI - 0.064 to 0.048). For the presence of activity on noradrenaline reuptake, the coefficient was 0.006 (95% CI ‒0.042 to 0.082). The coefficients examining the predictive value of 5HT2 antagonism did not predict the outcome in the included trials (see Table 3 and Fig. 1).
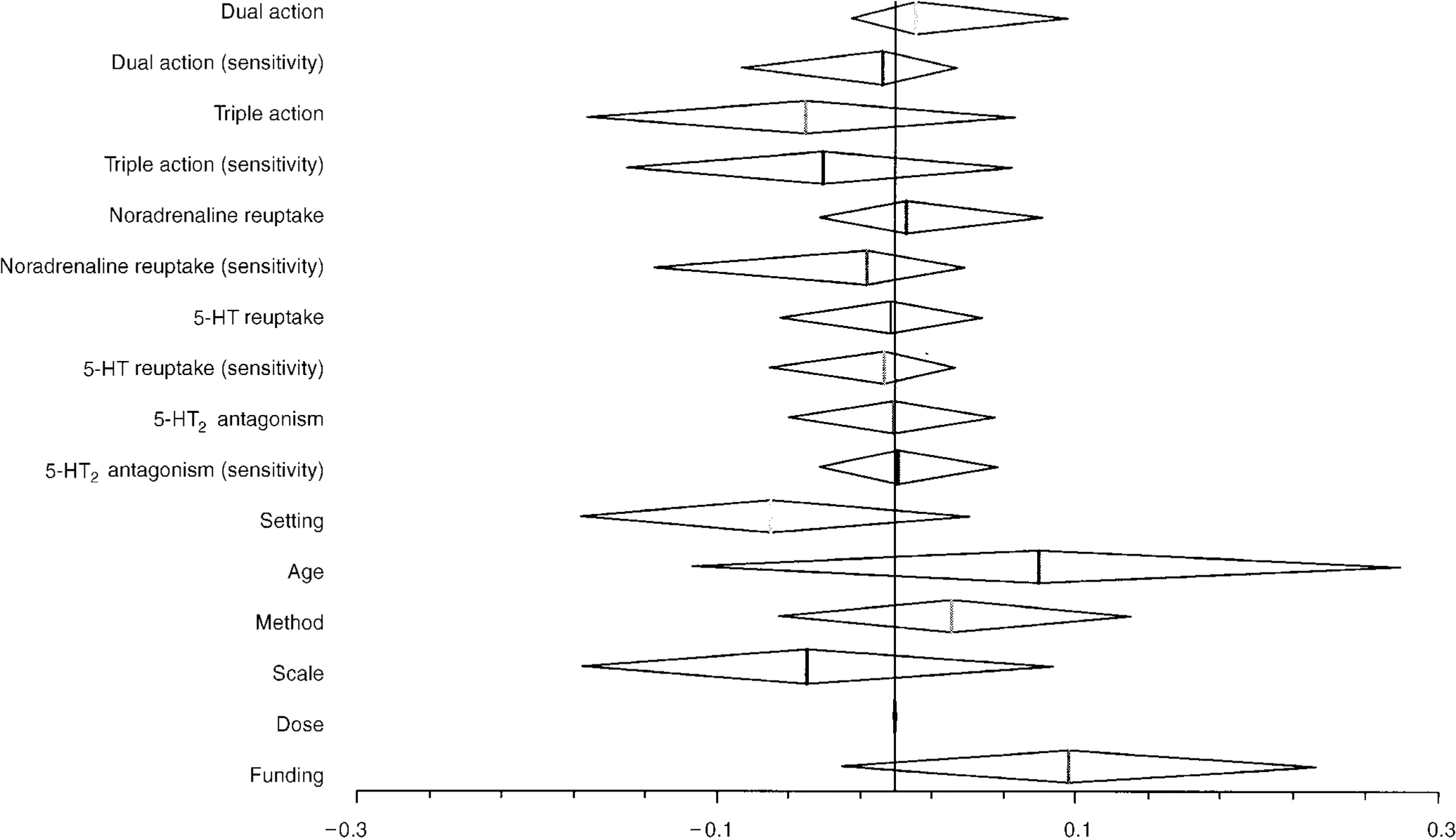
Fig. 1 Coefficient values for predictive value of receptor site activity.
For each coefficient described, the vertical line describes the point estimate of effect, and the diamond describes the limits of the 95% confidence intervals. The approach to estimation does not force assumptions of symmetry for confidence intervals. For pharmacological activity, a coefficient value less than zero implies an advantage for the presence of the factor described.
For the structural factors examined:
-
Setting: a positive value would suggest an increased efficacy for selective serotonin reuptake inhibitors (SSRIs) in in-patients
-
Age: a positive value would imply an increased efficacy for SSRIs where only those over 65 years are included
-
Method: a positive value would imply an increased efficacy for SSRIs in studies that used last observation carried forward instead of end-point analysis
-
Scale: a positive value would imply an advantage for SSRIs where the Hamilton Depression Rating Scale was used
-
Dose: a positive result would imply an advantage for SSRIs when a higher dose comparator was used
-
Funding: a positive result would imply an advantage for the sponsor's drug.
