Generalised social phobia (GSP) is the most prevalent anxiety disorder in the community (Reference Kessler, McGonagle and ZhaoKessler et al, 1994). Most patients with this disorder receive treatment in primary care (Reference Weiller, Bisserbe and BoyerWeiller et al, 1996). While pharmacological treatment is frequently used, non-pharmacological therapies, even with well-documented efficacy (Reference van Dyckvan Dyck, 1996), are generally considered to be too time-consuming to be a treatment alternative for the primary care physician. However, no controlled trial, either with respect to pharmacological or non-pharmacological treatment, has to date been performed in this setting. This study primarily compared the efficacy of treatment with sertraline or placebo in a controlled, double-blind, randomised design in primary care patients with GSP. In addition, primary care physicians were trained in a brief exposure therapy programme that constituted a practical, short-term behavioural treatment intervention. The study also examined the efficacy of this brief exposure treatment performed by primary care physicians, as well as the efficacy of combined sertraline and exposure therapy.
METHOD
Subjects aged 18-65 years with GSP according to DSM—IV criteria study (American Psychiatric Association, 1994) of at least 1 year's duration and rated as moderately ill (≥4) on the overall severity subscale of the Clinical Global Impression — Social Phobia scale (CGI—SP, range 1-7; Reference GuyGuy, 1976) were included in the study. The investigator made the diagnosis of GSP based on a clinical interview. The revised version of the MINI International Neuropsychiatric Interview (MINI—R; Reference Sheehan, Lecrubier and SheehanSheehan et al, 1998) was used to reliably diagnose DSM—IV comorbid psychiatric disorders. Patients with comorbid dysthymia or specific phobias were allowed to enter the study. Patients with panic disorder with onset before social phobia or any other current anxiety, major depressive, substance use or eating disorder were not eligible. In addition, patients with a lifetime history of bipolar disorder or psychosis were excluded. At each visit, specific inquiry was made to identify adverse experiences. After complete descriptions of the study to the subjects, written informed consent was obtained. Ethics committees in Norway and Sweden approved the protocol.
Training of investigators
Fifty physicians were included in a 30-hour training programme over three weekends. All participants were trained in DSM—IV criteria and MINI—R interviewing to identify GSP and comorbid disorders through lectures, videotapes and group supervision. The training is described in more detail elsewhere (Reference Haug, Hellström and BlomhoffHaug et al, 2000). Exposure therapy training, a videotape demonstrating an eight-session exposure therapy programme and a manual were given to all investigators. Additionally, exposure therapy supervision was offered in local groups throughout the study.
The physicians were trained in rating of the CGI—SP severity scale. Consensus ratings between five trained psychiatrists/ psychologists and one general practitioner of five videotaped patient interviews were defined as the gold-standard severity rating. Based on a minimum of 12 videotaped patient interviews, the intraclass correlation (ICC 1,1) as a measure of interrater reliability of at least 0.70 compared with the gold standard on the CGI—SP overall severity sub-scale was required (Reference Friis, Sundsvold, Dahl, Haug and SvartbergFriis & Sundsvold, 1987). Forty-five physicians (mean ICC 1,1=0.78, range 0.70-0.91) met this requirement. After repeated training two physicians treating a total of 12 patients did not reach the goal (ICC 1,1=0.62 and 0.65, respectively). However, since their patients already were randomised they were included in the intent-to-treat population. Three physicians withdrew during the training period.
The group of investigators constituted 12 females and 35 men with a median age of 49 (range 32-64) years. All had private clinical practice as their main occupation, with a median of 16 (range 3-33) years in this position. Two were private practising psychiatrists, 28 specialists in family medicine, the others non-specialist primary care physicians. The median time in clinical work was 40 (range 10-60) hours/week, and physicians treated a median of 93 (range 15-200) patients weekly. The physicians were not informed of the exposure therapy component in the study design during the process to recruit investigators, but learned about this additional element prior to entering the training programme.
Recruitment and assignment
Patients were consecutively recruited from subjects seeking medical consultations with one of the 47 participating physicians located at 41 different private primary care centres in Norway or Sweden. To recruit potential patients for the study, a form listing DSM—IV criteria for generalised social phobia and informing about the ongoing trial was distributed in the waiting room of all investigators. Based on the response to the form and the symptoms presented in the consultation, 289 primary care patients were included in the screening process (see Fig. 1). Eighteen investigators additionally screened 159 patients recruited through newspaper or other media advertisements.
