The discovery of imipramine created a ‘founder effect’ that still influences antidepressant drug development. The key pharmacological action of imipramine is believed to be blockade of the membrane transporters that terminate the actions of noradrenaline and serotonin (5-hydroxytryptamine, 5-HT) released into the synaptic cleft at central monoamine synapses. Many currently used antidepressants have been designed to achieve these effects more potently and selectively. However, blockade of transporters can be detected immediately after drug administration, whereas the therapeutic effect of antidepressant treatment requires a number of weeks to become clinically important. Reference Frazer and Benmansour1 This apparent discrepancy has stimulated an abundance of experimental work over the past three decades that has focused on the neurobiological effects of repeated antidepressant treatment. Secondary pharmacological effects whose time course requires repeated administration are numerous, and seem natural candidates to explain how antidepressants produce their therapeutic effects.
Such theories have emphasised the potential therapeutic importance of downstream neuroadaptive changes for antidepressant drug action, including downregulation of subsets of post-synaptic 5-HT and noradrenergic receptors as well as desensitisation of autoreceptors located on 5-HT and noradrenaline cell bodies. Reference Frazer and Benmansour1 More recently attention has shifted to antidepressant-induced activation of second messengers and consequent changes in gene expression. This is associated with increased production of neurotropic factors resulting ultimately in changes in synaptic plasticity and neurogenesis. Reference Manji, Quiroz, Sporn, Payne, Denicoff and Gray2 Such downstream effects of established antidepressants are taken to provide alternative molecular targets for potential new antidepressant agents.
Although of great potential interest, purely neurochemical theories of antidepressant action cannot explain how these pharmacological mechanisms lead to the remission of clinical symptoms in a depressed person. When it is discussed at all, it is generally assumed that the primary psychological action of antidepressants is to elevate mood and that improvement in the various symptom domains is, broadly, a secondary consequence of this effect. This view, however, appears incomplete because antidepressant drugs do not reliably elevate mood in non-depressed people and the psychostimulant drugs that can produce such mood elevation do not appear to be clinically useful antidepressants. Reference Satel and Nelson3 Hence, the mechanisms by which the neurochemical and neural changes induced by antidepressant drugs are translated into clinically meaningful effects in depression are still broadly unknown. This review summarises evidence for a cognitive neuropsychological hypothesis of antidepressant drug action which aims to integrate what we know about the neurochemistry and psychology of depression and its treatment and provide an alternative explanation for the therapeutic delay in antidepressant drug action.
Cognitive neuropsychological hypothesis of antidepressant drug action
We propose the hypothesis that, at a neuropsychological level, antidepressants work by remediating negative affective biases in depression and anxiety and that these actions occur relatively quickly following drug administration. Such changes in bias are probably not accessible to subjective state, but the effects of processing emotional and social stimuli in a more positive manner would be expected to lead to gradual changes in social reinforcement, behaviour and mood over time and experience of these cues. As illustrated in Fig. 1, this view suggests that the critical time lag in antidepressant drug action does not result from a delay in relevant neuropharmacological actions, but is imposed between the effects of antidepressants on emotional processing and the subsequent effects on mood. In other words, changes in affective bias with antidepressant drug administration do not directly enhance mood, but may provide a platform for subsequent cognitive and psychological reconsolidation. This view is consistent with cognitive theories of depression which emphasise the role of negative biases in information processing in the aetiology and maintenance of depression and the importance of correcting such biases in successful treatment of this disorder. Reference Beck, Rush, Shaw and Emery4
