The consistently high lifetime prevalence of substance use (SU) has made the United States a worldwide leader in the severity of this biopsychosocial problem (Degenhardt et al., Reference Degenhardt, Chiu, Sampson, Kessler, Anthony, Angermeyer, Bruffaerts, de Girolamo, Gureje, Huang, Karam, Kostyuchenko, Lepine, Mora, Neumark, Ormel, Pinto-Meza, Posada-Villa, Stein and Wells2008). Recreational use of illicit substances often leads to substance use disorder (SUD). The latter results in social degradation and an increased likelihood of severe negative health outcomes. These include neuropsychiatric, cerebro- and cardiovascular disorders, infections, trauma, and genotoxicity resulting in chromosomal anomalies and cancer in offspring (Pastor et al., Reference Pastor, Conn, MacIsaac and Bonomo2020; Reece & Hulse, Reference Reece and Hulse2021; Schulte & Hser, Reference Schulte and Hser2013; Thomas et al., Reference Thomas, Diamond, Vieco, Chaudhuri, Shinnar, Cromer, Perel, Mensah, Narula, Johnson, Roth and Moran2018). While the level of detail in knowledge about the effects of SU has been deepening, the harm to the individual who uses/abuses psychoactive substances and to the society has been documented for centuries.
As the SU problem is constantly growing, the countermeasures have been so drastic that military terminology has been often used to describe them. Two NIH institutes, National Institute on Drug Abuse (NIDA) and National Institute on Alcohol Abuse and Alcoholism (NIAAA), established over 45 years ago, deal specifically with substance-related disorders. In addition to NIDA’s over $1 billion budget, the HEAL initiative (Helping to End Addiction Long-term) was launched in 2018 ‘to provide scientific solutions to the national opioid overdose crisis’, funded to the tune of billions of dollars (over $500 million just in 2019 [NIH, 2019]) spread over numerous NIH institutes and centers. The research supported by these resources should have resulted in substantial progress in scientific understanding of response to drugs and alcohol.
There is, however, no subsiding of the SU problem. Moreover, with all the efforts to control supply and discourage or reduce demand, the measures taken have been inconsistent. Access to psychoactive substances in the U.S. is expanding. It is hardly by chance that the current ‘opioid epidemic’ arose in parallel with another change in mass drug use behavior, reflected in lifting its legal barriers: the spreading decriminalization and legalization of cannabis. In addition to the rising frequency of cannabis use disorder (Cerdá et al., Reference Cerdá, Mauro, Hamilton, Levy, Santaella-Tenorio, Hasin, Wall, Keyes and Martins2019) and the coterminous use of other drugs, other correlates of likely causal origin include nervous system disorders (Reece, Reference Reece2018; Reece & Hulse, Reference Reece and Hulse2019a, Reference Reece and Hulse2019b, Reference Reece and Hulse2021) and psychopathology (Paul et al., Reference Paul, Hatoum, Fine, Johnson, Hansen, Karcher, Moreau, Bondy, Qu, Carter, Rogers, Agrawal, Barch and Bogdan2020). Over 20% of new cases of psychosis could be attributed to daily cannabis use (Di Forti et al., Reference Di Forti, Quattrone, Freeman, Tripoli, Gayer-Anderson, Quigley, Rodriguez, Jongsma, Ferraro, La Cascia, La Barbera, Tarricone, Berardi, Szöke, Arango, Tortelli, Velthorst, Bernardo and Del-Ben2019). While the addiction relapse rate upon treatment is reported to ‘resemble those of other chronic diseases such as diabetes’, it reaches 60% (compared to 50% in diabetes; NIDA, 2018). As SU legislation continues to relax and the ability of any state or federal agency to discourage distribution and enforce current laws remains limited, the U.S. is expected to experience a further continued increase in SU problems.
SUD, which is a frequent focus of research, practice and publicity, is also only part of the problem. In fact, the currently most dramatic outcome of SU, acute poisoning, usually termed ‘overdose’ and frequently fatal, does not require the severity of use to reach a diagnosable disorder. This outcome may occur in the absence of a career of chronic use that is generally necessary for the development of a disorder. The number of deaths related to illicit drugs, particularly but not exclusively opioids, has more than doubled since 2015 and exceeded 100,000 annually in the U.S. (Ahmad et al., Reference Ahmad, Cisewski, Rossen and Sutton2022). Alcohol poisoning regularly occurs among college freshmen (White et al., Reference White, Kraus and Swartzwelder2006), many of whom drink immoderately without being physiologically dependent on alcohol, often when peer influence outweighs internalized norms, if any, in the newly acquired freedom from external (e.g., family; age-related) restrictions.