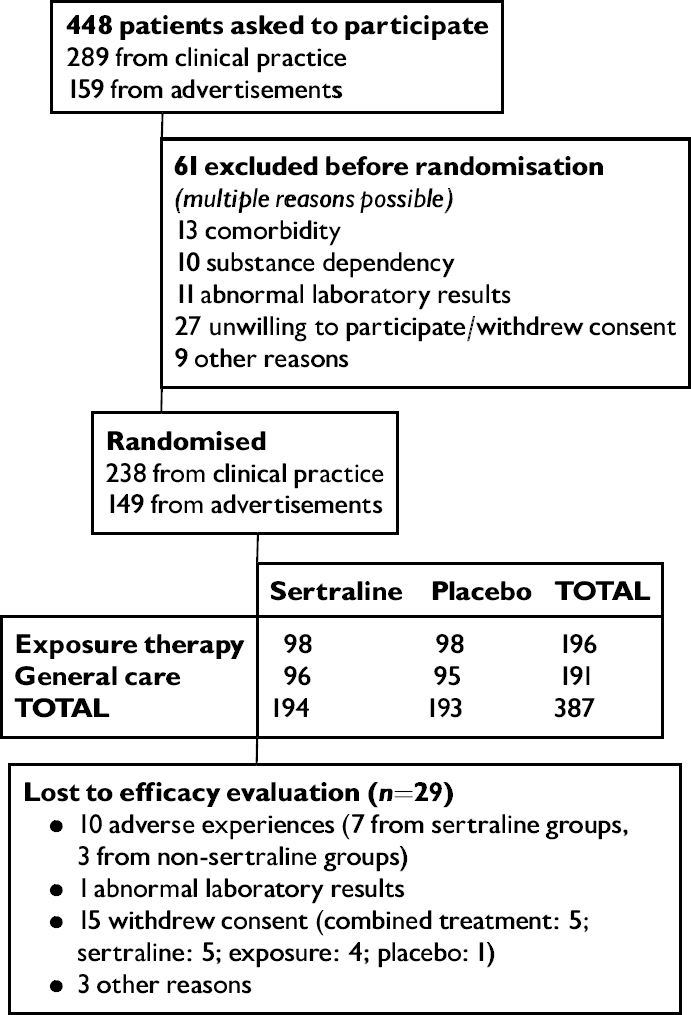
Fig. 1 Trial profile.
Three hundred and eighty-seven patients were randomly assigned by a computer to receive double-blind sertraline or placebo in blocks of eight subjects so that four patients in each block were randomised to each of the treatments. No other stratification factors were used. Each block was assigned to a specific general practitioner. In both the sertraline and placebo groups half of the patients were randomly allocated to exposure or general medical care. A separate randomisation list was made for exposure or non-exposure treatment. Sealed envelopes of allocations from this list were kept by the investigators and opened after the inclusion of the patient into the study. During this procedure equal numbers of subjects were assigned to each treatment option in each block. Tablets were packaged and numbered by the sponsor and personally delivered to each investigator.
Patient characteristics
Two hundred and thirty-four females and 153 males (mean age of 40.4 years (s.d.=10.4)) comprised the study population. Two hundred and fifty-eight (67%) were married or living with a partner. The median age at symptom onset was 13 years (range 3-48 years), duration of illness 23.6 years (s.d.=12.2). A comorbid psychiatric disorder was diagnosed in 135 (35%) patients: 101 (26%) phobic anxiety disorder, six (2%) panic disorder, six (2%) dysthymia and 13 (3%) other diagnoses.
The mean physician-rated CGI—SP disease severity was 5.0 (s.d.=0.9) on the subscale for anxiety attacks, 4.9 (s.d.=0.9) for avoidance, 4.8 (s.d.=0.8) for performance anxiety, 4.7 (s.d.=0.8) for disability and 4.7 (s.d.=0.7) for overall severity. The mean patient-rated severity as assessed by the Social Phobia Scale (SPS, range 20-100; Reference Mattick and ClarkeMattick & Clarke, 1998) was 54.0 (s.d.=16.2). The mean level of depression at baseline as rated with the Montgomer—Åsberg Depression Rating Scale (MADRS, range 0-60; Reference Montgomery and ÅsbergMontgomery & Åsberg, 1979) was 7.3 (s.d.=4.9).
Design and medication
All patients were scheduled for nine visits with the investigator during the first 16 weeks of treatment and a final efficacy visit after 24 weeks. For ethical reasons, all patients rated as not improved (CGI—SP overall improvement ≥4) according to the protocol criteria at the Week 16 visit completed the final study assessment at this time and were withdrawn from the study. All patients received general medical care according to the National Institute of Mental Health (NIMH) study guidelines for clinical management of mood disorders (Reference Fawcett, Epstein and FiesterFawcett et al, 1987). These guidelines recommend the physician to give general therapeutic support, reassurance and encouragement. The physician should give information and simple advice and permit the patient to ventilate fears and doubts. Specific behavioural instructions or psychological interpretations are not allowed.
Following a 1-week single-blind placebo period to identify fast placebo responders all subjects received either one tablet of sertraline 50 mg or placebo once daily. If the CGI—SP overall improvement score was not rated at least minimally improved (≤3) after 4 weeks of treatment, the dose was increased to two tablets of sertraline (100 mg daily) or placebo. Further dose escalations were allowed after 8 and 12 weeks to a maximum dose of 150 mg. In the absence of tolerability problems requiring dose reduction, the dose level achieved after 12 weeks of treatment was maintained for the remainder of the study. Adherence to treatment was assessed by returned-tablet counts and serum level assessments of sertraline and its metabolite desmethylsertraline.
