Long-term efficacy has not been well demonstrated for most currently available treatment regimens for bipolar disorder. Pharmacotherapy increasingly has involved combination therapy, typically consisting of a combination of lithium and another psychotropic agent such as an antipsychotic. Historically, the use of conventional antipsychotics has been hampered by a risk of tardive dyskinesia (Reference Kane and SmithKane & Smith, 1982) and worsening of depression (Reference MorganMorgan, 1972). Atypical antipsychotics may hold promise in avoiding the drawbacks of the conventional antipsychotics, but there have been hardly any controlled studies of atypical antipsychotics in the prophylactic treatment of bipolar disorder. We report here the results of an 18-month, double-masked, relapse prevention study of patients with bipolar disorder who had achieved remission with olanzapine in combination with lithium or valproate. This phase of the study was designed to compare continuation of combination treatment v. monotherapy, using an analysis defined a priori involving both syndromic and symptomatic definitions of relapse.
METHOD
Participants were men and women aged 18–70 years who had achieved syndromic remission from an index manic or mixed episode after receiving olanzapine plus lithium or valproate during a 6-week, double-masked study that compared combination treatment with lithium or valproate monotherapy, as previously reported (Reference Tohen, Chengappa and SuppesTohen et al, 2002). Briefly, patients received open-label lithium or valproate plus additional olanzapine or placebo under double-masked conditions. All patients had been diagnosed with bipolar I disorder, manic or mixed episode, with or without psychotic features, using the Structured Clinical Interview for the DSM–IV (Reference First, Spitzer and GibbonFirst et al, 1997; American Psychiatric Association, 1994). Prior to enrolment in the acute phase, participants had to have experienced at least two previous mood episodes (depressed, manic or mixed). They were also required to have had a documented trial at a therapeutic blood level of lithium (0.6–1.2 mmol/l) or valproate (50–125 mg/ml) for at least 2 weeks immediately prior to enrolment and to have demonstrated persistent manic symptoms, as defined from a Young Mania Rating Scale (YMRS; Reference Young, Biggs and ZieglerYoung et al, 1978) total score of 16 or more at both enrolment (visit 1) and randomisation (visit 2). Any of the following was considered grounds for exclusion from entry: pregnancy; serious and unstable medical illness; DSM–IV substance dependence within the past 30 days (except nicotine or caffeine); documented history of intolerance to olanzapine; and serious suicidal risk. Patients who achieved remission with the combination therapy of olanzapine plus lithium or valproate were randomly reassigned using a unique drug kit number (via a call-in interactive voice response system) in a 1:1 ratio to treatment with either the combination of olanzapine plus lithium or valproate (A) or lithium or valproate monotherapy (B). All patients, study site personnel and sponsor investigators were masked to the randomisation codes. Prior to participation, all patients received a complete description of the study and signed an informed consent document approved by their study site's institutional review board. To enter the relapse prevention phase of the study, patients receiving co-therapy during the acute phase had to demonstrate syndromic remission according to established research definitions (Reference Tohen, Stoll and StrakowskiTohen et al, 1992; Reference Strakowski, Keck and McElroyStrakowski et al, 1998) at the end of the acute phase (week 6), as follows:
-
(a) DSM–IV ‘A’ criteria for current manic episode no worse than mild (score ≤ 3 in a range of 1 to 7), ‘B’ criteria no worse than mild (≤3, range 1–7), and no more than two ‘B’ criteria that were mild (3, range 1–7);
-
(b) all DSM–IV ‘A’ criteria for current major depressive episode no worse than mild (≤3, range 1–7), and no more than three ‘A’ criteria mild (3, range 1–7).
Patients who received combination treatment during the acute phase and had achieved syndromic remission of both mania and depression as defined above were randomly reassigned at visit 8 (week 6 of the acute phase) in a 1:1 ratio to receive an additional 18 months of double-masked therapy, consisting of either olanzapine (flexible dosage range of 5 mg, 10 mg, 15 mg or 20 mg per day) in combination with lithium or valproate (combination therapy), or placebo added to lithium or valproate (monotherapy). Patients continued taking the same mood stabiliser that they had received during the acute phase, the choice of which was determined by the site investigator. As in the acute phase, mood stabiliser therapy was not masked; only the addition of olanzapine or placebo was conducted under double-masked conditions. Patients in the combination therapy group began treatment with 10 mg per day of olanzapine, given on the evening of visit 8. The period between visits was 1 week for the first two assessments (visits 9 and 10), 2 weeks for the next assessment (visit 11), 4 weeks for the assessment after that (visit 12), and 8 weeks for the remainder of the study (visits 13 to 20). To maintain masking, treatment took the form of two 5 mg capsules (either olanzapine or placebo), titrated up in increments of one capsule, or down by any number of decrements at the investigator's discretion, as indicated by each patient's symptom improvement and treatment tolerance. Patients unable to tolerate the minimum dose (one capsule) were withdrawn from the study. During this relapse prevention phase of the study, the blood levels of lithium and valproate remained within the therapeutic range (lithium 0.6–1.2 mmol/l, valproate 50–125 μg/ml), as previously defined. If lithium or valproate levels deviated from this therapeutic range, the investigator adjusted the dosage of either drug to re-establish blood levels within the range. Patients were permitted adjunctive use of benzodiazepines (≤2 mg lorazepam equivalent per day) for no more than 5 consecutive days, or 60 days cumulatively. Anticholinergic therapy (benzatropine mesylate ≤2 mg per day) was permitted throughout the study for treatment of extrapyramidal side-effects but not for prophylaxis. Aside from study drugs, benzodiazepines and anticholinergics, no other psychiatric drug was permitted during the study.