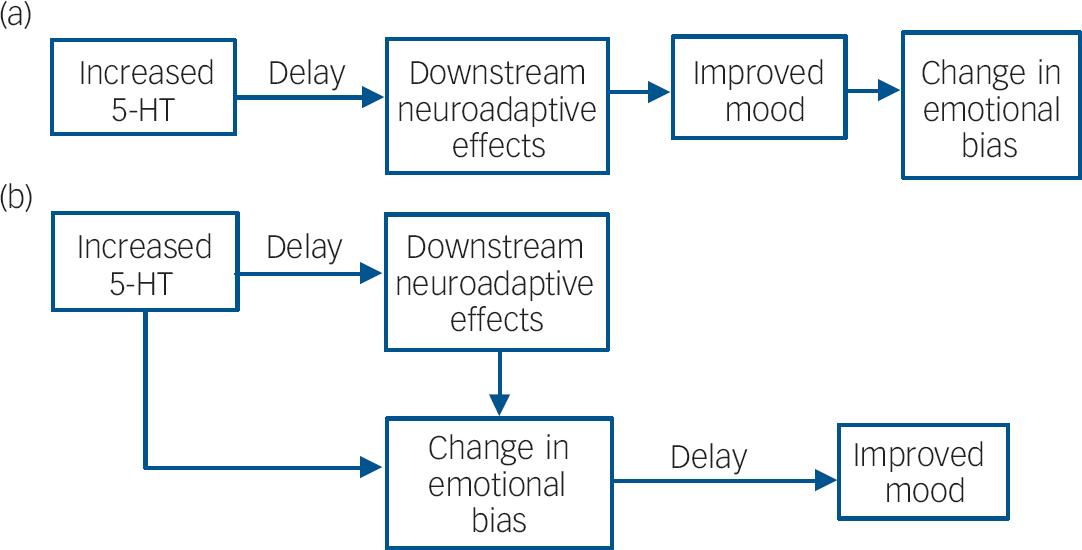
Fig. 1 (a) Accepted view of the mechanisms underlying the delay in antidepressant drug action; (b) proposed delay in antidepressant action being largely mediated by the translation of changes in emotional bias to mood, although some effects of antidepressants on emotional processing may also require downstream neuroadaptive effects and repeated administration of antidepressant drugs (5-HT, 5-hydroxytryptamine, serotonin).
This account suggests that the effects of antidepressants on emotional processing should be seen much earlier in treatment than previously suggested. Although present from the start of treatment, these effects may be boosted or affected further by slowly emerging downstream changes. For example, changes in emotional bias may affect social behaviour and mood through repeated experience of these cues and this will involve relearning a range of emotional associations. The need for such relearning effects sits intriguingly with the observation that antidepressants promote synaptic plasticity and learning in animal experimental studies. Reference Manji, Quiroz, Sporn, Payne, Denicoff and Gray2 Such processes may be expected to facilitate the effects of changed bias on mood and symptoms in depression. As such, the effects of enhanced synaptic plasticity may only be relevant when combined with increased positive processing induced by current antidepressant therapies.
Depression is a complex clinical syndrome characterised not only by lowered mood but also by vegetative and cognitive symptoms. How far can changes in emotional processing account for the resolution of these multiple symptom domains during the course of successful antidepressant treatment? We suggest that the changes in emotional processing could also explain this kind of global improvement because attention and appraisal are of fundamental importance in how the brain decides what matters to it. Thus the ability of antidepressant drugs to interfere with a preferential focus on negative stimuli means that the brain is once again open to emotionally positive information and positive interpretation of experience. We have argued above that this effect, over time, could lead to greater reactivity and improvement in mood and a decrease in social and occupational withdrawal. In the same way the lessening of preoccupation with negative themes could free up processing resources for tasks such as episodic memory, which are typically impaired in the depressive state. Equally, vegetative symptoms can be strongly influenced by attention and appraisal: for example, fluvoxamine treatment of patients with depression was associated with improvement in reported subjective sleep quality even though objectively measured sleep parameters showed a trend to worsen. Reference Wilson, Bell, Coupland and Nutt5 Such a notion suggests that changes in the processing of external and internal emotional cues have the potential to affect a wide range of symptoms in depression.
This account is supported by evidence from behavioural and neuroimaging investigations in healthy volunteers, at-risk groups and depressed patients. It builds upon many decades of research in cognitive psychology which has explored the role and importance of negative affective biases in depression. Here, we review the evidence for the cognitive neuropsychological theory of antidepressant drug action and identify the challenges that remain to establish it.
Testing the cognitive neuropsychological hypothesis
Are negative biases important in depression?