Exposure therapy
Instructions for exposure therapy were given in eight sessions during the first 12 weeks of treatment, each with an estimated duration of 15-20 minutes. Further encouragement and advice were given at the Week 16 visit. A description of the exposure therapy is published in more detail elsewhere (Reference Haug, Hellström and BlomhoffHaug et al, 2000). Briefly, in the first session patients were informed of the rationale for treatment and the main problem areas were identified. In the next session agreement was made about homework assignments, and the use of a symptommonitoring diary during exposure training was explained. In the remaining sessions, the patients were instructed to gradually expose themselves to feared situations, and thus learn new coping strategies. They were told to stay as long as they could in the phobic situation, ideally until the anxiety decreased. All patients received homework between the sessions and brought a report of the training with them to the next session. The patients were told to continue the exposure therapy according to the individually designed treatment programme in the last 12 weeks of the study. The physician helped the patients to identify goals and new coping strategies and supported them in the self-exposure training.
Primary efficacy measures
The patients were defined as responders, partial responders or non-responders based on assessments on the investigator-rated CGI—SP and the patient-rated SPS. Investigators made intermediate efficacy ratings after 4, 8, 12 and 16 weeks, and final efficacy assessment after 24 weeks of treatment.
Response was defined as a reduction of at least 50% on SPS-assessed symptom burden compared with baseline, a CGI—SP overall severity score at the final visit in the ‘no mental illness’ to ‘mild severity’ range (≤3), and a CGI—SP overall improvement score of very much or much improved (≤2). Non-response was defined as less than 25% reduction on SPS compared with baseline, or CGI—SP overall improvement rating of no change or worse (≥4). Partial response was defined as all responses between the criteria for response and non-response.
Secondary efficacy measures
The Brief Social Phobia Scale (BSPS; Reference Davidson, Potts and RichichiDavidson et al, 1991), the social phobia sub-scale of the Marks Fear Questionnaire (Reference Marks and MathewsMarks & Mathews, 1997), Fear of Negative Evaluation Scale (Reference Watson and FriendWatson & Friend, 1969), Sheehan Disability Inventory (Reference Leon, Shear and PorteraLeon et al, 1992), and the mental health subscale of the MOS 36 Short-Form Health Survey (SF—36; Reference McHorney, Ware and RaczekMcHorney et al, 1993) were employed as secondary efficacy measures.
Statistical procedures
The SAS version 6.12 (SAS Institute, 1997) was employed in all analyses. All efficacy analyses were on the intent-to-treat patient population. This population was defined as those who received at least one dose of medication and with at least one post-baseline efficacy evaluation. All statistical tests were two-tailed with α =0.05. Sample size calculation was based on an estimated 20% difference between active drug and placebo. This required at least 340 patients to detect a significant difference, if β=0.10 and the drop-out rate 35%. This procedure made the study primarily powered for the sertraline v. non-sertraline and exposure v. non-exposure analyses, but it also allowed pairwise comparisons between the specific groups. In these analyses, however, the power was reduced and the risk of false-negative results consequently increased. Data are reported as mean values, and 95% CIs are reported when appropriate. Ordinal logistic regression analyses were employed in the response analyses.
Multiple ordinal logistic regressions were also used to identify any statistical interactions between treatment groups on response. This was done by comparing models with and without interaction terms (drug treatment × exposure therapy) by like-lihood ratio tests. To fully utilise the power of the 2 × 2 factorial design, the specified strategy of analysis required that in case of no such interaction the main effects of sertraline should be tested by pooling exposed and non-exposed patients, and vice versa, with respect to the main effects of exposure therapy. Significant differences were followed up with pairwise comparisons.
In the time-point analyses the groups were compared with respect to response at each point in time. Differences in change from baseline scores on the continuous efficacy scales between all four groups were examined by parametric analyses of variance. Baseline scores and country were used as covariates.
RESULTS
In the period from 9 September 1996 to 13 May 1997, 256 patients from Norway and 131 patients from Sweden were included in the study. Sixty-one per cent were recruited from physicians' clinical practices and the remainder from advertisements. Forty-four patients were withdrawn from further treatment at Week 16 owing to non-response, as required in the protocol (11%). Two hundred and fifty-three patients completed 24 weeks of treatment (65%). Three hundred and fifty-four patients were included in the intent-to-treat efficacy population (93%).
Analysis of response
In individual analyses, no interaction was observed between response and each of the variables gender, age, country, recruitment method, medication or exposure therapy. There was no indication of better outcome in patients treated by the psychiatrists than by the other participating physicians.