Assessments
Relapse was assessed as:
-
(a) syndromic, meeting DSM–IV criteria for a manic, mixed or depressive episode (American Psychiatric Association, 1994)
-
(b) symptomatic, using the total score on the YMRS and the 21-item Hamilton Rating Scale for Depression (HRSD–21; Reference HamiltonHamilton, 1967).
Patients who not only met the requirements for syndromic remission at the end of the acute phase but also met the symptomatic remission criteria (YMRS total score ≤12 and HRSD–21 total score ≤8) were assessed for symptomatic relapse during this extension phase. Symptomatic relapse of mania was defined as a YMRS total score rating of 15 or greater after having previously met the criteria for symptomatic and syndromic remission. Symptomatic relapse of depression was defined as an HRSD–21 total score rating of 15 or greater after having previously met the criteria for both symptomatic and syndromic remission.
To assess relapse prevention, survival analyses were conducted to determine the times to syndromic relapse (the study's primary outcome measure) and symptomatic relapse of any mood episode, whether manic, depressive or mixed. Times to relapse were based on the date of the assessment when relapse criteria were first met or, if censored to relapse, the final assessment date, each relative to the date of randomisation. Patient assessments were conducted by mental health care professionals, including psychiatrists, psychologists, nurses and other mental health caregivers with an advanced clinical degree or certification. Raters were trained in the use of symptom rating scales before the study began. Changes in dosage levels of randomised therapy were made by the prescribing principal investigator at each site. This investigator might have also been responsible for outcomes assessment, but was not necessarily the rater.
Serum concentrations of lithium or valproate were assessed at every visit to determine if the therapeutic blood level was maintained. To estimate the mean blood level, the area under the serum concentration curve (AUC) was determined for each patient. The AUC was calculated with a weighted average of mean serum concentrations at each pair of consecutive visits, weighted by the number of days between those two visits.
Scales for assessment of extrapyramidal side-effects included the Simpson–Angus Scale (Reference Simpson and AngusSimpson & Angus, 1970), the Barnes Akathisia Rating Scale (BARS; Reference BarnesBarnes, 1989) and the Abnormal Involuntary Movement Scale (AIMS; Reference GuyGuy, 1976). Treatment-emergent parkinsonism was defined as a Simpson–Angus Scale score greater than 3 at any time following a score of 3 or less during the acute period. Treatment-emergent akathisia was defined as a BARS score of 2 or more at any time following a score of less than 2 during the acute period. A score of 3 or more on any of the first seven AIMS items, or a score of 2 or more on any two of the first seven AIMS items, was taken to be indicative of long-term treatment-emergent dyskinetic symptoms, given that neither of these criteria was met during the acute period. This definition of treatment-emergent dyskinetic symptoms is consistent with the cross-sectional symptom severity criteria suggested by Schooler & Kane (Reference Schooler and Kane1982) as research diagnostic criteria. Assessment of vital signs and weight and a full blood analysis (including prolactin and non-fasting glucose levels) were performed at each visit.
Statistical analyses
The sample size was planned on the assumption that 75% of patients receiving olanzapine plus lithium or valproate during the preceding acute phase would be in syndromic remission, yielding approximately 168 eligible patients for the relapse prevention phase of the study. With equal allocation into the combination and monotherapy treatment groups, this sample size provided 96% power to detect a difference in time to relapse using the log-rank test assuming an 18-month syndromic relapse of bipolar disorder of 30% and 60% for combination therapy and monotherapy respectively, and a 10% loss to follow-up in each group. Time-to-relapse curves for the therapy groups were estimated with the Kaplan–Meier technique and the curves were compared using the log-rank test. Median length of follow-up to the point of relapse or censoring is used to describe time to relapse in each group. Similar analyses were performed to examine time to discontinuation. A Cox proportional hazards regression approach allowed examination of differential time to relapse comparing the therapies based on subgroup factors (age, gender, racial origin, psychotic features, history of cycling course, type of index episode) by inclusion of a therapy–subgroup interaction. For rating scales, total scores were derived from the individual items; if any item was missing, the total score was treated as missing. Analysis of variance was used to evaluate quantitative change, including terms for treatment, investigator, and treatment–investigator interaction; treatment differences are characterised with 95% confidence intervals about the mean change difference. Fisher's exact test was used to compare rates of relapse and discontinuation; incidence rates of treatment-emergent adverse events and scale-based extrapyramidal side-effects are shown with an asymptomatic 95% confidence interval about the risk difference (combination therapy minus monotherapy). Tests of treatment differences were two-sided, with an α level of 0.05; significance of interactions was tested at an α level of 0.10. Measurements taken at the last acute-phase visit (end-point, week 6 of acute phase) served as the baseline for all continuous measures of efficacy and safety. Categorical treatment-emergent events were defined as those that worsened or first occurred after the acute phase of therapy.
RESULTS
Sample characteristics and disposition
The study was conducted at 29 sites in the USA and Canada between September 1997 and October 2000. Of those assessed at the end of the preceding acute phase (Fig. 1), 99 patients who had received olanzapine combined with either lithium or valproate during the double-masked acute phase achieved syndromic remission of bipolar disorder and were reassigned randomly either to continue with double-masked therapy consisting of olanzapine in combination with lithium or valproate (combination therapy, n=51) or to discontinue olanzapine and receive placebo added to lithium or valproate (monotherapy, n=48). The treatment groups were comparable with respect to mean age, racial origin and gender (Table 1).