The role of negative biases in information processing in the aetiology and maintenance of depressive disorders has long been hypothesised. Reference Beck, Rush, Shaw and Emery4 Early theories suggested that these biases affect all aspects of information processing, with patients with depression showing enhanced attention, interpretation and memory for all negative emotional material. Reference Beck, Rush, Shaw and Emery4 More recent evidence indicates that cognitive processes are not uniformly biased in depression and that distinctions between implicit and explicit aspects of performance, attentional engagement and disengagement and perceptual and conceptual levels of processing are relevant. However, there is evidence to support a consistently enhanced selective memory for negative material particularly seen in explicit memory paradigms. Reference Matt, Vacquez and Campbell6 For example, if patients are asked to recall positive and negative self-descriptors encoded in a classification task they show a tendency to remember negative rather than positive words. Interpretation of ambiguous textual or visual stimuli has also been reported to be negatively biased in depression. Reference Leppanen7 Measures of facial expression recognition have typically revealed a bias towards labelling ambiguous facial expressions as negative and/or the perception of positive cues of happiness in patients with depression may be decreased. Reference Gur, Erwin, Gur, Zwil, Heimberg and Kraemer8 Finally, at longer exposure durations there is also evidence of an attentional bias in depression, which may represent a difficulty in disengaging attention from negative emotional information. Reference Mogg, Bradley and Williams9
Studies using functional magnetic resonance imaging (fMRI) provide converging evidence for the role of limbic circuitry in negative affective processing biases in major depression. Reference Leppanen7 Thus, for example, in implicit face processing paradigms depression is associated with exaggerated responses in the amygdala, ventral striatum and insula to negative expressions of emotion, Reference Surguladze, Brammer, Keedwell, Giampietro, Young and Travis10,Reference Fu, Williams, Cleare, Brammer, Walsh and Kim11 whereas responses to happy facial expressions in the thalamus, amygdala, hippocampus and putamen appear to be reduced. Reference Lawrence, Williams, Surguladze, Giampietro, Brammer and Andrew12,Reference Fu, Williams, Brammer, Suckling, Kim and Cleare13 Although these subcortical areas are usually regarded as important in the initial evaluation of emotion, some of the observed effects may underpin attentional bias. Thus, patients with depression also show increased responses to sad facial expressions and decreased responses to happy facial expressions in extrastriate areas including the fusiform and lingual gyrus and precuneus. Reference Surguladze, Brammer, Keedwell, Giampietro, Young and Travis10 The visual mechanisms for increasing attention to salient and important stimuli are thought to be modulated by signals from both amygdala and frontoparietal circuitry. Reference Vuilleumier and Driver14 Hence, the coordinated responses to affective stimuli may provide a neural assay of emotional bias or the salience level of affective stimuli and inform us of the neural mechanisms underlying behavioural biases. Such biases may therefore be driven by enhanced negative evaluation within limbic areas coupled with deficient higher-order emotional modulation of cognitive processes within areas such as the ventromedial prefrontal cortex. Reference Elliott, Rubinsztein, Sahakian and Dolan15
Are negative biases important in vulnerability to depression?
Although the existence of cognitive biases in the state of depression is well established and might contribute to maintaining depression, such biases could be a secondary phenomenon to lowered mood. However, there is growing evidence that such biases may be present outside episodes of major depression and could represent trait vulnerability markers for these disorders. For example, we have seen increased negative v. positive emotional processing in healthy volunteers at high risk of developing depression by virtue of high scores on neuroticism Reference Chan, Goodwin and Harmer16 or personal history of major depression. Reference Hayward, Goodwin, Cowen and Harmer17 Although some biases appear to be resolved or absent in high-risk volunteers outside a depressive episode, Reference Mannie, Bristow, Harmer and Cowen18 they can be triggered following a negative mood induction (see, for example, Joormann et al) Reference Joormann, Talbot and Gotlib19 or via depletion of plasma tryptophan, the amino acid precursor to serotonin (see, for example, Hayward et al). Reference Hayward, Goodwin, Cowen and Harmer17 In other words, far from being a simple symptom of depression, processing biases may be latent vulnerability mechanisms which can be easily triggered or exaggerated by small decreases in mood or lowered serotonin function.
Neuroimaging studies also support the existence of negative bias in high-risk volunteer groups and may be a more sensitive assay than overt behaviour, since the output of aberrant processes may be gated in some cases before behavioural expression. Consistent with this, aberrant responses to emotional material in the anterior cingulate, fusiform gyrus and amygdala have been seen in volunteers at high risk of depression, even in the absence of observable behavioural bias. Reference Haas, Omura, Constable and Canli20
These results therefore suggest that emotional processing biases, seen both at a behavioural level and at a neural level, are integral to depression and vulnerability to depression. Although it is well established that psychological treatments such as cognitive–behavioural therapy explicitly aim to remediate such biases, the effect of antidepressants on these processes has only recently started to be addressed.