Significantly more sertraline-treated patients than non-sertraline-treated patients (P=0.001) responded (Table 1). No significant difference between exposure— and non-exposure-treated patients was observed (P=0.140). Combined sertraline and exposure (40/88 response, 21/88 partial response; P<0.001) and sertraline alone (35/87 response, 25/87 partial response; P=0.002) were significantly superior to placebo (21/88 response, 18/88 partial response). Trends towards increased efficacy of exposure alone compared with placebo (30/91 response, 22/91 partial response; P=0.083) and combined sertraline and exposure compared with exposure alone (P=0.059) were additionally observed.
Table 1 Outcome after 24 weeks of treatment (n=354)
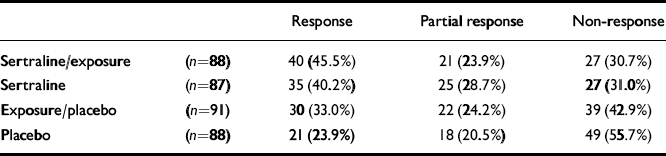
| Response | Partial response | Non-response | ||
|---|---|---|---|---|
| Sertraline/exposure | (n=88) | 40 (45.5%) | 21 (23.9%) | 27 (30.7%) |
| Sertraline | (n=87) | 35 (40.2%) | 25 (28.7%) | 27 (31.0%) |
| Exposure/placebo | (n=91) | 30 (33.0%) | 22 (24.2%) | 39 (42.9%) |
| Placebo | (n=88) | 21 (23.9%) | 18 (20.5%) | 49 (55.7%) |
Although the MADRS score at baseline was in the normal range in the sample as a whole (7.3, s.d.=4.9), a significant reduction (mean change 2.9, 95% CI 2.4-3.5) was observed during treatment. No significant interaction between MADRS-assessed level of depression and response was observed (P=0.67).
Time-point analysis
Significantly more patients in the combined sertraline and exposure group fulfilled the response criteria compared with placebo from Week 12 and in all further assessments (see Fig. 2). Sertraline alone was significantly superior to placebo at the final visit in this analysis.
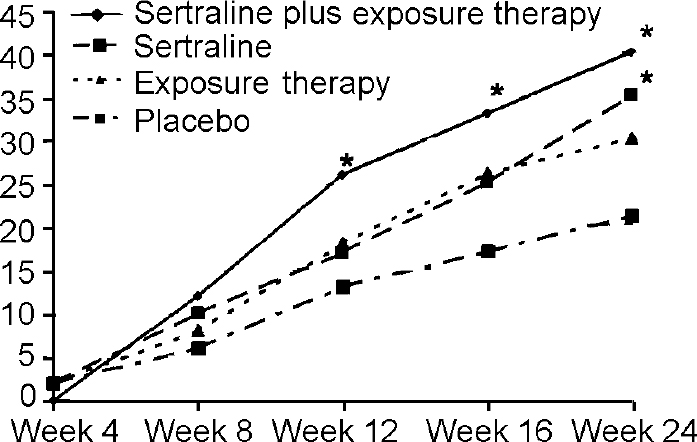
Fig. 2 Number of participants approaching response during treatment. * denotes P<0.05 compared with placebo. Weeks 12, 16: sertraline plus exposure therapy; Week 24: sertraline plus exposure therapy, sertraline. Response criteria: reduction of at least 50% on Social Phobia Scale; Clinical Global Impression — Social Phobia (CGI—SP) scale overall severity score ‘no mental illness’ to ‘mild severity’ (<3); and CGI—SP overall improvement score very much or much improved (<2).
Patients in all active treatment groups were significantly more improved (partial response or response) than placebo-treated patients at the Week 12 visit (see Fig. 3). However, only the sertraline groups remained significantly superior to placebo at the Week 16 and Week 24 visits. No significant difference between the active treatment groups appeared in any of the analyses.
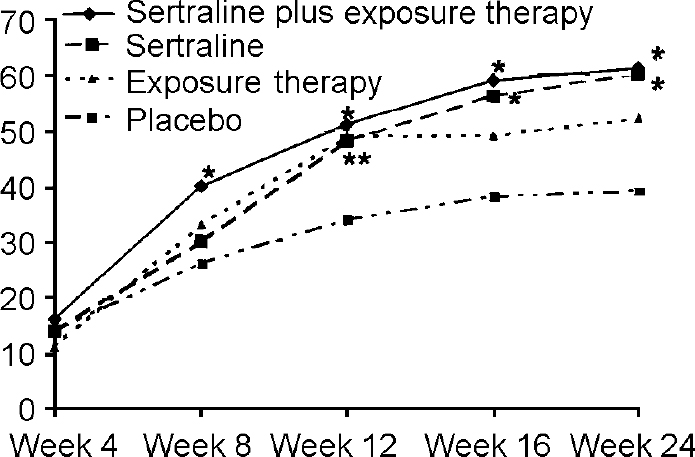
Fig. 3 Number of participants improved (responders or partial responders) during treatment. * denotes P<0.05 compared with placebo. Week 8: sertraline plus exposure therapy; Week 12: sertraline plus exposure therapy, sertraline, exposure therapy; Weeks 16, 24: sertraline plus exposure therapy, sertraline. Improvement criteria: all responses better than non-response (defined as less than 25% reduction on Social Phobia Scale, or Clinical Global Impression — Social Phobia overall improvement rating of no change or worse).