This article is intended to review the literature and expand on prior publications introducing a perspective on SU covering premorbid and morbid forms as well as ‘normal’ phenotypes and changing the traditional focus of biomedical and clinical research. Based on the human genetics concept of liability to a disorder (Falconer, Reference Falconer1965), this perspective differentiates between the two aspects of this quantitative trait, risk and resistance, referenced by the two opposite poles of the liability distribution. We discuss the limitations of the current focus on risk and lay a conceptual foundation for reversing the research perspective. The resistance aspect is developed into a research strategy that is applicable not only to SU and addiction, but also to other complex traits and disorders. This approach, currently implemented in a NIDA-funded Resist! Project (Prom-Wormley et al., Reference Prom-Wormley, Maes and Vanyukov2022) and enabling the identification of factors enhancing resistance, may facilitate findings that are readily translatable into prevention and clinical practice.
Substance Use and Substance Use Disorder: The Taxonomical Context
‘SUD’ and ‘addiction’ are used interchangeably herein, although the current version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association [APA], 2013), points out that ‘addiction’ is not applied in its classification as a diagnostic term. As defined by the DSM-5, SUD is a ‘pattern of behaviors related to use of substances’ (p. 483), persistent SU despite negative consequences (e.g., taking the substance in larger amounts or over a longer period than was originally intended, failure to fulfill major role obligations at work, school, or home, or giving up important social, occupational, or recreational activities). This behavioral phenotype is reflected in 8 out of the 11 current DSM-5 diagnostic criteria, any two of which are enough for a positive SUD diagnosis.
The three remaining criteria are not behavioral but pharmacological phenomena pertaining to physical (physiological) dependence. Reflecting a lower level of biological organization than behavior, these symptoms — withdrawal, tolerance, and craving — unsurprisingly do not fit the underlying unidimensional construct conceptually and statistically (Kirisci & Vanyukov, Reference Kirisci, Vanyukov and Vanyukov2022). Conceptually, as manifestations of the adaptive physiological response to drug exposure, they are not necessarily indicative of a disorder. For instance, these symptoms are not recognized in the DSM-5 as diagnostic criteria for SUD if they develop during treatment under medical care. They are also not documented and are thus not used diagnostically for all substances (e.g., not for hallucinogens). When withdrawal (and, similarly, intoxication, another physiological response) is observed, it is also categorized by the DSM as a separate ‘substance-induced’ rather than ‘substance use’ disorder. As this physiological response develops, it increases the probability of the behavioral symptoms of addiction and, reciprocally, becomes more severe as that SU behavior continues and intensifies.
The presence of the physiological symptoms has been misinterpreted: as stated in the DSM-5 manual, it ‘has been known to lead to an erroneous diagnosis of “addiction” even when these were the only symptoms present’ (APA, 2013, p. 484). Equating these symptoms with the disorder has resulted in unjustifiably denying pain medication to patients (Heit & Gourlay, Reference Heit and Gourlay2009; O’Brien, Reference O’Brien2011; O’Brien et al., Reference O’Brien, Volkow and Li2006). Statistically, in the most optimal representation of the covariance structure of SUD symptoms for the variety of drugs, a bifactor model, the physiological symptoms are indicators of neither the global factor (reflecting nondrug-specific causes of symptom variation) nor substance-specific factors, but are in a causal relationship with both (Kirisci & Vanyukov, Reference Kirisci, Vanyukov and Vanyukov2022), in line with the conceptual biobehavioral considerations.
Quantitative Perspective: Liability to Addiction
Once initiated, SU is clearly a quantitative trait, involving both the qualitative variety of the psychoactive drugs used and the frequency-quantity facet of this behavior. It is this trait that is represented by the above global factor (reflecting variation in mechanisms common to SU in general), with additional sources of variance that are specific to drugs (e.g., those reflecting variation in drug-specific metabolism). Importantly, however, even before drug use starts, a phenotype for this trait does exist, albeit unobserved. More generally, when this trait is expressed as actual SU, the latter provides only a group of directly observable indicators of that trait (symptoms; other characteristics of use), but the trait itself remains a latent, unobserved, not directly measurable variable.
This quantitative behavioral trait is termed ‘liability’ (to a disorder [Falconer, Reference Falconer1965], such as SUD, or, wider, to any phenotype that is considered morbid). If measured, ‘it would give us a graded scale of the degree of affectedness or of normality’; it is ‘intended to express not only the individual’s innate tendency to develop or contract the disease, i.e. his susceptibility in the usual sense, but also the whole combination of external circumstances that makes him more or less likely to develop the disease’ (Falconer, Reference Falconer1965, p. 52). The categorical diagnosis dichotomizes the liability dimension into two phenotypic classes, unaffected and affected. The unaffected and affected individuals have liability phenotypes that are generally below or above, respectively, a certain point on the liability scale, termed the threshold. The threshold is artificial for a behavioral disorder like addiction: there is no natural division between affected and unaffected individuals in the liability distribution, a long-known observation for psychiatric disorders (Kent & Rosanoff, Reference Kent and Rosanoff1910), and the diagnosis is defined by hundreds of unique combinations of symptoms (Vanyukov, Tarter et al., Reference Vanyukov, Tarter, Kirisci, Kirillova, Maher and Clark2003). The diagnostic systems in the field of mental/behavioral conditions may be needed for clinical work and the economic aspect of healthcare, but the ‘[d]iagnostic categories based on clinical consensus fail to align with findings emerging from clinical neuroscience and genetics’ (Insel et al., Reference Insel, Cuthbert, Garvey, Heinssen, Pine, Quinn, Sanislow and Wang2010, p. 748). The liability-threshold model is a prevailing conceptualization for the multifactorial disorders (e.g., Bucholz et al., Reference Bucholz, Heath, Reich, Hesselbrock, Kramer, Nurnberger and Schuckit1996; Neale & Cardon, Reference Neale and Cardon1992; Reich et al., Reference Reich, Cloninger and Guze1975).