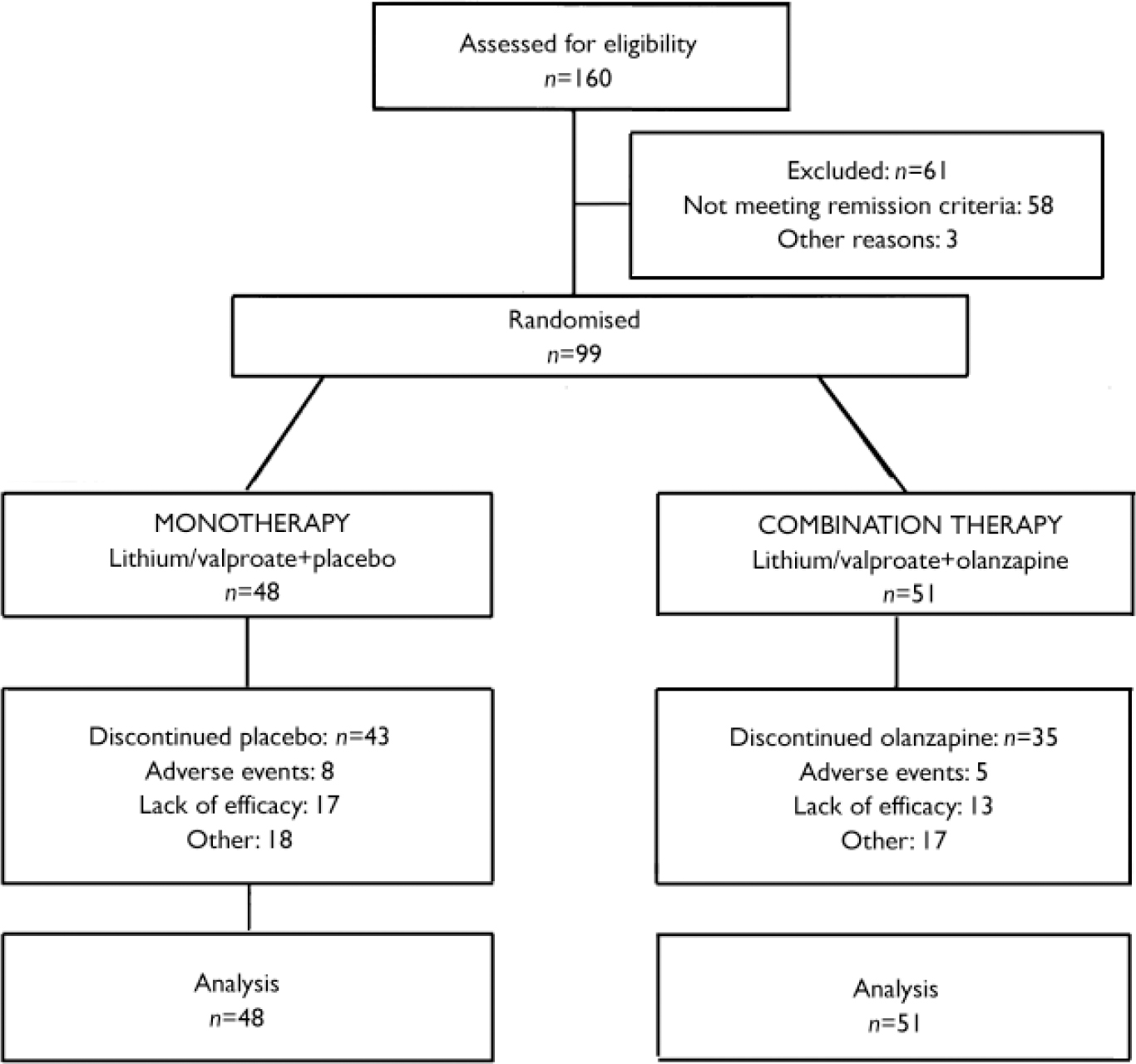
Fig. 1 Study profile.
Table 1 Illness and demographic characteristics of the study sample

| Characteristic | Olanzapine combination therapy (n=51) | Lithium/valproate monotherapy (n=48) |
|---|---|---|
| Age (years): mean (range) | 43.5 (19-69) | 39 (20-65) |
| Males (%) | 52.9 | 43.8 |
| White (%) | 84.3 | 85.4 |
| Mixed index episode (%) | 49.0 | 50.0 |
| Index episode without psychotic features (%) | 72.5 | 75.0 |
| Rapid-cycling course (%) | 43.1 | 39.6 |
| Receiving valproate (%) | 64.7 | 62.5 |
| Age at onset of bipolar disorder (years): median | 22 | 20 |
| Previous episodes (n): median | ||
| Manic | 10 | 6 |
| Mixed | 7 | 5 |
| Depressed | 3 | 1.5 |
| Length of index episode at entry (days): median | 58 | 78 |
| Currently employed (%) | 14.0 | 14.6 |
Prior to randomisation in the acute treatment period, the median duration of mood stabiliser therapy immediately before study entry was 67 days, and 203 of the 344 patients enrolled in the acute phase had a duration of therapy greater than 6 weeks. Of the 99 patients entering the relapse prevention phase, almost two-thirds (n=63) were receiving valproate. As characterised by their index episode at study initiation, patients with mania (n=50) and mixed states (n=49) were about equally represented. A rapid-cycling course was present in 41 patients, and 26 exhibited psychotic features in their index episode of mania. The duration and nature of the illness appear consistent between treatment groups (Table 1).