Psychometric scales
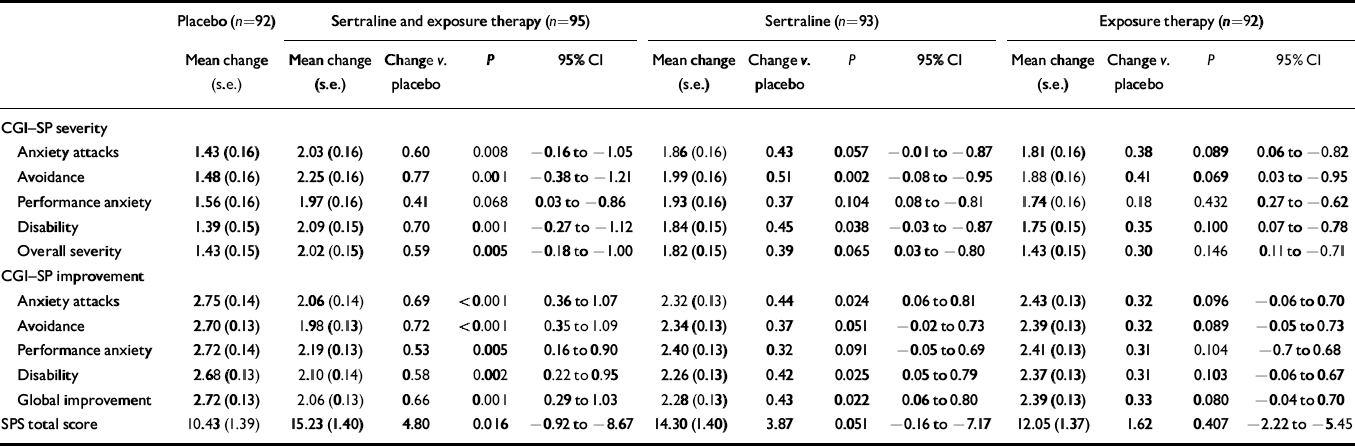
On the primary efficacy variables, subjects in the combined sertraline and exposure group were significantly more improved than placebo-treated subjects on all CGI—SP sub-scales, with the exception of CGI—SP severity-assessed performance anxiety (see Table 2). None of the treatments was significantly better than placebo on this subscale. In those receiving sertraline alone, borderline significances were observed on CGI—SP severity-assessed anxiety attacks and overall severity, as well as on CGI—SP improvement-assessed avoidance and SPS total score. No significant differences between exposure alone and placebo were found on any of the primary efficacy variables.
Table 2 Primary efficacy variables: changes in Clinical Global Impression — Social Phobia (CGI—SP) severity sub-scale and Social Phobia Scale (SPS) during treatment and CGI—SP improvement sub-scale at final examination
| Placebo (n=92) | Sertraline and exposure therapy (n=95) | Sertraline (n=93) | Exposure therapy (n=92) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean change (s.e.) | Mean change (s.e.) | Change v. placebo | P | 95% Cl | Mean change (s.e.) | Change v. placebo | P | 95% Cl | Mean change (s.e.) | Change v. placebo | P | 95% Cl | |
| CGI—SP severity | |||||||||||||
| Anxiety attacks | 1.43 (0.16) | 2.03 (0.16) | 0.60 | 0.008 | −0.16 to −1.05 | 1.86 (0.16) | 0.43 | 0.057 | −0.01 to −0.87 | 1.81 (0.16) | 0.38 | 0.089 | 0.06 to −0.82 |
| Avoidance | 1.48 (0.16) | 2.25 (0.16) | 0.77 | 0.001 | −0.38 to −1.21 | 1.99 (0.16) | 0.51 | 0.002 | −0.08 to −0.95 | 1.88 (0.16) | 0.41 | 0.069 | 0.03 to −0.95 |
| Performance anxiety | 1.56 (0.16) | 1.97 (0.16) | 0.41 | 0.068 | 0.03 to −0.86 | 1.93 (0.16) | 0.37 | 0.104 | 0.08 to −0.81 | 1.74 (0.16) | 0.18 | 0.432 | 0.27 to −0.62 |