The liability distribution thus covers both manifest SU and the subthreshold phenotypes, including the asymptomatic ones. In the affected class, the dimensionality can be observed in the gradient of severity, with varying numbers of symptoms (in SUD as per DSM-5, the number of symptoms defines categorical gradations of ‘mild’, ‘moderate’ and ‘severe’). However, the unaffected phenotypes, in which, lacking face-valid indicators, liability is expressed as the varying probability of the disorder, are usually collapsed into one ‘normal’ class.
Obviously, the threshold can be moved if the end-phenotype of interest is changed; for example, as related to the number of diagnostic criteria. Both that number and the sets of those criteria change with every revision of the DSM. A change in the phenotypic definitions likely changes the liability dimension itself, involving its sources of variation (e.g., as shown by differences in genetic associations for opioid use and opioid dependence [Polimanti et al., Reference Polimanti, Walters, Johnson, McClintick, Adkins, Adkins, Bacanu, Bierut, Bigdeli, Brown, Bucholz, Copeland, Costello, Degenhardt, Farrer, Foroud, Fox, Goate, Grucza and Gelernter2020]), while leaving it correlated with that of liability to SUD as a clinical diagnosis. The complexity of liability to SU/addiction as a trait is compounded by its developmental malleability and nonlinear ontogenetic trajectory (Tarter & Vanyukov, Reference Tarter and Vanyukov1994), with the changing composition of its variance (the relative contribution of genetic and environmental sources to the phenotypic variation in the population) (Hicks et al., Reference Hicks, Blonigen, Kramer, Krueger, Patrick, Iacono and McGue2007), requiring longitudinal research approaches.
Despite a high degree of heterogeneity due to the chemical class, mode of administration, mechanism of action, biotransformation, availability and so forth, there is substantial commonality, both genetic and environmental, among mechanisms of variation in liabilities to substance-specific behaviors (Karkowski et al., Reference Karkowski, Prescott and Kendler2000; Kendler et al., Reference Kendler, Jacobson, Prescott and Neale2003; Palmer et al., Reference Palmer, Button, Rhee, Corley, Young, Stallings, Hopfer and Hewitt2012; Tsuang et al., Reference Tsuang, Lyons, Meyer, Doyle, Eisen, Goldberg, True, Lin, Toomey and Eaves1998; Vanyukov, Reference Vanyukov2012). This is consistent with underlying general/common liability to addiction (GLA; Conway et al., Reference Conway, Levy, Vanyukov, Chandler, Rutter, Swan and Neale2010; Hicks et al., Reference Hicks, Iacono and McGue2012; Vanyukov, Tarter et al., Reference Vanyukov, Tarter, Kirisci, Kirillova, Maher and Clark2003; Vanyukov et al., Reference Vanyukov, Tarter, Kirillova, Kirisci, Reynolds, Kreek, Conway, Maher, Iacono, Bierut, Neale, Clark and Ridenour2012; Vanyukov, Kirisci et al., Reference Vanyukov, Kirisci, Tarter, Simkevitz, Kirillova, Maher and Clark2003), reflected in the above-mentioned global factor (Kirisci & Vanyukov, Reference Kirisci, Vanyukov and Vanyukov2022). Furthermore, there are high correlations, including genetic, between addiction liabilities and behavior dysregulation (disinhibition), externalizing, or antisocial characteristics (Iacono et al., Reference Iacono, Carlson, Taylor, Elkins and McGue1999; Iacono et al., Reference Iacono, Malone and McGue2008; Kendler et al., Reference Kendler, Jacobson, Prescott and Neale2003; Kirisci et al., Reference Kirisci, Tarter, Ridenour, Reynolds, Horner and Vanyukov2014; Krueger et al., Reference Krueger, Hicks, Patrick, Carlson, Iacono and McGue2002).