Can effects of antidepressants on emotional processing be seen early on in treatment?
Healthy volunteer studies
There is a growing body of evidence that antidepressants can affect emotional processing very early on in treatment and independently from changes in subjective mood. For example, a week's treatment with the selective serotonin reuptake inhibitor (SSRI) citalopram and the selective noradrenaline reuptake inhibitor reboxetine in healthy volunteers produced biases in emotional processing that were generally the opposite of those seen in depression. Reference Harmer, Shelley, Cowen and Goodwin21 Both drugs decreased the recognition of fearful facial expressions, and people treated with citalopram were more likely to classify equivocal facial expression of negative emotions as happy. In addition, both reboxetine and citalopram significantly increased the number of positive words recalled in a self-descriptor memory task. These changes occurred in the absence of any subjective alteration in mood (Table 1). Reference Harmer, Shelley, Cowen and Goodwin21 Positive biases in emotional processing were apparent after 1 week of treatment, rather earlier than the therapeutic effects of antidepressants are often thought to occur, but at a time when steady-state blood levels would have been attained. However, we have also seen comparable effects after single doses of reboxetine, duloxetine and citalopram, all of which increased the recognition of happy facial expressions. Reference Harmer, Bhagwagar, Perrett, Vollm, Cowen and Goodwin22,Reference Harmer, Hill, Taylor, Cowen and Goodwin23 In addition, citalopram increased attention to positive socially relevant stimuli in a visual probe task after a single administration. Reference Browning, Reid, Cowen, Goodwin and Harmer24 Such effects currently appear to be specific to antidepressant and/or anxiolytic agents. For example, dopaminergic drugs such as amisulpiride or sulpiride appear to have no effect on attention to affective stimuli, Reference Gibbs, Naudts, Spencer and David25 whereas the effects on facial expression processing are restricted to the identification of anger. Reference Lawrence, Calder, McGowan and Grasby26
Table 1 Effects of depression and antidepressants on emotional processing
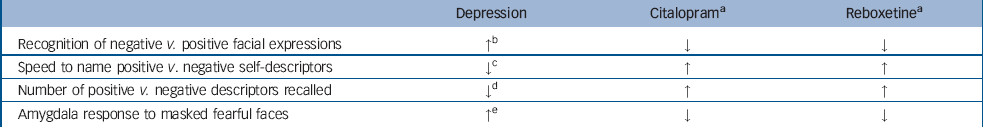
| Depression | Citalopram a | Reboxetine a | |
|---|---|---|---|
| Recognition of negative v. positive facial expressions | ↑ b | ↓ | ↓ |
| Speed to name positive v. negative self-descriptors | ↓ c | ↑ | ↑ |
| Number of positive v. negative descriptors recalled | ↓ d | ↑ | ↑ |
| Amygdala response to masked fearful faces | ↑ e | ↓ | ↓ |
a. Citalopram and reboxetine were administered to healthy participants for 7 days in studies with double-blind, placebo-controlled designs Reference Harmer, Shelley, Cowen and Goodwin21,Reference Harmer, Mackay, Reid, Cowen and Goodwin30,Reference Norbury, Mackay, Cowen, Goodwin and Harmer31
b. See reference Reference Leppanen7
c. Further information available from the authors
d. See reference Reference Matt, Vacquez and Campbell6
e. See reference Reference Whalen, Shin, Somerville, McLean and Kim32
The effects of antidepressants on the interpretation of positively reinforcing facial expressions may be expected to affect social function and behaviour. Consistent with this, Knutson et al saw an increase in cooperative behaviour in a problem-solving task following 1 week's treatment with paroxetine, Reference Knutson, Wolkowitz, Cole, Chan, Moore and Johnson27 whereas Tse & Bond have reported increased cooperation and assertive behaviour following antidepressant drug administration in healthy volunteers both acutely and after a week's treatment. Reference Tse and Bond28,Reference Tse and Bond29 Such actions are compatible with effects on emotional processing and suggest a mechanism by which antidepressants could produce an early remediation of some of the interpersonal deficits associated with depression. Our further suggestion is that these effects have a role in improving mood, with the delay characteristic of antidepressant action in depression.