Table 3 Predictive effects of pharmacological action and other study factors

| Covariate | Coefficient | 95% Credibility limits | |
|---|---|---|---|
| Lower limit | Upper limit | ||
| Dual action | 0.011 | -0.025 | 0.096 |
| Dual action (sensitivity) | -0.007 | -0.086 | 0.034 |
| Triple action | -0.05 | -0.172 | 0.067 |
| Triple action (sensitivity) | -0.040 | -0.15 | 0.065 |
| Noradrenaline reuptake | 0.006 | -0.042 | 0.082 |
| Noradrenaline reuptake (sensitivity) | -0.016 | -0.134 | 0.039 |
| 5HT reuptake | -0.003 | -0.064 | 0.048 |
| 5HT reuptake (sensitivity) | -0.006 | -0.070 | 0.033 |
| 5HT2 antagonism | -0.001 | -0.060 | 0.055 |
| 5HT2 antagonism (sensitivity) | 0.002 | -0.042 | 0.057 |
| Setting | -0.069 | -0.176 | 0.041 |
| Age | 0.080 | -0.113 | 0.28 |
| Method | 0.032 | -0.065 | 0.13 |
| Scale | -0.049 | -0.175 | 0.088 |
| Dose | 0 | > 0.0010 | <0.001 |
| Funding | 0.097 | -0.03 | 0.23 |
We also examined the predictive value of the presence of dual action (5-HT and noradrenaline reuptake inhibition) and triple action (dual action plus 5-HT2 antagonism) on the model. Neither predicted an increase in effectiveness.
None of the identified structural factors that may have confounded the results of the analyses had statistically significant predictive value and, perhaps surprisingly, the dose of the comparator had no influence, with the results being particularly precise (very narrow confidence interval). The most important structural predictor of outcome was trial sponsorship, which demonstrated a trend towards increased efficacy of the sponsor's drug, although this did not reach statistical significance.
DISCUSSION
We have shown that, in this data-set, there is no evidence to support the increased efficacy of specific combinations of actions at 5-HT and noradrenaline transporter and 5-HT2 receptor sites, compared to a single action in inhibiting the reuptake of 5-HT. The results of our review suggest that great caution needs to be taken in ascribing any possible efficacy advantages of particular antidepressants over SSRIs to acute pharmacological properties.
Scope
We did not examine the efficacy of MAOIs, moclobemide or mirtazapine because their actions to increase 5-HT and noradrenaline function, while presynaptic, cannot be compared directly with single or dual action reuptake inhibition. Neither did we examine effects at other receptors, based on the principle of limiting the analysis to factors for which there is evidence of involvement in antidepressant efficacy. Our results indicate that the argument that a dual action (in inhibiting 5-HT and noradrenaline reuptake) could account for the results of selected trials in which superior efficacy is shown by one drug over another should be accepted with caution, and emphasise the difficulty in establishing the superiority of one antidepressant over another in studies such as these. The term ‘dual action’ has become a marketing concept for a number of antidepressants, and this study raises the question as to whether it has a legitimate scientific basis, in considering mechanisms behind antidepressant efficacy.
The role of 5-HT2 receptor antagonism in antidepressant action is unclear, but is suggested because it is the principal pharmacological property of the antidepressants trazodone and nefazodone. The picture is further complicated by the differentiation of this receptor into 5-HT2A and 5-HT2C subtypes. Our analysis is based on antagonism of the 5-HT2A subtype, and there is a lack of good data on the binding of antidepressants to the human 5-HT2C receptor. Animal studies suggest that most, but not all, antidepressants bind with similar affinity to the two subtypes (Reference Pälvimäki, Roth and MajasuoPälvimäki et al, 1996). However, this analysis has not made a specific examination of the role of 5-HT2C receptor antagonism.
Issues in the analysis of the data
Our findings show that appropriate meta-regression techniques can be useful in examining the importance of different factors across a range of trials examining a common goal, but differing in potentially important characteristics. Standard ordinary least-squares regression is inadequate in an analysis such as this, as the method assumes that the observed outcomes in the trials (the estimate of the size of effect) are the true outcomes. It is important to recognise that the outcomes in clinical trials involve considerable uncertainty, and that standard statistical techniques would fail to include an adequate estimate of measurement error.
Each of the factors was entered individually in the analysis, and only if a significant predictive effect had been found would its influence on other factors have been examined. A potential limitation of our study is that factors without a uniform influence on outcome could have been missed. For instance, the effect of in-patient treatment setting could be to favour one group of comparators but disadvantage others, giving no overall effect. Addressing this type of limitation requires strong a priori hypotheses, such as that for the category of ‘dual action’, and goes beyond this analysis.