The phenotypic commonality points to sources of common mechanisms in behavior regulation and socialization. This is not surprising, because, in sharp contrast to other psychiatric disorders, addictions often result from voluntary behavior choices and require willful goal-directed behavior to be maintained. Stable cessation of drug use is virtually synonymous with remission or recovery. General (common) liability to SU/addiction, as opposed to liabilities pertaining to specific substances, is the trait that underlies their concurrent and/or consecutive use that has been frequently misconstrued within ‘gateway theory’ (GT; Kandel, Reference Kandel1975). As previously discussed (Vanyukov, Reference Vanyukov2022a, Reference Vanyukov2022b; Vanyukov et al., Reference Vanyukov, Tarter, Kirillova, Kirisci, Reynolds, Kreek, Conway, Maher, Iacono, Bierut, Neale, Clark and Ridenour2012), the order of SU initiation — the essence of GT — is opportunistic, starting with what is available as the least personal cost.
Resistance vs. risk
Inasmuch as the main target of health research and practice is the disease, to be cured or prevented, it is the risk aspect of liability that has commonly been under study. In other words, the focus is on the factors that can elevate the probability and severity of the disorder, known as ‘risk factors’. A quick perusal of the literature in SU confirms the predominance of this perspective. For instance, a third of PubMed-listed publications containing ‘substance use disorder’ in their titles or abstracts also contain ‘risk’. The implied reason for this focus is the assumption that identifying a ‘risk factor’, a variable related to the probability of the disorder or a specific individual influence contributing to risk elevation, may help decrease this probability.
A different perspective, however, is possible, with a potentially higher likelihood of reaching that goal. That perspective, focused on resistance (and health) rather than risk (and disease), is based on the other aspect of liability — that which is opposite to risk (Figure 1; Vanyukov, Tarter et al., Reference Vanyukov, Tarter, Conway, Kirillova, Chandler and Daley2016). Albeit implemented historically — for example, in the development of vaccination — the resistance perspective is seldom employed in current biomedical research, including studies in mental disorders and addictions. Where applied, it is, as a rule, inadvertently so, as a byproduct of risk research rather than as the objective, or serendipitous, like the finding of the alcohol-aversive disulfiram effect (Williams, Reference Williams1937). The risk focus also limits the potential of developing resistance-specific analytic approaches. Based on the application of the resistance paradigm, we propose a methodology to reverse the research perspective with the explicit primary goal of detecting resistance factors.
It is noteworthy, to avoid confusion, that resistance as discussed herein substantially differs from the long-standing concept of resilience. The latter is commonly defined as the nonspecific ‘“successful” adaptation to life tasks in the face of social disadvantage or highly adverse conditions’ (Windle, Reference Windle, Glantz and Johnson1999, p. 163), ‘process of, capacity for, or outcome of successful adaptation despite challenging or threatening circumstances’ (Masten et al., Reference Masten, Best and Garmezy1990, p. 426), ‘where individuals display positive adaptation despite experiences of significant adversity or trauma’ (Luthar & Cicchetti, Reference Luthar and Cicchetti2000, p. 2). As such, resilience is an ex post facto registration of a conventionally ‘good’ outcome upon experiencing what is conventionally viewed as adversity. Resilience generally does not map on the liability dimension and is also hard to apply to SU (see Tarter & Vanyukov, Reference Tarter, Vanyukov, Glantz and Johnson1999; Vanyukov, Tarter et al., Reference Vanyukov, Tarter, Conway, Kirillova, Chandler and Daley2016). When viewed as disorder-specific, however, it is subsumed by resistance, which also covers effects that precede and prevent exposure to drugs, as well as dynamic changes of the liability phenotype (Tarter & Vanyukov, Reference Tarter, Vanyukov, Glantz and Johnson1999), including remission, relapse, and recovery from a disorder.
Even when quantitatively evaluated, resilience is conceptualized as the general ability of the organism to withstand adversities (Sheerin et al., Reference Sheerin, Bustamante, Bountress, Cusack, Aggen, Kendler and Amstadter2021) rather than referring to a specific undesirable outcome. In rare publications where the term ‘resilience’ is used in a dimensional sense (e.g., Belcher et al., Reference Belcher, Volkow, Moeller and Ferre2014), thus somewhat departing from its classical definition, it is understood as a derivative of ‘endophenotypes of SUD’ and ‘protective factors’ that are nonspecific to SU/addiction. Moreover, many traits that have been examined as potential endophenotypes of SUD are not characteristics at the level of ‘biochemical test or microscopic examination’, the endophenotype definition (Gottesman & Gould, Reference Gottesman and Gould2003). Instead, they are observed at the same high level of organization — behavioral, psychological — as liabilities to addiction or to other psychiatric disorders. Even the physiological-level traits that have been proposed as their endophenotypes (e.g., the P300 event-related potential) are complex alike SUD liability (Iacono, Reference Iacono2018).
