Prematurity and low birthweight have been linked to IQ, attention deficit disorder and emotional problems in children (Reference Bhutta, Cleves and CaseyBhutta et al, 2002). Two recent studies have suggested an association between birthweight and psychological distress in early adulthood (Reference CheungCheung, 2002) and middle age (Reference Cheung, Khoo and KarlbergCheung et al, 2002). The present report addresses the relationship between intra-uterine growth, as measured by prematurity and low birthweight, and depressive disorder in late adolescence, a period of peak incidence for first episodes. It also tests the mediating role of three established risk factors: pre-existing symptoms of depression and anxiety, negative life events and parental bonding.
METHOD
Sampling, assessment and data analytical procedures for the state-wide case-control study are described elsewhere (Reference Patton, Coffey and PosterinoPatton et al, 2003). Between August 1992 and July 1995 we conducted a six-wave cohort study of adolescent health in Victoria, Australia. The current nested study took place between waves 2 and 6. The nested study was open to participants with at least one previous data wave, resulting in 6653 available observations. The mean age (s.d.) at wave 2 was 15.0 (0.4) and at wave 6 it was 17.4 (0.4) years.
The computerised revised Clinical Interview Schedule (CIS-R) identified putative episodes of ICD-10 (World Health Organization, 1992) depressive disorder in the cohort study (Reference Lewis and PelosiLewis & Pelosi, 1992) for a second-phase face-to-face interview. A sample of CIS-R non-cases was also selected at random from participants in the same school in a 2:1 ratio to cases.
Blinded face-to-face interview included:
-
(a) Depression and hypomania modules of the Composite International Diagnostic Interview (CIDI, Core Version 1.1; Reference Robins, Wing and WittchenRobins et al, 1988) were used to generate lifetime ICD-10 diagnoses for affective disorder. The CIDI cases were excluded from reselection at later study waves. Two diagnostic categories for depressive disorder were defined: all cases diagnosed by the CIDI and a second category of ‘stable’ depressive disorder that concurrently fulfilled criteria on both the CIS-R and the CIDI.
-
(b) Recent life events measured using an adapted List of Threatening Experiences Questionnaire (Reference Brugha, Bebbington and TennantBrugha et al, 1985).
-
(c) The Parental Bonding Instrument (PBI; Reference Parker, Tupling and BrownParker et al, 1979) was administered immediately prior to the CIDI.
The CIS-R data, from 6 months earlier, provided an index of pre-existing depressive and anxiety symptoms.
Parental telephone interviews (blinded) took place after completion of wave 6 of the cohort. All parents of participants diagnosed with depressive disorder were approached, together with the parents of two controls drawn from the equivalent school and wave. Maternal interviews were completed in 66 (73%) of the CIDI cases and 150 (81%) of the controls. Paternal interviews were completed in 53 (62%) of the cases and 130 (74%) of the controls. The parental interview consisted of:
-
(a) A diagnostic interview of each parent using the CIDI, with parental disorder defined as either parent having ever experienced a depressive episode.
-
(b) Gestational age and birthweight were based on maternal report (Reference Ison, Ross and PendergrassIson et al, 1997). When mothers were unavailable (dead or uncontactable) this information was collected from fathers (5) or adoptive parents (1). Birthweight of <2.5 kg was categorised as low birthweight and delivery ≥3 weeks prior to term was categorised as premature.
Associations with depressive disorder were examined using conditional logistic regression (Stata, release 7.0), with matching defined by school and wave of selection. Inverse probability weighting was used to estimate the cumulative prevalence of depressive disorder, with 95% CIs obtained using Stata's survey estimation procedures.
RESULTS
The cumulative rate of stable depressive disorder (CIS-R and CIDI) over 30 months was 2.1% (95% CI 1.8-2.5) in females and 0.33% (95% CI 0.19-0.54) in males. Cumulative rates of stable depressive disorder in those born at term and those of birthweight ≥2500 g were low: 0.25% (95% CI 0.13-0.5) in males and 1.8% (95% CI 1.6-2.1) in females. Rates were 1.0% (95% CI 0.8-1.3) in premature low-birthweight males and substantially higher at 15.2% (95% CI 11.1-20.5) in premature low-birthweight females.
Matched pairs or triplets for CIDI-defined depressive disorder (i.e. at least one case and one control from a specific school and wave of selection) were available with complete (parental response) data for 63 cases and 112 controls. For stable depressive disorder, 49 cases were matched with 102 controls. On bivariate analysis, prematurity and low birthweight were associated with approximately sixfold (OR=5.7; 95% CI 1.4-2.3) and approximately threefold (OR=2.9; 95% CI 0.6-1.4) increases in the odds of CIDI-defined depressive disorder, respectively.