The mean drug dosages and serum concentrations in the two groups are shown in Table 2. Concomitant use of benzodiazepines in the combination therapy group (10 of 51, 20%) was similar to that in the monotherapy group (14 of 48, 29%). In addition, concomitant use of anticholinergics was similar in the two groups (combination treatment 5 of 51, 10%; monotherapy 7 of 48, 15%).
Table 2 Drug dosages and serum concentrations in patients receiving olanzapine plus lithium or valproate (combination therapy) compared with those receiving lithium or valproate alone

| Combination therapy Mean (95% CI) | Monotherapy Mean (95% CI) | |
|---|---|---|
| Olanzapine | ||
| Modal daily dose (mg) | 8.6 (7.4-9.9) | |
| Lithium | ||
| Daily dose (mg) | 1064.6 (954.6-1174.5) | 1023.8 (891.2-1156.3) |
| Blood level (mmol/l) | 0.76 (0.66-0.86) | 0.74 (0.67-0.81) |
| Valproate | ||
| Daily dose (mg) | 1264.6 (1119.5-1409.6) | 1286.5 (1060.4-1512.6) |
| Blood level (μg/ml) | 67.8 (61.8-73.8) | 66.3 (60.1-72.5) |
The percentage of patients completing the 18-month follow-up period was nearly three times higher in the combination treatment group than in the monotherapy group (combination treatment 31%, monotherapy 10%; P=0.014). Moreover, time to discontinuation differed significantly between treatment groups (χ2 1=3.86, P=0.049, log-rank test), with median length of follow-up of 111 days for combination therapy compared with 82 days for monotherapy. The proportions of specific reasons for discontinuation were not significantly different between treatment groups.
Relapse prevention
At the start of the relapse prevention phase, 99 patients were assessed as being in syndromic remission of bipolar disorder. Time to relapse into a syndromic affective episode, whether mania or depression, was not significantly different between the treatment groups (χ2 1=0.11, P=0.742, log-rank test; hazard ratio 1.13, 95% CI 0.55–2.31), with median times to relapse occurring at 40.5 days for the monotherapy group and 94 days for the combination therapy group. Rates of syndromic relapse into either mania or depression were also not significantly different between treatment groups: combination therapy 15 of 51 (29%), monotherapy 15 of 48 (31%); P>0.99.
Of the 99 patients in syndromic remission at entry to the relapse prevention phase, 68 were assessed to be free from either manic or depressive symptoms (specified a priori as YMRS score ≤12 and HRSD–21 score ≤8) at randomisation. During the maintenance phase, time to symptomatic relapse into either mania or depression was significantly longer for the combination therapy group compared with the monotherapy group (χ2 1=5.19, P=0.023, log-rank test, hazard ratio 2.29, 95% CI 1.10–4.78), with median times to relapse of 163 days for combination treatment and 42 days for monotherapy (Fig. 2). Rates of symptomatic relapse into either mania or depression were not significantly different between the treatment groups: combination therapy 11 of 30 (37%); monotherapy 21 of 38 (55%); P=0.149.
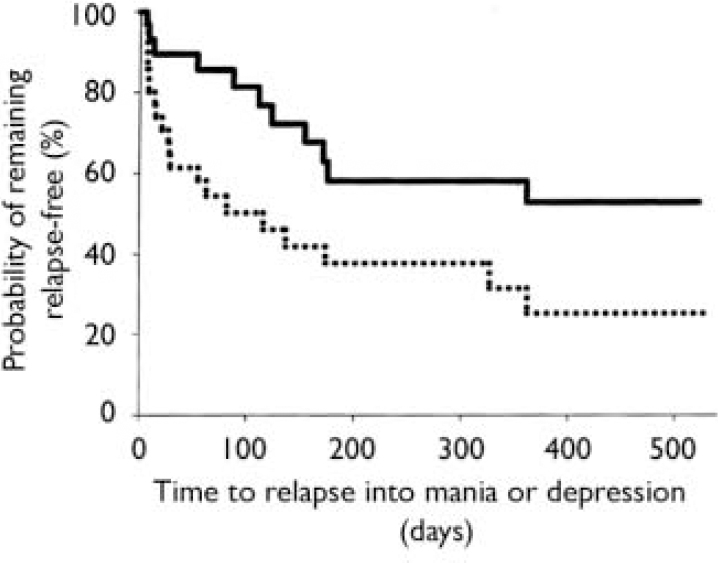
Fig. 2 Time to symptomatic relapse (mania or depression) of patients previously meeting symptomatic remission criteria was significantly longer (P=0.023, log-rank test) for the olanzapine combination therapy group (n=30; solid line) than for the monotherapy group (n=38; dotted line). Median time to relapse was 163 days for combination therapy and 42 days for monotherapy.