On their surface, resistance and risk are simply the alternative points of view on the same trait, liability (Kirisci & Vanyukov, Reference Kirisci, Vanyukov and Vanyukov2022; Vanyukov, Reference Vanyukov2021; Vanyukov, Cornelius et al., Reference Vanyukov, Tarter, Conway, Kirillova, Chandler and Daley2016; Vanyukov, Tarter et al., Reference Vanyukov, Tarter, Conway, Kirillova, Chandler and Daley2016). Just as individual liability may grow under the influence of risk factors (e.g., availability of addictive substances; a gene’s allele related to faster addiction-driving neurobiological changes), it may decrease under the influence of resistance factors (e.g., a dramatic example of a relative dying of drug poisoning; a gene’s allele that causes a noxious response to a substance, such as ALDH2*2 for alcohol; Goedde et al., Reference Goedde, Agarwal, Harada, Meier-Tackmann, Ruofu, Bienzle, Kroeger and Hussein1983; Harada et al., Reference Harada, Agarwal, Goedde, Tagaki and Ishikawa1982). Whereas risk and resistance are the symmetric liability aspects (Figure 1; Vanyukov, Tarter et al., Reference Vanyukov, Tarter, Conway, Kirillova, Chandler and Daley2016), resistance factors are not limited to protective factors. The latter are often considered as merely alternative to risk factors (e.g., an allele that is more common among the controls), defined as those at ‘opposite ends of the same continuum’ (Marco et al., Reference Marco, Dragioti, Arango, Radua, Ostinelli, Kilic, Yilmaz, Yalcinay-İnan, Soares, Mariano, Mosillo, Cortese, Correll, Carvalho, Shin and Fusar-Poli2021, p. 57) and influencing the effects of specific risk factors (Hawkins et al., Reference Hawkins, Catalano and Miller1992; Marco et al., Reference Marco, Dragioti, Arango, Radua, Ostinelli, Kilic, Yilmaz, Yalcinay-İnan, Soares, Mariano, Mosillo, Cortese, Correll, Carvalho, Shin and Fusar-Poli2021; Schulenberg & Maggs, Reference Schulenberg and Maggs2002), thus again referencing the risk concept. Resistance factors also include those precluding exposure to a pathogen (e.g., to addictive substances), facilitate recovery upon disorder onset, prevent relapse, and so on. In other words, resistance factors are any that lower liability (Kirisci & Vanyukov, Reference Kirisci, Vanyukov and Vanyukov2022).
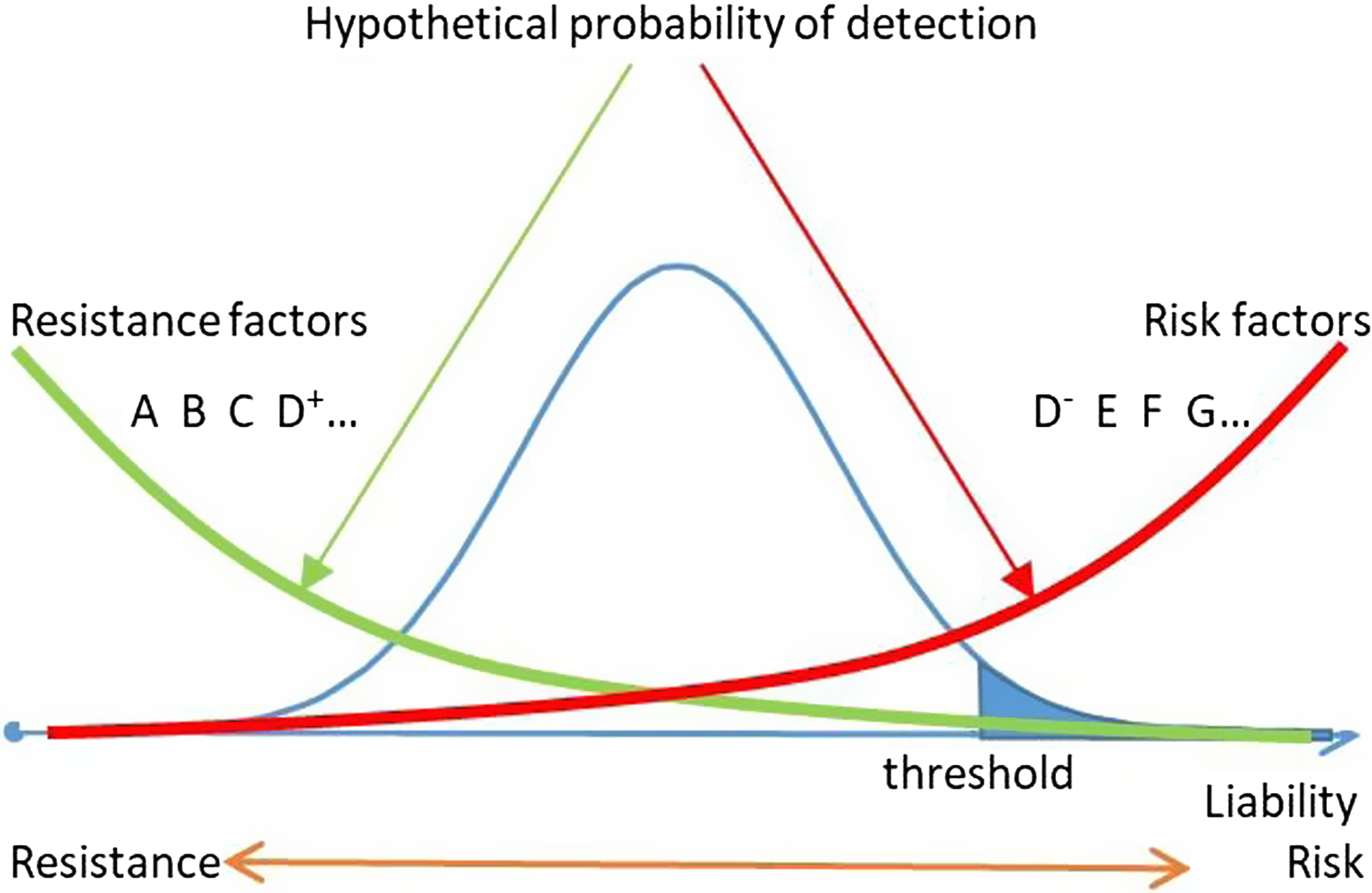
Fig. 1. SUD liability, risk, and resistance: detectability of risk and resistance factors. Resistance and risk factors are aggregated at opposite ends of liability distributions and only partially overlap (D+ and D−).