| Disability | 1.39 (0.15) | 2.09 (0.15) | 0.70 | 0.001 | −0.27 to −1.12 | 1.84 (0.15) | 0.45 | 0.038 | −0.03 to −0.87 | 1.75 (0.15) | 0.35 | 0.100 | 0.07 to −0.78 |
| Overall severity | 1.43 (0.15) | 2.02 (0.15) | 0.59 | 0.005 | −0.18 to −1.00 | 1.82 (0.15) | 0.39 | 0.065 | 0.03 to −0.80 | 1.43 (0.15) | 0.30 | 0.146 | 0.11 to −0.71 |
| CGI—SP improvement | |||||||||||||
| Anxiety attacks | 2.75 (0.14) | 2.06 (0.14) | 0.69 | <0.001 | 0.36 to 1.07 | 2.32 (0.13) | 0.44 | 0.024 | 0.06 to 0.81 | 2.43 (0.13) | 0.32 | 0.096 | −0.06 to 0.70 |
| Avoidance | 2.70 (0.13) | 1.98 (0.13) | 0.72 | <0.001 | 0.35 to 1.09 | 2.34 (0.13) | 0.37 | 0.051 | −0.02 to 0.73 | 2.39 (0.13) | 0.32 | 0.089 | −0.05 to 0.73 |
| Performance anxiety | 2.72 (0.14) | 2.19 (0.13) | 0.53 | 0.005 | 0.16 to 0.90 | 2.40 (0.13) | 0.32 | 0.091 | −0.05 to 0.69 | 2.41 (0.13) | 0.31 | 0.104 | −0.7 to 0.68 |
| Disability | 2.68 (0.13) | 2.10 (0.14) | 0.58 | 0.002 | 0.22 to 0.95 | 2.26 (0.13) | 0.42 | 0.025 | 0.05 to 0.79 | 2.37 (0.13) | 0.31 | 0.103 | −0.06 to 0.67 |
| Global improvement | 2.72 (0.13) | 2.06 (0.13) | 0.66 | 0.001 | 0.29 to 1.03 | 2.28 (0.13) | 0.43 | 0.022 | 0.06 to 0.80 | 2.39 (0.13) | 0.33 | 0.080 | −0.04 to 0.70 |
| SPS total score | 10.43 (1.39) | 15.23 (1.40) | 4.80 | 0.016 | −0.92 to −8.67 | 14.30 (1.40) | 3.87 | 0.051 | −0.16 to −7.17 | 12.05 (1.37) | 1.62 | 0.407 | −2.22 to −5.45 |
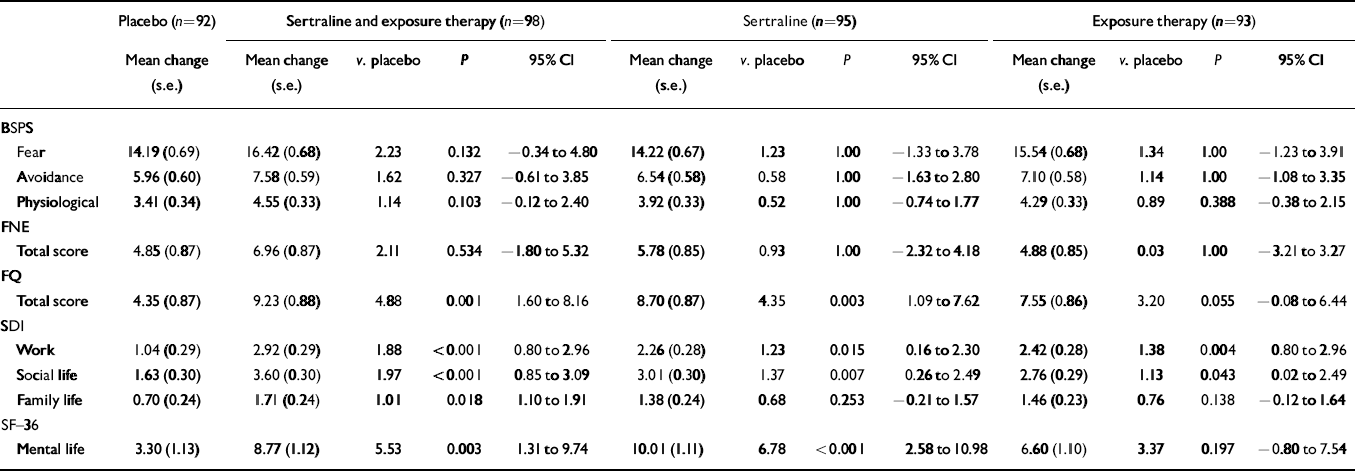
In the Bonferroni-adjusted analyses of the adjusted secondary efficacy scales, combined sertraline and exposure treatment was significantly superior to placebo on five out of nine assessments, sertraline alone on four assessments and exposure alone on two assessments (see Table 3).