The pharmacological classification of antidepressants we used needs comment. A difficulty permeating our analysis, and relatively unrecognised, is how limited our knowledge of even the acute pharmacology of antidepressants remains. Commonly held views about the pharmacology of antidepressants, at least in vivo, and in humans, probably go beyond the evidence. We are uncertain about whether many of the putative pharmacological properties of drugs are translated into effects in the human brain for many reasons, including continuing advances in our understanding of how neurotransmission may be modified, the lack of true selectivity of drugs (including the action of metabolites), lack of knowledge of the pharmacology of drugs in humans as opposed to other animals, and ignorance about neuronal concentrations of drugs and their metabolites at doses employed clinically. This suggests that the scientific question of whether particular putative actions or combinations of putative actions of drugs may relate to efficacy still awaits better understanding of what the actions really are. We have tried to use the best data available, including those obtained in experiments with human tissues, but these are relatively limited. Uncertainties about the classification of some drugs are inevitable, and for some there is evidence of a dose relationship across the doses used in the studies, which could not easily be accounted for in the analysis (for example, noradrenaline reuptake inhibition occurring only at a higher venlafaxine dose). A final important point is the recognition that the acute effects of antidepressants do not directly account for antidepressant action, which is believed to be due to secondary changes arising as a consequence of the primary effects. The acute pharmacology, even if it can be known, therefore stands as a crude proxy for as yet unknown changes that are crucial for antidepressant action. It is quite possible that it is not simply the presence or absence of an acute pharmacological effect but the balance between different ones that is important in determining later changes and, finally, response to antidepressants.
Quality of data
Our data-set is both large and systematically assembled, which means that the power to detect significant effects is high and that bias is minimised, although in interpreting our results it is important to recognise the limitations inherent in the data. The quality of the trials was variable and likely to have added ‘noise’ to the results. In addition, there is uncertainty about optimum doses for the comparators in relation to SSRIs, which will influence the analysis of dose; this may be particularly true for the comparator drugs in which there is uncertainty about pharmacological activity at specific sites, as discussed above. In our model there was strong evidence that the dose of comparator antidepressant had no effect on the relative effectiveness compared with that of an SSRI. Hence we believe it is unlikely that a major effect attributable to the chosen pharmacological actions, singly or in combination, has been obscured in the data, although we cannot exclude an effect of dose for some individual drugs or an interaction between factors. For example, as discussed above, drugs such as venlafaxine may cross from single to dual reuptake inhibition with increasing dose.
Most studies involved TCAs, and the lack of effect of dose on efficacy potentially adds to the debate about the supposed dangers of ‘subtherapeutic’ prescribing of TCAs, which has been seen as a factor influencing choice between antidepressants (Reference Donaghue and TyleeDonaghue & Tylee, 1996). In clinical practice, it is not uncommon to see individual patients, often with more severe illness, whose depression only responds to higher doses of TCAs. The evidence that this is generally true is extremely limited (Reference Blashki, Mowbray and DaviesBlashki et al, 1971; Reference Thompson and ThompsonThompson & Thompson, 1989) and should not be accepted uncritically. The trials included in this analysis were not designed to look at the effect of dose, and differed as to whether a fixed or variable dose was employed. Nevertheless, not only was no effect of dose on relative efficacy detected, but the precision of the estimate was extremely high, making it very unlikely that a true effect was obscured, taking the cut-off between high and low dose that we employed. As nearly all ‘low-dose’ studies used TCA doses of 75 mg or above, this suggests that one needs to keep an open mind about whether the minimum therapeutic dose of TCAs may be 75 mg or below in populations such as these.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Currently, there is uncertainty about whether some antidepressants display superior efficacy.
-
▪ In our present state of knowledge of the pharmacology of individual drugs, there does not seem to be a simple relationship between acute pharmacological properties and efficacy.
-
▪ When choosing antidepressants on the basis of efficacy, clinicians should consider the properties of individual drugs rather than make assumptions about efficacy based on their acute pharmacological actions. Safety, tolerability and patients' preference are likely to be more important for most patients.
LIMITATIONS
-
▪ Differences in the reporting of outcomes between studies require standardisation of many outcomes, resulting in a reduction in interpretation of the practical importance of the results.
-
▪ Data on the relative effectiveness of different antidepressants remain limited for individual agents.
-
▪ Our knowledge of the acute pharmacology of individual antidepressants in humans is limited; this is even more true of the secondary effects believed to underlie the antidepressant action.
ACKNOWLEDGEMENTS
We are grateful to Wyeth UK for supporting this research, and to those investigators and sponsors who provided unpublished data. The views expressed are those of the investigators and not necessarily those of the sponsor. We are grateful also to Anne Burton, for her assistance in retrieving relevant studies and her persistent attempts to locate unpublished data.







eLetters
No eLetters have been published for this article.