Note: SUD, substance use disorder.
Moreover, from the semantic standpoint, ‘resistance’ is the more appropriate designation than ‘protection’ for the opposite-to-risk end of the continuum: both risk and resistance refer to the same undesirable phenotype (e.g., risk for, and resistance to, a disorder), whereas protection would refer to some disorder-causing agents (protection from them) rather than the phenotype itself. It is also hard to conceptualize as ‘protective’ some of the factors that may enhance resistance to SU — for example, severe stress, as in the above example with the dying relative. ‘Protective’ also implies only one — positive — modality of a factor’s action, whereas ‘resistance’ allows both positive and negative changes. That also fits well the potential individual ambiguity of a factor’s effect. For instance, parental SU as an environmental factor may promote similar behavior in offspring — by providing a ready access to alcohol or other drugs and by modeling behavior that the child may conclude is socially acceptable. Alternatively, parental SU may produce aversion by demonstrating its negative effects (Goodwin, Reference Goodwin1974). There are few known actionable protective factors in mental disorders (Marco et al., Reference Marco, Dragioti, Arango, Radua, Ostinelli, Kilic, Yilmaz, Yalcinay-İnan, Soares, Mariano, Mosillo, Cortese, Correll, Carvalho, Shin and Fusar-Poli2021). Even when known, such as religiosity that may be in relation to drug use (Maes et al., Reference Maes, Woodard, Murrelle, Meyer, Silberg, Hewitt, Rutter, Simonoff, Pickles, Carbonneau, Neale and Eaves1999), they may be difficult to implement.
Resistance and risk factors may partially overlap but also include asymmetric ones. For instance, whereas phenylketonuria is caused by a mutation, its prevention, raising resistance, is not genetic but environmental — a phenylalanine-poor diet. The smallpox vaccine had been developed thanks to the identification of a highly resistant population, milkmaids exposed to cowpox and immune to smallpox (Riedel, Reference Riedel2005), long before the variola virus was discovered. Notably, neither variola nor cowpox virus is even involved in the development of the contemporary smallpox vaccine. Biologically, resistance to smallpox that arises in response to that vaccine is consequent to resistance to the vaccinia virus, of unknown origin (Sánchez-Sampedro et al., Reference Sánchez-Sampedro, Perdiguero, Mejías-Pérez, García-Arriaza, Di Pilato and Esteban2015). The vaccines are a long-standing prototype of the resistance approach.
While vaccination would be difficult to apply to a drug (although such attempts have been made, they have been to counteract effects rather than prevent use of drugs — e.g., Bremer & Janda, Reference Bremer and Janda2017), there are other ways to employ the resistance paradigm. An approach implemented in a recently funded project (Prom-Wormley et al., Reference Prom-Wormley, Maes and Vanyukov2022) is presented below.
Implementing a Resistance Perspective
Measures directed at lowering the prevalence of SU have been premised on reducing the influence of risk factors (Kellam et al., Reference Kellam, Wang, Mackenzie, Brown, Ompad, Or, Ialongo, Poduska and Windham2014; Vanyukov, Tarter et al., Reference Vanyukov, Tarter, Conway, Kirillova, Chandler and Daley2016). Much effort has been directed at several such factors in school-age children (Kellam et al., Reference Kellam, Wang, Mackenzie, Brown, Ompad, Or, Ialongo, Poduska and Windham2014; Botvin, Reference Botvin1995; Sussman et al., Reference Sussman, Dent and Stacy2002) and their parents (Kumpfer & Hansen, Reference Kumpfer, Hansen, Scheier and Hansen2014; Walden et al., Reference Walden, McGue, Lacono, Burt and Elkins2004); for example, diminished self-control, deficient decision making, peer pressure. Even when known, however, risk factors are difficult to deal with (see Kirisci & Vanyukov, Reference Kirisci, Vanyukov and Vanyukov2022; Vanyukov, Reference Vanyukov2021). Drug supply, the obvious risk factor necessary for drug use regardless of any other factors, is an example.