Table 3 Secondary efficacy variables: changes in Brief Social Phobia Scale (BSPS), Fear of Negative Evaluations (FNE), Fear Questionnaire (FQ), Sheehan Disability Inventory (SDI), and the mental sub-scale of Short-Form—36 (SF—36) during treatment
| Placebo (n=92) | Sertraline and exposure therapy (n=98) | Sertraline (n=95) | Exposure therapy (n=93) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean change (s.e.) | Mean change (s.e.) | v. placebo | P | 95% CI | Mean change (s.e.) | v. placebo | P | 95% CI | Mean change (s.e.) | v. placebo | P | 95% CI | |
| BSPS | |||||||||||||
| Fear | 14.19 (0.69) | 16.42 (0.68) | 2.23 | 0.132 | −0.34 to 4.80 | 14.22 (0.67) | 1.23 | 1.00 | −1.33 to 3.78 | 15.54 (0.68) | 1.34 | 1.00 | −1.23 to 3.91 |
| Avoidance | 5.96 (0.60) | 7.58 (0.59) | 1.62 | 0.327 | −0.61 to 3.85 | 6.54 (0.58) | 0.58 | 1.00 | −1.63 to 2.80 | 7.10 (0.58) | 1.14 | 1.00 | −1.08 to 3.35 |
| Physiological | 3.41 (0.34) | 4.55 (0.33) | 1.14 | 0.103 | −0.12 to 2.40 | 3.92 (0.33) | 0.52 | 1.00 | −0.74 to 1.77 | 4.29 (0.33) | 0.89 | 0.388 | −0.38 to 2.15 |
| FNE | |||||||||||||
| Total score | 4.85 (0.87) | 6.96 (0.87) | 2.11 | 0.534 | −1.80 to 5.32 | 5.78 (0.85) | 0.93 | 1.00 | −2.32 to 4.18 | 4.88 (0.85) | 0.03 | 1.00 | −3.21 to 3.27 |
| FQ | |||||||||||||
| Total score | 4.35 (0.87) | 9.23 (0.88) | 4.88 | 0.001 | 1.60 to 8.16 | 8.70 (0.87) | 4.35 | 0.003 | 1.09 to 7.62 | 7.55 (0.86) | 3.20 | 0.055 | −0.08 to 6.44 |
| SDI | |||||||||||||
| Work | 1.04 (0.29) | 2.92 (0.29) | 1.88 | <0.001 | 0.80 to 2.96 | 2.26 (0.28) | 1.23 | 0.015 | 0.16 to 2.30 | 2.42 (0.28) | 1.38 | 0.004 | 0.80 to 2.96 |
| Social life | 1.63 (0.30) | 3.60 (0.30) | 1.97 | <0.001 | 0.85 to 3.09 | 3.01 (0.30) | 1.37 | 0.007 | 0.26 to 2.49 | 2.76 (0.29) | 1.13 | 0.043 | 0.02 to 2.49 |
| Family life | 0.70 (0.24) | 1.71 (0.24) | 1.01 | 0.018 | 1.10 to 1.91 | 1.38 (0.24) | 0.68 | 0.253 | −0.21 to 1.57 | 1.46 (0.23) | 0.76 | 0.138 | −0.12 to 1.64 |
| SF-36 | |||||||||||||
| Mental life | 3.30 (1.13) | 8.77 (1.12) | 5.53 | 0.003 | 1.31 to 9.74 | 10.01 (1.11) | 6.78 | <0.001 | 2.58 to 10.98 | 6.60 (1.10) | 3.37 | 0.197 | −0.80 to 7.54 |
Adverse experiences
Nausea (61/196 v. 27/191; χ2=9.75, P=0.002), malaise (37/196 v. 17/191; χ2=5.26, P=0.022) and sexual dysfunctions (14/196 v. 1/191; χ 2=9.78, P=0.002) were observed significantly more frequently in sertraline than non-sertraline treatment groups. The nausea and malaise were generally mild and occurred most frequently during the first weeks of treatment. No unusual adverse experiences were reported.
Dosage
In the sertraline groups the mean dose level at Week 16, before the non-responders were excluded from the study, was 98.6 (87.5, 109.7) mg daily in the response group, 131.6 (121.2, 142.0) mg in the partial responder group and 140.2 (131.4, 149.0) mg in the non-responder group. In the placebo group, the mean dose was 2.5 (0.0, 3.9) tablets daily.
DISCUSSION
Methodological considerations
The rater non-blindedness to exposure therapy constitutes a potential bias with respect to the outcome of the behavioural intervention. Since many of the general practitioners included as investigators worked in single practices, it was not possible to obtain blinded efficacy assessments. We thus required agreement between investigatorrated CGI—SP overall severity and patient-rated SPS score in order to classify a patient as responder. If agreement did not emerge, the response was classified as partial or non-response.
The high response we found in the placebo group is probably in part explained by the study design. ‘General medical care’ is not equivalent to ‘no treatment’, particularly in a 24-week study comprising 10 sessions with a physician. It is also possible that the physicians, all freshly trained in exposure therapy, may have included some information and encouragement with respect to self-exposure in their general medical care.