Time to symptomatic relapse into mania alone during the prevention phase following both syndromic and symptomatic remission of both mania and depression was longer for the combination therapy group, but not significantly so (χ2 1=2.27, P=0.132, log-rank test; hazard ratio 2.12, 95% CI 0.78–5.77), with median times to relapse of 171.5 days for combination therapy v. 59 days for monotherapy. Rates of symptomatic relapse into mania only were also lower in the combination therapy group (6 of 30, or 20%) than in the monotherapy group (11 of 38, or 29%), but not significantly so (P=0.574). Median times to symptomatic relapse into depression alone during the extension phase (combination therapy 163 days, monotherapy 55 days) were not statistically significantly different between treatment groups (χ2 1=3.27, P=0.071, log-rank test; hazard ratio 2.24, 95% CI 0.91–5.50). Likewise, rates of relapse into depression alone, although lower in the combination therapy group (7 of 30, or 23%) than in the monotherapy group (15 of 38, or 40%), were not significantly different (P=0.197).
Subgroup analyses
Stratification of data regarding time to relapse into either symptomatic mania or depression was conducted using the patient characteristics of age, gender, racial origin, presence of psychotic features, type of index manic episode, presence of rapid-cycling course, and mood stabiliser used. Differences in treatment responsiveness were noted between patients stratified by gender or racial origin (Table 3). Reflecting the overall group results, women receiving combination therapy were found to have significantly longer times to a symptomatic affective (mania or depression) relapse than did women receiving monotherapy (Fig. 3): median time to relapse for combination therapy 177 days v. monotherapy 27.5 days; P=0.001, log-rank test. However, men (Fig. 4) showed no such treatment difference (median time to relapse for combination therapy 84 days v. monotherapy 67 days; P=0.811, log-rank test). Similarly, stratifying the group by racial origin revealed possible differences in treatment responsiveness, with White patients showing significantly longer times to symptomatic relapse with combination therapy than with monotherapy, whereas non-White patients showed no significant difference in treatment responsiveness. No significant interaction was seen between treatment and any other subgroup characteristic (presence v. absence of psychotic features, manic v. mixed index episode, presence v. absence of history of rapid cycling, use of valproate v. lithium).
Table 3 Subgroup analyses of efficacy: time to recurrence of symptomatic mania or depression

| Subgroup | Stratum | Therapy | n | Median follow-up (days) | Within-stratum χ2 statistic1 | Within-stratum P value | Hazard ratio | Interaction P value2 |
|---|---|---|---|---|---|---|---|---|
| Age | < 40 years | Monotherapy | 20 | 59.5 | 2.593 | 0.107 | 3.75 | 0.660 |
| Combination | 13 | 182 | ||||||
| ≥ 40 years | Monotherapy | 18 | 28.5 | 6.583 | 0.010 | 2.53 | ||
| Combination | 17 | 155 | ||||||
| Gender | Male | Monotherapy | 18 | 67 | 0.057 | 0.811 | 0.86 | 0.020 |
| Combination | 16 | 84 | ||||||
| Female | Monotherapy | 20 | 27.5 | 10.700 | 0.001 | 5.49 | ||
| Combination | 14 | 177 | ||||||
| Origin | White | Monotherapy | 33 | 28 | 8.049 | 0.005 | 3.07 | 0.090 |
| Combination | 24 | 171 | ||||||
| Other | Monotherapy | 5 | 72 | 0.609 | 0.435 | 0.38 | ||
| Combination | 6 | 89 | ||||||
| Psychotic features | No | Monotherapy | 29 | 29 | 2.681 | 0.102 | 2.00 | 0.595 |
| Combination | 20 | 139.5 | ||||||
| Yes | Monotherapy | 9 | 56 | 2.390 | 0.122 | 3.15 | ||
| Combination | 10 | 272 | ||||||
| Manic episode type | Manic | Monotherapy | 20 | 28.5 | 8.349 | 0.004 | 3.57 | 0.208 |
| Combination | 17 | 172 | ||||||
| Mixed | Monotherapy | 18 | 59 | 0.192 | 0.661 | 1.38 | ||
| Combination | 13 | 108 | ||||||
| Rapid-cycling course | No | Monotherapy | 23 | 56 | 2.965 | 0.085 | 2.17 | 0.837 |
| Combination | 16 | 163 | ||||||
| Yes | Monotherapy | 15 | 15 | 1.821 | 0.177 | 2.54 | ||
| Combination | 14 | 144 | ||||||
| Mood stabiliser | Valproate | Monotherapy | 25 | 56 | 2.571 | 0.109 | 2.26 | 0.984 |
| Combination | 21 | 171 | ||||||
| Lithium | Monotherapy | 13 | 28 | 2.055 | 0.152 | 2.30 | ||
| Combination | 9 | 155 |
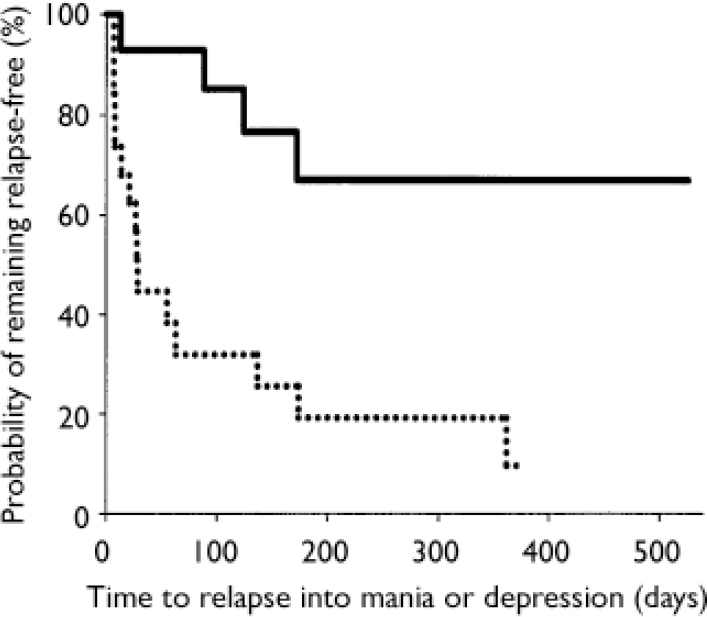
Fig. 3 Time to symptomatic relapse (mania or depression) in women participants. Women meeting symptomatic remission criteria who were treated with olanzapine in combination with lithium or valproate (n=14; solid line) had a significantly longer time to relapse (P=0.001, log-rank test) than women receiving lithium or valproate plus placebo (n=20; dotted line); median time to relapse was 177 days for combination therapy and 27.5 days for monotherapy.