The potential mediating roles of adverse parenting, heightened responses to social adversity and high levels of pre-existing symptoms were considered in further conditional logistic regression models (Table 1). The baseline odds ratio after adjustment for parental education, parental separation, maternal age at birth, maternal smoking in pregnancy, serious illness in the first year of life and parental depressive disorder suggested that either prematurity or low birthweight increased the odds for depressive disorder by >11-fold. Both low maternal care and high control were independently associated with depressive disorder but adjustment did not markedly reduce the association with prematurity/low birthweight. Adjustment for negative life events reduced the association with depressive disorder but the clearest reduction in association occurred with the addition of pre-existing depressive and anxiety symptoms to the model. Models using stable depression as the outcome produced similar findings.
Table 1 Association of prematurity/low birthweight with depressive disorder: (a) after adjustment for background factors; and (b) with further adjustment for parenting style, negative life events and level of earlier depressive and anxiety symptoms as putative mediators
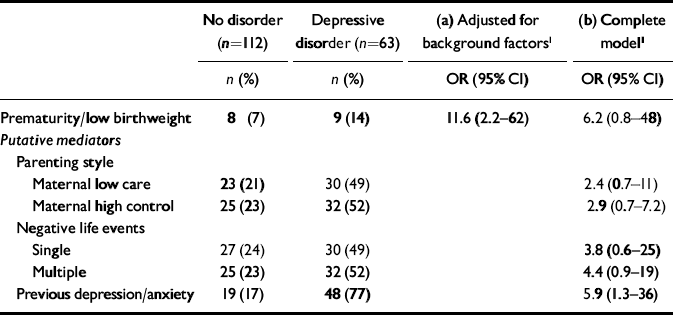
| No disorder (n=112) | Depressive disorder (n=63) | (a) Adjusted for background factors1 | (b) Complete model1 | |
|---|---|---|---|---|
| n (%) | n (%) | OR (95% CI) | OR (95% CI) | |
| Prematurity/low birthweight | 8 (7) | 9 (14) | 11.6 (2.2—62) | 6.2 (0.8—48) |
| Putative mediators | ||||
| Parenting style | ||||
| Maternal low care | 23 (21) | 30 (49) | 2.4 (0.7—11) | |
| Maternal high control | 25 (23) | 32 (52) | 2.9 (0.7—7.2) | |
| Negative life events | ||||
| Single | 27 (24) | 30 (49) | 3.8 (0.6—25) | |
| Multiple | 25 (23) | 32 (52) | 4.4 (0.9—19) | |
| Previous depression/anxiety | 19 (17) | 48 (77) | 5.9 (1.3—36) |
DISCUSSION
Prematurity and low birthweight were associated with a substantially higher rate of depressive disorder in adolescence. After adjustment for potential confounders, the odds for depressive disorder were elevated by >11-fold in adolescents born premature or of low birthweight. For females, rates of stable disorder in those born premature or with low birthweight prevalence estimates were 15%, compared with <2% in those with normal deliveries.
Poor maternal bonding through childhood did not appear to be a mechanism for the association between prematurity and depressive disorder. However, adjustment of regression models for pre-existing depressive and anxiety symptoms and recent negative life events did reduce the association, suggesting that a heightened sensitivity to social adversity might play a role in depressive disorder in this group.
Foetal genotype, maternal physiology and placental function may affect early brain development through nutritional and hormonal mechanisms (Reference LeonLeon, 2001). For those born prematurely, perinatal stress and suboptimal nutrition in the early weeks ex utero also play a role. Early physiological adaptation, particularly of the hypothalamic-pituitary-adrenal axis (HPA), to intra-uterine nutritional deficiencies has been one explanation for the links between low birthweight and later cardiovascular and diabetes risks (Reference Phillips, Barker and FallPhillips et al, 1998). The HPA and hypothalamo-pituitarygonadal axes have been implicated in early depression (Reference Patton, Hibbert and CarlinPatton et al, 1996; Reference Goodyer, Herbert and TamplinGoodyer et al, 2000) and both are affected by low birthweight (Reference Phillips, Barker and FallPhillips et al, 1998; Reference Ibanez, Potau and de ZegherIbanez et al, 2000). Thus, a tendency of premature/low-birthweight subjects to have high circulating glucocorticoids may indicate an early acquired and persisting neurophysiological vulnerability. Such a vulnerability may lower the threshold for depressive and anxiety symptoms in response to adversity and, in time, lead to a more negative appraisal and heightened vulnerability to life events.
Acknowledgements
National Health and Medical Research Council and Australian Rotary Health. Research Fund provided funding for the study.




eLetters
No eLetters have been published for this article.