Exposure therapy in primary care
This study is the first to assess the efficacy of a behavioural treatment intervention performed by primary care physicians for an anxiety disorder. The exposure therapy proved easy to learn and appeared suitable for use in general practice. The behavioural intervention seemed to be comparable in efficacy with that seen in exposure therapy studies in specialist settings (Reference van Dyckvan Dyck, 1996). A trend towards significance between exposure alone and placebo was observed in the final efficacy evaluation. Time-point improvement analyses (see Fig. 3) revealed that exposure was superior to placebo at the end of the physician-assisted exposure training (i.e. after 12 weeks) and that most exposure therapy patients that were improved (i.e. partial response or response) at the final visit also were improved at the Week 12 assessment. Only marginal symptom improvement was seen during the self-training period in non-responders at the Week 12 assessment, but the number of patients that fulfilled the criteria for response increased. The study design presupposed that the patients would learn the principles of graded exposure and continue the training on their own. Although patients were encouraged to continue to practise and were given an additional exposure session at the Week 16 visit, we do not know that the exposure patients actually performed the training. It is thus possible that if physician-administered exposure therapy had been continued throughout the study, the statistical efficacy of exposure therapy at the final efficacy evaluation might have been enhanced.
Sertraline in the primary care treatment of GSP
This study demonstrated that sertraline, both alone and combined with exposure therapy, is effective and well tolerated in the treatment of generalised social phobia carried out in primary care. Other pharmacological treatments, such as irreversible monoamine oxidase inhibitors (MAOI) (Reference Liebowitz, Schneier and CampeasLiebowitz et al, 1992; Reference Heimberg, Liebowitz and HopeHeimberg et al, 1998) and benzodiazepines (Reference Davidson, Potts and RichichiDavidson et al, 1993) have primarily shown efficacy in GSP treatment in psychiatric out-patients. However, tolerability and safety problems such as dietary restrictions and dependence limit the use of these drugs. It is possible that sertraline (Reference van Ameringen, Mancini and Streinervan Ameringen et al, 1994), as well as other selective serotonin reuptake inhibitors that have shown efficacy in GSP treatment (Reference Baldwin, Bobes and SteinBaldwin et al, 1999; Reference Stein, Fyer and DavidsonStein et al, 1999) may be more acceptable drugs in primary care settings, owing to their milder side-effect profiles.
What treatment should be used in primary care?
The study suggested an enhanced efficacy of combined sertraline and exposure treatment, primarily through increasing the number of patients who achieved response. Since we did not find any statistical interaction between sertraline and exposure therapy, the finding most probably must be interpreted as the result of additive treatment effects, as also suggested in treatment studies in anxiety disorders performed in other health care settings (Reference GelderGelder, 1998; Reference Lader and BondLader & Bond, 1998; Reference O'Connor, Todorov and RobillardO'Connor et al, 1999). If our findings are confirmed in further research, it may be argued that good primary care treatment should include a brief structured behavioural intervention such as exposure therapy as an adjuvant to drug treatment.
Some primary care physicians might argue that even this brief behavioural intervention is too time-consuming to be acceptable in their practice. However, patients with GSP are long-term patients requiring considerable use of time in primary care. A combined treatment programme that includes eight sessions of 15-20 minutes' duration may be acceptable with respect to the use of time. A trial of exposure therapy may be relevant in patients not suited to pharmacological treatment, or who do not fully respond to drug treatment. The combination of sertraline and exposure therapy seems to be particularly effective, mainly by increasing the number of patients reaching response, and may constitute the treatment of choice in primary care.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• Sertraline is effective in the treatment of generalised social phobia. However, combined treatment of sertraline and exposure therapy seems to be the most efficient treatment in primary care.
-
• Primary care physicians can learn to practise exposure therapy effectively during a brief training course.
-
• Long-standing generalised social phobia may be successfully treated in primary care. However, the response is increasing over a considerable time span.
LIMITATIONS
-
• The rating of effect of the exposure therapy was made by the investigator in agreement with the patient.
-
• No quality control of exposure therapy performance was obtained.
-
• Patients were recruited both from primary care patients and from newspaper advertisements.
Acknowledgements
Participating investigators: Norway: I. Bach-Gansmo, C. Bonnevie, C. J. Christensen, A. Dehli, T. Eikeland, H. Fonnelop, K. Fossvik, K. A. Graaboe, H. J. Helgesen, I. Haestad, H. O. Hoeivik, K. Hoeye, A. G. Hanshuus, B. Jordet, T. Karsrud, T. M. Lundgren, O. Loeland, S. Madsbu, J. G. Melby, I. E. Moland, K. B. Molteberg, R. Rekve, J. Rud, S. Roenbeck, T. A. Roevik, K. P. Solvaag, O. Soerensen, M. J. Taule, S. Thomassen, T. Thomassen, L. Tjeldflaat, I. H. Udnaes. Sweden: A. Blomberg, O. Borgholst, K. Ekenstierna, P. Hellke, C. Lütz, T. Nordlund, L. Paulsson, O. Sjoeberg, V. Stan, M. Staahlberg-Ekborg, S. B. Sundqvist, C. Tillberg, A. C. Weibull, V. Aahgren, I. Aakerman.
We thank F. Bendiksen, K. A. Moe, K. Lagermalm, M. Groensleth, T. Smedsrud, A. Sjoeberg, W. L. Andersen, T. Stamnes at Pfizer, Norway and Sweden for giving practical and administrative support. We also thank U. F. Malt for giving scientific advice.









eLetters
No eLetters have been published for this article.