Imaging studies with fMRI show neural effects that are congruent with the behavioural changes produced by antidepressants on emotional processing. We found that 1 week's treatment with both reboxetine and citalopram attenuated the amygdala response to masked fearful faces; reboxetine also increased the activity of the fusiform gyrus to presentation of happy faces. Reference Harmer, Mackay, Reid, Cowen and Goodwin30,Reference Norbury, Mackay, Cowen, Goodwin and Harmer31 These effects of antidepressants seen in healthy volunteers are opposite to the neural biases reported in depression (see above). A reduction in amygdala response to threat may reduce aberrant hypervigilance to negative affective stimuli in depression and anxiety, Reference Whalen, Shin, Somerville, McLean and Kim32 whereas an increased response of the fusiform face area to happy facial expressions may reflect increased attentional processing of positive and affiliative cues. Similar effects have been found in response to self-referent verbal stimuli; in particular, repeated administration of reboxetine affected frontoparietal responses during the presentation and retrieval of emotional trait words. Reference Norbury, Mackay, Cowen, Goodwin and Harmer33 Memory effects may be particularly relevant to antidepressant action, and encoding and retrieval of positive words was symmetrically influenced (more neural activity in encoding, less required in retrieval).
Together, these results suggest that antidepressants are able to modify behavioural and neural responses to emotional information without any change in subjective mood. Moreover, the changes in emotional processing can be seen across different stimuli types and extend outside conscious awareness. The effect sizes of these antidepressant manipulations appear broadly comparable to the magnitude of the biases measured as a function of depression and anxiety. Although demonstrated in the laboratory, these biases have the potential to affect behaviour and responses to stressors. For instance, biases induced through requiring volunteers to habitually attend to negative information have been reported to increase negative mood response to aversive stressful stimuli. Reference MacLeod, Rutherford, Campbell, Ebsworthy and Holker34 The neural circuitry affected by antidepressant drug administration includes areas believed to be involved in the automatic monitoring of threat such as the amygdala, frontal systems involved in the interface between emotion and cognition and visual processing regions involved in translating emotional signals into increased attention to salient and important stimuli (Fig. 2).
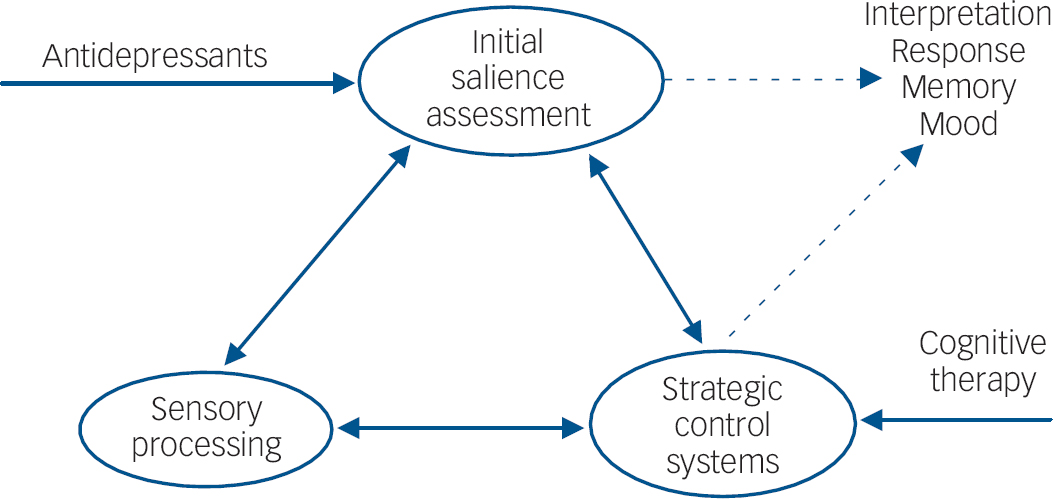
Fig. 2 Hypothetical location of antidepressant drug action. Antidepressants may affect early stages of emotional processing during initial valence assessment (underpinned by limbic areas including the amygdala). These early effects on valence assessment affect emotional processing by virtue of resources and attention devoted to these emotional cues. For example, positive cues such as happy facial expressions are given priority, thereby increasing initial visual processing in processing areas such as the fusiform gyrus. The appraisal of emotional stimuli and attention devoted to these stimuli would also be regulated by more strategic control areas such as the frontoparietal attentional network. It is proposed that although the effects of antidepressants and cognitive therapy would ultimately overlap, the initial site of cognitive therapy might particularly target the strategic control of emotional processing.
