Rosenbaum et al (Reference Rosenbaum, Fava and Hoog1998) demonstrated an important clinical correlate of differences in elimination times of selective serotonin reuptake inhibitors (SSRIs). They reported a definite relation between the interruption of therapy by placebo substitution and rebound-like worsening of depressive symptoms and somatic distress in patients who were treated previously with paroxetine or sertraline. Michelson et al (Reference Michelson, Fava and Amsterdam2000), in a similarly designed trial, confirmed that a significant increase in symptoms occurred after as little as 2 days of placebo substitution. Other authors also have reported rebound-like effects after abrupt discontinuation or periods of non-dosing with SSRIs (Reference Barr, Goodman and PriceBarr et al, 1994; Reference HaddadHaddad, 1997; Reference Lejoyeux and AdesLejoyeux & Ades, 1997).
These findings raise the question of the frequency and duration of spontaneously occurring omissions of scheduled doses of SSRIs during routine clinical management of depression. The aim of this observational prospective study, therefore, was to assess the incidence and duration of missed doses for patients prescribed SSRIs in clinical practice.
METHOD
Patients
We designed an observational prospective cohort study to assess the incidence, frequency and length of dosing lapses in patients prescribed an SSRI. Patients were included randomly in 1999 by their pharmacist, who asked and received informed consent. Patients were eligible for the study when they presented a repeat-prescription for fluoxetine, fluvoxamine, paroxetine or sertraline and were 18 years of age or older. They had to take their medication independently, without having to rely on a caregiver. No further inclusion or exclusion criteria were applied. A total of seven pharmacies participated in the study.
Study design
Relevant clinical information was provided by the prescribing physician, including indication and psychiatric comorbidity. Information on the dosing regimen of the SSRI was recorded at the pharmacy and validated by the prescriber. Data on compliance were gathered through electronic drug exposure monitors (eDEMs): medicine containers with a built-in microchip that register the time and date of each opening and closing of the package. During the observation period of 3 months, the eDEM was filled at the pharmacy at the time of each refill. All patients were instructed by the pharmacist on the use of the eDEM. Information was presented to the patient, stating the goal of the study and the use of the eDEM.
The observation period started with the first opening of the eDEM by the patient after the first dispensing in the pharmacy. Refill date or dosing changes were evaluated and compared with the computerised medication history available from the pharmacy. The observation period continued until 3 months after the first opening by the patient, or earlier if therapy ended or the patient dropped out of the study.
Electronic monitoring
The eDEM information included the times and dates of individual bottle openings provided as calendar and chronology plots. A calendar plot reflects the number of openings of the eDEM. In a chronology plot, the hour and number of openings during each successive 24-h period are depicted. Patients with eDEM information were categorised according to the number of (consecutive) days of non-dosing found. Lapses of 2, 3, 4 and 5 or more consecutive days of non-dosing were counted per patient.
RESULTS
Patient characteristics
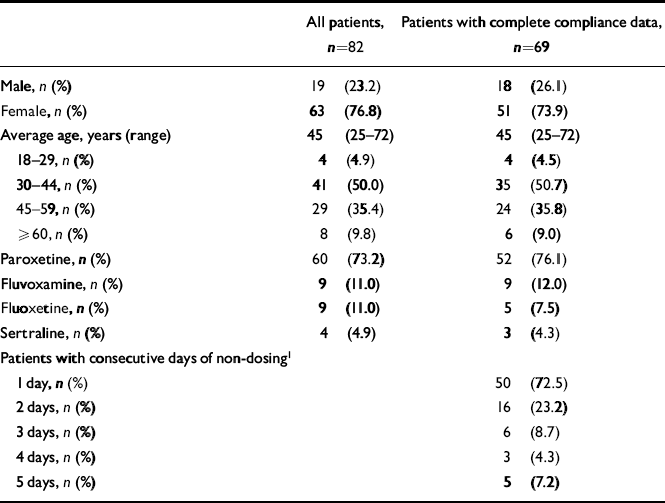
A total of 82 patients treated with an SSRI (fluoxetine, fluvoxamine, sertraline or paroxetine) were recruited at their pharmacy. Table 1 shows the basic characteristics of the population. For 13 patients no dosing information from the eDEM was available, owing to loss of the device (9), relocation of the patient (2), hospitalisation of the patient (1) or preliminary discontinuation of therapy (1). For 69 patients (84.1%) complete compliance data were available, including electronic monitoring data and information from the patient. The majority of the patients were female (73.9%) and the average age was 45 years (range 25-72 years). Paroxetine was used most frequently (76.1%), followed by fluvoxamine (12.0%), fluoxetine (7.5%) and sertraline (4.3%). Characteristics of patients with no dosing information did not differ significantly from patients with complete dosing data.