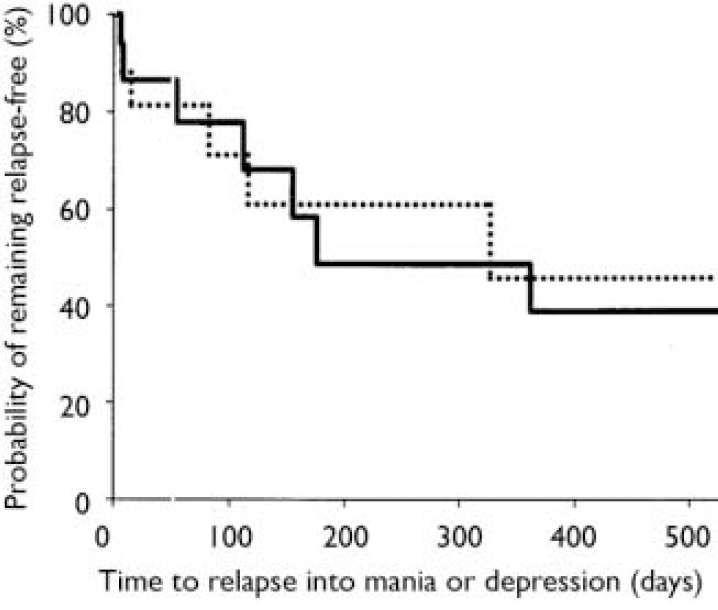
Fig. 4 Time to symptomatic relapse (mania or depression) in men. No treatment difference was seen between men receiving combination therapy (84 days, n=16; solid line) and men receiving monotherapy (67 days, n=18; dotted line); P=0.811, log-rank test.
Safety measures
Incidences of common treatment-emergent adverse events (Table 4) were for the most part not dissimilar in the two treatment groups, with the exception of insomnia, which occurred in more than a quarter (n=13) of monotherapy patients compared with only 2 (4%) combination treatment patients, and weight gain, which was more common with combination therapy than monotherapy (combination therapy 20%, monotherapy 6%).
Table 4 Treatment-emergent adverse events1

| Event | Olanzapine combination therapy (n=51) n (%) | Lithium/valproate monotherapy (n=48) n (%) | Risk difference (95% CI)2 |
|---|---|---|---|
| Depression | 19 (37.3) | 14 (29.2) | 8.1 (-10.4 to 26.6) |
| Somnolence | 10 (19.6) | 4 (8.3) | 11.3 (-2.1 to 24.7) |
| Weight gain | 10 (19.6) | 3 (6.3) | 13.4 (0.5 to 26.2) |
| Anxiety | 7 (13.7) | 7 (14.6) | -0.8 (-14.6 to 12.9) |
| Tremor | 7 (13.7) | 4 (8.3) | 5.4 (-6.9 to 17.6) |
| Apathy | 5 (9.8) | 8 (16.7) | -6.8 (-20.2 to 6.5) |
| Asthenia | 5 (9.8) | 6 (12.5) | -2.7 (-15.1 to 9.7) |
| Diarrhoea | 5 (9.8) | 8 (16.7) | -6.9 (-20.2 to 6.5) |
| Insomnia | 2 (3.9) | 13 (27.1) | -23.2 (-36.8 to -9.5) |
| Abnormal thinking | 1 (2.0) | 5 (10.4) | -8.5 (-17.9 to 1.0) |
On measures of extrapyramidal sideeffects, mean within-group changes and treatment differences in changes on the AIMS and the Simpson–Angus and Barnes scales were generally small and not clinically meaningful (Table 5). Incidence rates of scale-based treatment-emergent parkinsonism (combination treatment 6.4%, monotherapy 8.9%; risk difference –2.5%, 95% CI –13.4 to 8.4), akathisia (combination therapy 7.5%, monotherapy 5.9%; risk difference 1.6%, 95% CI –9.7 to 13.0) and dyskinesia (combination treatment 0%, monotherapy 4.2%; risk difference –4.2%, 95% CI –9.8 to 1.5) also did not differ in a clinically meaningful manner.