Acute v. repeated administration of serotonergic agents
As well as enhancing positive bias in emotional processing, acute SSRI administration also appears to increase threat processing in healthy volunteers. In particular, fearful face recognition and startle responses are enhanced following acute SSRI administration. Reference Harmer, Bhagwagar, Perrett, Vollm, Cowen and Goodwin22,Reference Browning, Reid, Cowen, Goodwin and Harmer24,Reference Grillon, Levenson and Pine35 This early increase in threat processing is consistent with clinical descriptions of increased anxiety and agitation in a subgroup of patients early in SSRI treatment before anxiolytic actions are seen, Reference Kent, Coplan and Gorman36 and this same sequence of effects has been described in animal models of anxiety. Reference Burghardt, Sullivan, McEwen, Gorman and LeDoux37 Performance in these human models of emotional processing may therefore tap into similar underlying processes to those seen clinically and could be useful in our attempts to understand the mechanisms that mediate unwanted as well as wanted effects.
The difference in time course of the positive affective changes (seen immediately) and the reduced threat processing (only seen after repeated SSRI administration) lend support to the idea that these may be dependent on dissociable cognitive and neural processes. Indeed, unlike citalopram, reboxetine did not reduce startle responses following repeated administration and did not enhance fear recognition following a single administration. Reference Harmer, Shelley, Cowen and Goodwin21,Reference Harmer, Hill, Taylor, Cowen and Goodwin23 It is tempting to suppose that the effects of antidepressants on threat processing are relevant to the therapeutic actions on anxiety symptoms in depression and in anxiety disorders, whereas the positive affective changes (positive biases in facial expression recognition and emotional recall) may be more relevant to mood disturbance and anhedonia seen in depression. Certainly, SSRIs such as citalopram are more broadly used in the treatment of anxiety disorders than catecholamine agents such as reboxetine (see the National Institute for Health and Clinical Excellence guidelines 38 ), consistent with their effects seen in models of emotional processing above. Further research is required to identify the neural basis of these different actions and whether they are related to the resolution of different symptoms in both depression and anxiety.
Studies in patients with depression or anxiety
The positive biasing effects of antidepressants described in healthy volunteers with acute administration also appear to occur in depression. Thus, a single 4 mg dose of reboxetine reversed negative biases seen in facial expression recognition, emotional categorisation and memory in acutely depressed patients in the absence of changes in mood, anxiety or other measures of subjective state (further details available from the authors). These results confirm that effects on emotional processing are seen much earlier than changes in mood in depression and suggest that results from healthy volunteer models are equally relevant to patients with depression. Furthermore, the magnitude of reboxetine's effect on emotional categorisation on day one of treatment was associated with eventual therapeutic improvement measured after 6 weeks of continued reboxetine administration. These results are therefore consistent with the hypothesis that early changes in emotional processing are instrumental in later changes in mood and depression and also imply that at least some aspects of treatment non-response may be related to a failure to induce sufficient changes in emotional processing early on in treatment.