Table 1 Characteristics of the study population and summary of missed doses
| All patients, n=82 | Patients with complete compliance data, n=69 | |
|---|---|---|
| Male, n(%) | 19 (23.2) | 18 (26.1) |
| Female, n(%) | 63 (76.8) | 51 (73.9) |
| Average age, years (range) | 45 (25-72) | 45 (25-72) |
| 18-29, n(%) | 4 (4.9) | 4 (4.5) |
| 30-44, n(%) | 41 (50.0) | 35 (50.7) |
| 45-59, n(%) | 29 (35.4) | 24 (35.8) |
| ≥ 60, n (%) | 8 (9.8) | 6 (9.0) |
| Paroxetine, n(%) | 60 (73.2) | 52 (76.1) |
| Fluvoxamine, n(%) | 9 (11.0) | 9 (12.0) |
| Fluoxetine, n(%) | 9 (11.0) | 5 (7.5) |
| Sertraline, n(%) | 4 (4.9) | 3 (4.3) |
| Patients with consecutive days of non-dosing1 | ||
| 1 day, n(%) | 50 (72.5) | |
| 2 days, n(%) | 16 (23.2) | |
| 3 days, n(%) | 6 (8.7) | |
| 4 days, n(%) | 3 (4.3) | |
| 5 days, n(%) | 5 (7.2) |
Information about the indication was provided by the prescriber for a subset of the patients (n=51). Depressive disorder (45.1%), followed by anxiety disorder (17.6%) and other disorders (3.6%), including obsessive-compulsive disorder, bulimia or sleep disturbances, were indicated as single diagnoses. A substantial number of the patients received an SSRI for treatment of multiple diagnoses (33.3%), most often depressive disorder and anxiety disorder (17.6%) but also other combinations (15.7%). According to the patient, the main indication for which the SSRI was prescribed had been present for 4.2 years and the average duration of treatment had been 2.0 years.
Medication histories derived from computerised dispensing data in the pharmacies' computers were used to evaluate the use of psychotropic co-medication. Of all patients with dosing data, benzodiazepines were used in 23 patients (33%), including 16 (23%) chronic users. Antipsychotics were used by four patients (5.8%). Multiple use of antipsychotics and/or benzodiazepines was seen in three patients. In these patients, multiple days of non-dosing were recorded, resulting in only 51-64% of days on which the prescribed doses were taken.
Compliance data
The median duration of monitoring was 89 days (range 30-90). In total 5914 days of monitoring were registered. Patients were recorded as taking a single dose, as prescribed, on 89% of these monitored days. On 477 of the monitored days (8.1%), no dose was recorded as having been taken. On 164 of the monitored days (2.8%), more than the prescribed single dose was recorded as having been taken.
A total of 50 of the 69 patients (72.5%) were observed to have had at least 1 day on which a dose was omitted (range= 1-18 single days per patient; median=3 days). Sixteen patients had at least one lapse of 2 consecutive days of omitted doses (range=1-3; median=1). Nine patients had at least one lapse of 3 or more consecutive days of omitted doses, distributed between 3, 4 and 5 consecutive days. The range of lapses in dosing lasting 3 or more days was 1-6. A total of 29.0% (20/69) of patients had at least 2 consecutive days of non-dosing. These 20 patients had a total of 47 dosing lapses of at least 2 days (range= 1-8 lapses), resulting in an average of 2.4 lapses per patient during the 86-day follow-up period.
The distribution of interdose intervals using 24±6 h as the central category was recorded. The majority of the patients had a dosing time between 06.00 and 12.00 a.m.; a total of 20.3% of all patients had irregular intervals of administration, defined as >25% of the dosages taken outside a 6-h period. Patients with irregular dosing intervals showed significantly more days of non-dosing (10/14) compared with patients with regular dosing (10/55) (P<0.01).
The majority of the patients reported no problems with the eDEM and a minority found the device impractical compared with the blisterpack that they were used to (10.1%). The majority (71.0%) assessed their knowledge of disease and treatment as sufficient. About two-thirds of patients (63.8%) were positive about using the devices feeling that feedback from the physician or pharmacist might help them to improve their compliance.
DISCUSSION
Main results and background of this study
In this observational prospective cohort study we found that almost 73% of SSRI users occasionally miss a complete day of dosing and 29% occasionally miss 2 or more consecutive days. Since the introduction of electronic monitoring to compile dosing histories for ambulatory patients in the late 1980s, many studies have been performed (Reference UrquhartUrquhart, 1997), most of which describe a variety of aberrant dosing histories with little or no information on their clinical consequences. For the SSRIs, however, this omission has been partially addressed by Rosenbaum et al (Reference Rosenbaum, Fava and Hoog1998) and Michelson et al (Reference Michelson, Fava and Amsterdam2000), who showed the consequences of several-day lapses in dosing, but without information on the frequency with which such dosing errors occur and recur in normal clinical practice. The purpose of our study is to provide some of that missing information.