Table 5 Mean baseline to end-point changes in safety measures

| Outcome | Therapy | n 1 | Baseline | Baseline to end-point change Mean (95% CI) | Treatment difference2 (95% CI) |
|---|---|---|---|---|---|
| Extrapyramidal side-effects (score) | |||||
| Simpson—Angus Scale | Monotherapy | 47 | 0.53 | -0.13 (-0.4 to 0.2) | 0.35 (0.01 to 0.68) |
| Combination | 51 | 0.61 | 0.22 (-0.1 to 0.5) | ||
| AIMS | Monotherapy | 48 | 0.04 | 0.13 (-0.1 to 0.3) | -0.14 (-0.39 to 0.10) |
| Combination | 51 | 0.41 | -0.02 (-0.2 to 0.2) | ||
| Barnes Akathisia Rating Scale | Monotherapy | 48 | 0.29 | -0.06 (-0.3 to 0.1) | 0.20 (-0.06 to 0.46) |
| Combination | 51 | 0.16 | 0.14 (0.0 to 0.3) | ||
| Weight (kg) | Monotherapy | 48 | 86.8 | -1.8 (-3.2 to -0.4) | 3.8 (1.8 to 5.9) |
| Combination | 51 | 89.1 | 2.0 (0.3 to 3.7) | ||
| Laboratory analyses | |||||
| Cholesterol (mmol/l) | Monotherapy | 39 | 5.17 | -0.06 (-0.3 to 0.2) | 0.02 (-0.27 to 0.31) |
| Combination | 45 | 5.16 | -0.04 (-0.2 to 0.1) | ||
| Glucose (mmol/l) | Monotherapy | 38 | 5.93 | -0.50 (-0.9 to -0.1) | 0.65 (-0.26 to 1.57) |
| Combination | 45 | 6.30 | 0.15 (-0.6 to 0.9) | ||
| MCH (fmol Fe) | Monotherapy | 37 | 1.89 | 0.02 (0.00 to 0.05) | -0.01 (-0.04 to 0.01) |
| Combination | 42 | 1.89 | 0.01 (-0.01 to 0.03) | ||
| Platelets (109/l) | Monotherapy | 36 | 244.4 | 35.9 (4.7 to 67.0) | -36.7 (-64.3 to -9.1) |
| Combination | 42 | 242.6 | -0.86 (-11.9 to 10.2) |
There was no common or clinically relevant treatment-related difference in vital signs or electrocardiographic measure, including orthostasis and corrected QT interval. Mean change in body weight from baseline to end-point was 3.8 kg greater for combination therapy than for monotherapy (Table 5), and the clinically relevant increase in weight (≥7% change from baseline) was greater for patients receiving combination therapy: combination therapy 27%, monotherapy 6%; risk difference 21.2% (95% CI 7.2 to 35.2). In terms of laboratory measures (Table 5), patients in the monotherapy group showed an elevation of mean corpuscular haemoglobin and platelet count values that was greater than that seen among patients in the combination therapy group. Mean baseline to end-point changes in non-fasting glucose and non-fasting cholesterol levels were small, and there was no case of clinically relevant increase in non-fasting glucose concentration (≥11.1 mmol/l at any post-baseline assessment if less than 11.1 mmol/l at baseline) or in non-fasting cholesterol concentration (≥6.20 mmol/l at any post-baseline assessment if less than 5.17 mmol/l at baseline) in either therapy group.
DISCUSSION
To our knowledge, this is the first published report of a randomised, double-masked maintenance study of the use of the combination of lithium or valproate with any atypical antipsychotic agent in the prevention of relapse of bipolar disorder. To date, published reports of long-term use of atypical antipsychotics in bipolar disorder have consisted primarily of uncontrolled openlabel clinical trials, naturalistic prospective studies or retrospective chart reviews (Reference Banov, Zarate and TohenBanov et al, 1994; Reference Zarate, Rothschild and FletcherZarate et al, 2000; Reference Vieta, Goikolea and CorbellaVieta et al, 2001). The results of this study suggest that, in patients who achieved remission from a manic or mixed episode after addition of an atypical antipsychotic agent such as olanzapine to previous treatment with lithium or valproate, the continuation of combination treatment reduced the rate of relapse of symptomatic bipolar episodes, compared with patients who stopped taking olanzapine and who continued on lithium or valproate monotherapy. The observed difference in outcomes based on symptomatic or syndromic criteria may be related to the relatively more conservative definition of symptomatic remission used in our study. To achieve symptomatic remission, patients needed to achieve syndromic remission and attain a minimum score on the YMRS and HRSD–21. It is possible that the more conservative definition captured patients who were symptomatically more stable. In these patients, combination treatment was significantly more effective than monotherapy, with the median time to relapse increasing from 42 days to approximately 5 months. Investigators have underscored the importance of assessing sub-syndromal depressive and manic symptoms (Reference Judd, Akiskal and SchettlerJudd et al, 2002). Sub-syndromal symptoms may in fact be the most common expression of bipolar I disorder over its long-term course, and are seen at a rate three times that of syndromal symptoms (Reference Judd, Akiskal and SchettlerJudd et al, 2002). Thus, when presence or absence of a syndromic relapse is assessed, worsening of residual symptoms may not be detected, and a true difference between treatments may thereby be missed.
An interesting finding of this study was that women were more likely to relapse sooner with monotherapy than with combination therapy; this differential treatment response was not observed for men; nor did the treatment–gender interaction occur during the acute phase of this trial. There is some published evidence of gender differences in treatment response (Reference Tohen, Zarate and HennenTohen et al, 2003), and with respect to olanzapine, women patients with a first-episode psychosis appear to have a greater response to olanzapine than to haloperidol – an outcome not found with male patients (Reference Goldstein, Cohen and HortonGoldstein et al, 2002).