Functional MRI studies of antidepressant drug administration in depression have yielded results largely consistent with the effects of antidepressants in healthy volunteer models, although so far most of the studies have investigated the effects after longer-term treatment. Hence, the increased responses to negative facial expressions seen in the amygdala, ventral striatum and frontoparietal cortex in depression were normalised following 8 weeks of SSRI treatment. Reference Fu, Williams, Cleare, Brammer, Walsh and Kim11 Eight weeks' treatment with venlafaxine was also found to normalise decreased anterior cingulate responses seen to negative v. neutral affective stimuli in patients with depression as well as affecting insular responses as early as 2 weeks post-treatment. Reference Davidson, Irwin, Anderle and Kalin39 Recent evidence suggests that SSRI treatment can also normalise responses to positive affective stimuli, with increased extrastriate responses to happy facial expressions being seen after 8 weeks, Reference Fu, Williams, Brammer, Suckling, Kim and Cleare13 and widespread normalisation of hypoactive responses to positive affective stimuli after 22 weeks of treatment. Reference Schaefer, Putnam, Benca and Davidson40
These findings in healthy volunteers and in patients with depression suggest modulation of the same emotional processes following antidepressant drug administration. Early remediation of negative biases in emotional processing is seen in depressed patients and this is related to later clinical responses to drug treatment. Neuroimaging studies also highlight effects of drug treatment on limbic, prefrontal and visual/attentional processing mechanisms in patients with depression. Although these changes are seen almost immediately in the healthy volunteer studies, it is not known whether such effects are seen before mood change in depressed patients since assessments have always been carried out after the therapeutic effects of these drugs are also evident. Future work is required to assess the effects of antidepressants on the neural processing of emotional information very early on following antidepressant drug administration in depression.
Implications of the cognitive neuropsychological hypothesis
Implications for treatment
The actions of antidepressants on emotional processing suggest an intriguing convergence with cognitive theories of depression that emphasise the importance of increased negative v. positive processing in the underlying aetiology of this disorder. Reference Beck, Rush, Shaw and Emery4 The results presented here suggest that antidepressant drug treatments may target these underlying processes supporting mood rather than targeting mood directly. It is unclear, however, to what extent the effects of drug treatment and cognitive therapy are overlapping. Although they both ultimately lead to reappraisal of emotional experiences the initial locus of action must be different. It seems intuitive that antidepressants affect relatively early and automatic processing of emotional stimuli which over time influences conscious appraisal of these stimuli, whereas cognitive therapy changes conscious evaluation of emotional experience which then influences automatic patterns of processing (see Fig. 2). However, the precise mechanisms by which cognitive therapy, behavioural therapy and their combination lead to improvements in depression are still under debate. Reference Longmore and Worrell41 Similarly, more research is needed to understand fully how alterations in monoamine function act to bias emotional processing at a systems level. Thus, recent neuroimaging studies have started to examine the effects of drug v. psychological treatment in depression. In particular, Kennedy et al found that response to venlafaxine after 16 weeks of treatment was associated with larger decreases in subgenual cingulate (Brodmann area 25) activity than response to cognitive–behavioural therapy, perhaps consistent with greater effects on automatic responses to emotional information, whereas cognitive–behavioural therapy was associated with increases in a more dorsal area of the anterior cingulate (BA 32), consistent with altered cognitive control. Reference Kennedy, Konarski, Segal, Lau, Bieling and McIntyre42 This area of research may be a particularly fruitful way of understanding the components of effective interventions for depression and anxiety and how best different approaches may be combined.
If supported, this formulation has a number of implications. The most clinically relevant concerns the modest efficacy of antidepressant treatment or cognitive therapy given alone. In the case of antidepressants, the therapeutic effect of noradrenaline and serotonin enhancement may depend on how far positive shifts in automatic emotional processing can lead to altered conscious emotional appraisal and improved mood. Treatment failure may be particularly associated with an adverse interpersonal environment or long-standing negative attitudes, such that changes in automatic emotional biases are insufficient to produce a satisfactory antidepressant effect. In contrast, failure of cognitive therapy may arise because the primary automatic biases are too fixed to allow a conscious remodelling of appraisal and evaluation.
These interpretations predict that treatment failure in depression may have several potential causes. They also suggest that in these circumstances combining antidepressants with cognitive–behavioural therapy might be more effective than increasing the ‘dose’ of either therapy alone. The literature on treatment-refractory depression is currently too limited to judge how correct this speculation is; however, there is evidence that the combination of cognitive therapy and antidepressant medication is a useful approach, particularly for severe or chronic depression. Reference Simon, Pilling, Burbeck and Goldberg43 Increased knowledge of individual differences in the automatic and conscious aspects of emotional processing could lead to more effective personalisation of psychological and pharmacological treatments, especially in depression that is difficult to treat.