While removal of risk factors to decrease disorder probability is conceivable, it is rare. (In fact, the only example of the eradication of an infection, smallpox, resulted from the resistance approach.) A more direct approach would be to turn attention from risk towards resistance factors — those that counteract motives for using drugs, decrease the probability of use and its consequences, and lead to health rather than the disorder (Vanyukov, Tarter et al., Reference Vanyukov, Tarter, Conway, Kirillova, Chandler and Daley2016). It is resistance factors that promote the absence of drug problems and/or recovery from them in the majority of the population — on the current background of the facile and increasing availability of drugs.
The reversal of the perspective would help the establishment of the culture of prevention (Sloboda & David, Reference Sloboda and David2020) that has not been fully embraced for SU/addiction and could benefit other areas of medicine as well. For instance, up to 50% of cancer deaths in the world are attributable to behavioral and thus modifiable causes, including SU (mostly smoking and alcohol, but illicit substances as well; Tran et al., Reference Tran, Lang, Compton, Xu, Acheson, Henrikson, Kocarnik, enberthy, Aali, Abbas, Abbasi, Abbasi-Kangevari, Abbasi-Kangevari, Abbastabar, Abdelmasseh, Abd-Elsalam, Abdelwahab, Abdoli, Abdulkadir and Murray2022). Shrinking research budgets (in 2014 the real NIH resources were at least 25% less than in 2003 [Alberts et al., Reference Alberts, Kirschner, Tilghman and Varmus2014]) suggest the need to find ways to raise biomedical research efficiency in terms of practical benefits, which are often promised but seldom achieved. To develop new strategies that reduce SU, it is necessary to elucidate modifiable factors that enhance resistance.
The main distinction between the risk and resistance perspectives is in the differences in approaches to the identification of the respective factors. Research focused on risk factors and thus on the high end of the liability distribution (usually the affected individuals, 1–10% of the population) is likely to detect those with the strongest risk-increasing effects. Under the high-risk (e.g., case-control) designs, the phenotypes carrying strongest resistance-increasing effects — biological and/or environmental — are diluted by the heterogeneity of the typical unaffected control, sampled from 90–99% of the population. Factors enhancing resistance are aggregated among phenotypes at the low end of the liability distribution, requiring high-resistance rather than high-risk sampling for their detection (Kirisci & Vanyukov, Reference Kirisci, Vanyukov and Vanyukov2022; Vanyukov, Cornelius et al., Reference Vanyukov, Tarter, Conway, Kirillova, Chandler and Daley2016; Vanyukov, Tarter et al., Reference Vanyukov, Tarter, Conway, Kirillova, Chandler and Daley2016). A major obstacle for that is the absence of face-valid indicators of liability in the asymptomatic individuals and thus difficulty in quantifying and identifying phenotypes to ensure their location on the low end of the liability dimension.
This difficulty, however, can be overcome by using latent variable analysis techniques, such as item response theory (IRT), and specific sampling that refers to observable indicators of liability. We have developed a methodology enabling liability quantification on an interval scale of an index of that latent trait in the absence of its face-valid indicators. Its prototypic application has resulted in the Transmissible Liability Index (TLI; Kirisci et al., Reference Kirisci, Tarter, Mezzich, Ridenour, Reynolds and Vanyukov2009; PhenX, n.d.; Vanyukov et al., Reference Vanyukov, Kirisci, Moss, Tarter, Reynolds, Maher, Kirillova, Ridenour and Clark2009; Vanyukov, Kirisci et al., Reference Vanyukov, Kirisci, Tarter, Simkevitz, Kirillova, Maher and Clark2003; Vanyukov, Tarter et al., Reference Vanyukov, Tarter, Kirisci, Kirillova, Maher and Clark2003). Briefly, transmissible liability is the component of liability variance that is correlated between generations (Rice et al., Reference Rice, Cloninger and Reich1980; Vanyukov, Moss et al., Reference Vanyukov, Moss, Tarter, Sloboda and Bukowski2003). SUD liability is significantly heritable (Baker et al., Reference Baker, Maes, Larsson, Lichtenstein and Kendler2011; Haberstick et al., Reference Haberstick, Zeiger, Corley, Hopfer, Stallings, Rhee and Hewitt2011; Kendler et al., Reference Kendler, Jacobson, Prescott and Neale2003; Kendler et al., Reference Kendler, Myers and Prescott2007; Palmer et al., Reference Palmer, Button, Rhee, Corley, Young, Stallings, Hopfer and Hewitt2012; Palmer et al., Reference Palmer, Young, Corley, Hopfer, Stallings and Hewitt2013; Rhee et al., Reference Rhee, Hewitt, Young, Corley, Crowley and Stallings2003) and thus transmissible. The TLI is based on items extracted from standard behavior assessment instruments and