Tolerability
Both the lithium and valproate monotherapies and the combined treatment with olanzapine were generally well tolerated. During the 18-month study, patients receiving combination therapy gained 2.0 kg, compared with a loss of 1.8 kg in the monotherapy group. It should be noted, however, that patients had already gained an average of 3.1 kg during the 6-week acute phase (Reference Tohen, Chengappa and SuppesTohen et al, 2002). The loss of weight in the monotherapy patients was probably secondary to the discontinuation of olanzapine. Thus, the weight gain of 2.0 kg in the relapse prevention phase should be considered additional weight gain after a longer exposure to olanzapine. These mean long-term weight increases of 5–6 kg with the use of olanzapine in combination with lithium or valproate are similar to those reported during long-term monotherapy of bipolar disorder (Reference Bowden, Calabrese and SachsBowden et al, 2003) and in schizophrenia (Reference Kinon, Basson and GilmoreKinon et al, 2001). Extrapyramidal side-effects were minimal in both treatment groups. The association with extrapyramidal side-effects, particularly tardive dyskinesia, has long been regarded as a major drawback of typical antipsychotics, as patients with bipolar disorder appear to be more susceptible to such effects than are patients with schizophrenia (Reference Kane and SmithKane & Smith, 1982).
There was no abnormal increase in non-fasting blood glucose or cholesterol levels at any time in the study group on the 18-month follow-up period. This study might not have had sufficient power to determine treatment differences; furthermore, assessment of the potential impact of treatment on glucose homoeostasis was limited because the glucose measurements were non-fasting. Laboratory changes that were noted included an increase in platelet count with monotherapy, but not with combination therapy; the significance of this difference, however, is not readily apparent.
Methodological limitations
Several limitations of the current study bear mentioning. First, the statistical power of the study was based on the assumption that 168 patients receiving olanzapine plus lithium or valproate during the preceding acute phase would have met remission criteria; however, only 99 patients were available for the second randomisation. This reduced sample size provided approximately 79% power (using assumed relapse rates for combination therapy and monotherapy as in the original estimation) and might have prevented the primary outcome variable from being statistically significant. Second, the clinical characteristics of the patient sample might not have been representative of the general patient population treated in clinical settings, as it contained a high proportion of patients whose bipolar I disorder had had a rapid-cycling course in the previous year. Patients with a rapid-cycling course may be refractory to treatment with lithium (Reference Bench, Lammertsma and GrasbyBench et al, 1996); it would therefore be expected that patients in the monotherapy group would have a particularly poor outcome owing to a weak response to lithium treatment, and indeed we found that the median time to relapse in this group was shorter than that observed during prophylactic lithium treatment in other trials (Reference Cuesta, Peralta and ZarzuelaCuesta et al, 2001). Related to this point is the fact that the patients in this study represented a somewhat ‘enriched’ sample, inasmuch as they were required to show incomplete responses to a preceding 2-week treatment with lithium or valproate and then respond satisfactorily to concomitant treatment with olanzapine. This may limit our ability to generalise the results of the study to all patients. On the other hand, this study is thereby one of only a few to address the question of whether a particular treatment that produces an improvement acutely – in this case, olanzapine – is able to maintain that improvement; that is, whether what gets the patient well can keep the patient well. Another limitation of our study is that although plasma concentrations of both lithium and valproate were maintained well within the target range and were in line with those of other maintenance studies (Reference Cuesta, Peralta and ZarzuelaCuesta et al, 2001), the valproate levels were nevertheless towards the lower end of the therapeutic range. In addition, as was discussed in the report of the preceding study phase (Reference Tohen, Chengappa and SuppesTohen et al, 2002), assignment of patients to lithium or valproate was made on the basis of the treatment preferences of the attending clinicians, rather than through randomisation. Accordingly, the study was not powered to show significant differences in outcome variables stratified by lithium or valproate treatment. It was therefore not possible to determine the relative efficacies and safety consideration of these two agents during the course of this 18-month treatment period. Another limitation was the small sample size in several of the subgroup analyses, which might have prevented detection of differential treatment responses. Finally, a treatment group for olanzapine monotherapy was not included. Therefore, an assessment of any synergistic effect between olanzapine plus lithium or valproate cannot be made, as it would be necessary to demonstrate that the combination treatment is more effective than each of the monotherapies alone.
In summary, our results indicate that long-term use of the combination of olanzapine plus lithium or valproate may prolong the time spent in symptomatic remission compared with lithium or valproate monotherapy in patients who have achieved remission with the combination treatment. The most clinically meaningful adverse event was a mean increase in body weight in the combination therapy group amounting to a gain of 2.0 kg over the 18-month relapse prevention phase, compared with a loss of 1.8 kg in the monotherapy group. These findings may be useful to clinicians for evaluating the relative risks and benefits for each individual patient in determining the selection of pharmacological treatment.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Olanzapine in combination with lithium or valproate prolongs the time to relapse, based on symptom rating scale criteria, compared with lithium or valproate alone. The advantage of combination therapy was seen whether lithium or valproate was used.
-
▪ Clinical features (rapid cycling, index episode type, psychotic features) did not predict treatment difference outcomes; however, being female or White predicted a longer time to recurrence with combination therapy.
-
▪ Combination therapy is associated with greater weight gain compared with monotherapy.
LIMITATIONS
-
▪ Estimates of relapse vary depending on the criteria used to evaluate remission and relapse.
-
▪ The lack of an olanzapine monotherapy arm prevented assessment of any synergistic effect between olanzapine plus lithium or valproate.
-
▪ Results may have limited generalisability to the targeted clinical patient populations.












eLetters
No eLetters have been published for this article.