Implications for treatment development
Emotional processing in healthy volunteers is affected by conventional antidepressant drugs with different neurochemical actions, suggesting the possibility of a common overlapping mechanism which may be important in their therapeutic effects. If confirmed, then performance in these models should be a useful indicator of therapeutic potential of novel candidate medications. This could help overcome some of the problems associated with animal models currently used to provide proof of concept. For example, the substance P (neurokinin-1) receptor antagonist aprepitant showed broadly positive effects in preclinical animal models used to screen new agents but limited eventual clinical efficacy in depression. Reference Hafizi, Chandra and Cowen44 Emotional processing models are applicable to volunteers, high-risk individuals and depressed patients and in suitably designed studies may provide a better estimate of likely value in clinical populations than animal studies. Consistent with this hypothesis, aprepitant produced a limited profile of action across tasks of emotional processing in healthy volunteers compared with the effects reported with conventional antidepressants. Reference Chandra, Hafizi, Massey-Chase, Goodwin, Cowen and Harmer45 Further studies are required to assess to what extent these models are able to predict therapeutic actions and dosage of novel agents known to be acting via different neurochemical mechanisms.
Future challenges
The cognitive neuropsychological hypothesis provides a broad framework for considering how antidepressant drugs work in depression and why it takes so long before their therapeutic effects are clinically apparent. This view is supported by evidence that the effects of antidepressants on emotional processing are seen surprisingly early on in treatment in both healthy volunteer models and depressed patients. If, as the hypothesis suggests, these early changes in emotional processing are critical in the later expression of therapeutic actions of these drug treatments, then these two effects should be directly correlated with one another. There are currently two studies which have addressed this question, both of which found that clinical response at 6 weeks could be predicted by the magnitude of antidepressant drug effect on emotional processing earlier in treatment Reference Tranter, Bell, Gutting, Harmer, Healy and Anderson46 (further information on the second study is available from the authors). Replication of this effect in larger samples is required to test the basic premise of this account. Such studies may also be able to identify which aspects of emotional processing change are particularly related to symptom improvement in depression.
This position also implies that to be a successful antidepressant, an agent must be able to modify emotional bias. It is unclear, at present, whether all effective treatments for depression will have similar effects on emotional processing. Preliminary evidence suggests that even treatments with apparently different mechanisms of action, such as vagal nerve stimulation, also affect emotional processing prior to full clinical action. Reference Critchley, Lewis, Orth, Josephs, Deichmann and Trimble47 Further examination of the effects of other treatments on emotional processing would assess whether these changes are necessary or sufficient for all aspects of antidepressant drug response.
So far largely unexplored is the possibility that the psychological mechanisms underlying the risk of onset or relapse of depression might be used to guide individual treatments of remediation and prevention. In other words, if a neuropsychological theory of drug action is correct, an individual profile of vulnerability should predict response to a particular treatment with a complementary pattern of remedial effect. Little is known as yet about the individual differences determining vulnerability to mood disorder or relapse. Hence, our overall theory could be tested by showing that benefits from different treatments track individual or subgroup-specific neuropsychological vulnerability profiles. Such effects might also be relevant to the prediction of relapse following discontinuation of antidepressant drug treatment. Further assessment of whether these kinds of changes are particularly important for subgroups of depression, or different severity levels, is needed.
Conclusions
This review has summarised evidence for increased negative and decreased positive affective processing in depression which can be indexed in both behavioural and neuroimaging paradigms. Antidepressant drug administration was seen to affect these same processes in healthy volunteers and in depressed patients, often immediately following drug administration and prior to mood change. These findings are supported by clinical studies, which suggest that the neural biases towards negative and positive emotional information are normalised following longer periods of antidepressant drug treatment. The cognitive neuropsychological hypothesis presented here suggests that these effects are critical in antidepressant drug action: thus early changes in emotional processing are proposed to precede and ultimately contribute to later changes in mood and depressive symptoms following antidepressant drug treatment. Hence, rather than acting as direct ‘mood enhancers’, antidepressants may re-tune how we process personal and socially relevant affective information. The challenge remains to assess the predictive power of these emotional processing measures both in the development of novel therapeutic agents for depression and the early assessment of relative treatment utility in individual patient care.
Acknowledgements
The work of the authors is supported by the Medical Research Council.






eLetters
No eLetters have been published for this article.