Lapses in SSRI dosing in clinical practice
Michelson et al (Reference Michelson, Fava and Amsterdam2000) reported a significant increase in adverse events as early as the second day of placebo substitution in patients using paroxetine. These adverse events are generally mild, short-lived and self-limiting, but can be distressing and may lead to missed working days and decreased productivity. The symptoms may be somatic (e.g. dizziness, nausea, vomiting and/or flu-like symptoms) or psychological in nature (anxiety, agitation, crying spells). Thus, there is a well-defined set of clinical consequences of lapses in dosing taken by patients prescribed the shorter acting SSRI antidepressants (paroxetine and sertraline). We have no reason to assume that clinical effects of lapses in dosing differ in clinical practice compared with the controlled situation described by Rosenbaum et al (Reference Rosenbaum, Fava and Hoog1998) and Michelsonet al (Reference Michelson, Fava and Amsterdam2000). Although it would be interesting to study these effects in an observational design, clinical data cannot be captured without interrupting the naturally occurring dosing lapses.
Pharmacokinetics of individual SSRIs
Dosing time data are important in relation to the pharmacological duration of action. The individual antidepressant drugs studied here differ widely in their pharmacokinetic half-lives, from less than 24 h in the case of paroxetine (Reference Benet, Oie, Schwartz, Hardman, Molinoff and RuddonBenet et al, 1996) to several days in the case of fluoxetine and a week or more in the case of its active metabolite norfluoxetine (Reference ReynoldsReynolds, 1993). These differences in half-life are reflected in a widely different persistence of therapeutic drug action, after dosing interruptions, as shown by the placebo-substitution study of Rosenbaum et al (Reference Rosenbaum, Fava and Hoog1998). That study showed a surprisingly rapid reversal of the antidepressant action of paroxetine, within as little as 48 h of controlled substitution of placebos for active drug. A key question emerging from that study has been whether the multiday substitution of placebo for active drug was a realistic model of naturally occurring forms of non-compliant dosing. Our study clearly shows that, in this group of long-term users of SSRI antidepressants, the multiday lapse in dosing does indeed occur in about three out of ten patients. One might reasonably expect that such errors would occur even more often, and perhaps with longer lapses in dosing, in patients who have just started treatment with antidepressant drugs. Owing to the small sample size, we could not differentiate between the individual SSRIs but we have no reason to assume that the incidence of days of non-dosing differs between chronic users of individual SSRIs. The present prescribing pattern of Dutch general practitioners is reflected in the distribution of the SSRIs in this study. All SSRIs included in this study were daily formulations and the consequences of non-dosing in patients using the weekly formulation of fluoxetine, for example, now licensed in the USA, are unknown.
Obviously, the interpretation of interdose interval data depends upon the duration of action of the drugs in question, which were widely disparate in this study, ranging from paroxetine at the shortest end to fluoxetine at the longest end, based on the data of Rosenbaum et al (Reference Rosenbaum, Fava and Hoog1998). Although the numbers were too small to differentiate between the individual SSRIs, we found that patients with irregular dosing intervals showed significantly more lapses in dosing.
Underestimation of actual non-compliance
All patients were included by their pharmacist and, although we have no information about the number of patients refusing to participate, it might be possible that non-compliant patients were less motivated to participate in the study, creating a selection bias. Moreover, the nine patients who lost the device are more likely to be non-compliant patients.
Overall, the patients in this study were relatively healthy with little comorbidity, psychiatric or otherwise. However, we included a limited number of patients with concomitant use of antipsychotics, and these individuals showed low compliance with antidepressant medication. Patients included were under treatment for an average duration of 2.5 years with a relatively simple dosing scheme. Our study focused on patients prescribed SSRI antidepressants on a long-term basis, a group in which one might expect, if anything, somewhat better compliance than during the first several weeks of antidepressant therapy, when changes in prescription are frequent and medication-taking is as yet unrewarded by alleviation of depression. Our results therefore may be an underestimation of the actual non-compliance.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
• During a 3-month study period we found dosing lapses of at least 2 days occurring in 30% of chronically ill patients treated with selective serotonin reuptake inhibitors (SSRIs) in clinical practice.
• From earlier research we know that lapses of 2+ days are long enough to result in clinically relevant increases in the number and severity of somatic or psychological adverse events.
• The possibility of symptoms related to missed doses also should be considered when relapse of disease after early discontinuation or impairment during treatment are diagnosed.
LIMITATIONS
• Sample size in this study was too small to differentiate compliance patterns between individual SSRIs.
• Although it would be interesting to study the effects of lapses in dosing in an observational design, clinical data cannot be captured without interrupting the naturally occurring dosing lapses.
• In this study, non-compliance may have been underestimated through the selection of potentially more compliant participants and the loss of information on possibly non-compliant participants.




eLetters
No eLetters have been published for this article.