reflecting childhood behavior regulation (Vanyukov et al., Reference Vanyukov, Kirisci, Moss, Tarter, Reynolds, Maher, Kirillova, Ridenour and Clark2009), and on calibrating them and deriving the index by applying IRT. These items, by being selected to discriminate between groups of children with affected and unaffected parents, are indicators of transmissible SUD liability (Vanyukov, Kirisci et al., Reference Vanyukov, Kirisci, Tarter, Simkevitz, Kirillova, Maher and Clark2003), which is supported by the high heritability of the TLI (Hicks et al., Reference Hicks, Iacono and McGue2012; Vanyukov et al., Reference Vanyukov, Kim, Irons, Kirisci, Neale, Ridenour, Hicks, Tarter, Reynolds, Kirillova, McGue and Iacono2015; Vanyukov et al., Reference Vanyukov, Kirisci, Moss, Tarter, Reynolds, Maher, Kirillova, Ridenour and Clark2009). The TLI also has other advantages: (1) it estimates individual SUD liability along the full scale of values of the normally distributed trait, from high resistance to high risk; (2) it is highly predictive of adult SUD; (3) the genetic component of TLI variance assessed in children accounts for over half of the genetic variance in their adult SUD diagnosis and the entire relationship between TLI and diagnosis (Vanyukov et al., Reference Vanyukov, Kim, Irons, Kirisci, Neale, Ridenour, Hicks, Tarter, Reynolds, Kirillova, McGue and Iacono2015); (4) it is validated as a childhood measure of adult SUD liability in multiple samples (Arria et al., Reference Arria, Vincent and Caldeira2009; Ridenour et al., Reference Ridenour, Kirisci, Tarter and Vanyukov2011); and (5) it enables identification of groups with high as well as low SUD liability (Vanyukov, Cornelius et al., Reference Vanyukov, Tarter, Conway, Kirillova, Chandler and Daley2016).
While TLI is a valid measure of addiction liability, its items were selected based on a high-risk design. Its precision is thus greater in the high-risk portion of the childhood liability distribution. The standard psychological instruments also tend to have a focus on behavioral deviance rather than variation in the ‘normal’ behavior. To refocus and augment the liability measurement methodology, we will develop quantitative resistance indices, followed by tracking their individual trajectories in the data from two longitudinal studies of twins (the Virginia Twin Study of Adolescent and Behavioral Development, VTSABD (Eaves et al., Reference Eaves, Silberg, Meyer, Maes, Simonoff, Pickles, Rutter, Neale, Reynolds, Erikson, Heath, Loeber, Truett and Hewitt1997; Hewitt et al., Reference Hewitt, Silberg, Rutter, Simonoff, Meyer, Maes, Pickles, Neale, Loeber, Erickson, Kendler, Heath, Truett, Reynolds and Eaves1997; Maes et al., Reference Maes, Woodard, Murrelle, Meyer, Silberg, Hewitt, Rutter, Simonoff, Pickles, Carbonneau, Neale and Eaves1999), and the Minnesota Twin Family Study, MTFS (Iacono et al, Reference Iacono, Carlson, Taylor, Elkins and McGue1999; Iacono & McGue, Reference Iacono and McGue2002). Finally, we will determine the influence of specific potentially modifiable characteristics on these indices and their trajectories while controlling for genetic confounding of environmental potentially modifiable variables.
To derive resistance indices, from the available childhood data, we will select items that discriminate between the high-resistance groups and the average resistance (AR) group based on the individual TLI phenotypes (see below). The reason why the affected individuals are excluded from the control sample is the aggregation of high-risk factors among these individuals (see Figure 1). Because of the relative diagnostic certainty as compared with the more probabilistic definition of the high-resistance phenotype, the results of comparisons with the sample that includes affected individuals would be likely driven by high-risk rather than high-resistance factors. Removing those individuals from comparisons renders the average liability of the control close to the population mean and lowers the impact of risk factors.
After scoring both samples on the TLI, the following comparison groups will be identified (Vanyukov, Cornelius et al., Reference Vanyukov, Tarter, Conway, Kirillova, Chandler and Daley2016, Vanyukov, Tarter et al., Reference Vanyukov, Tarter, Conway, Kirillova, Chandler and Daley2016) (Table 1): (1) High Outset Resistance (HOR; individuals with a low TLI score, indicating high outset resistance to SU); (2) High Realized Resistance (HRR; individuals with a high TLI score, indicating high outset liability to SU), and (3) Average Resistance (AR; intermediate TLI). TLI will be used only for group identification and item selection for resistance indices, and the TLI and resistance index item sets will not overlap. We will calibrate those resistance indicator items using IRT, validate their parameters, and employ them to generate the resistance indices, HORI and HRRI, across the sample. These indices will enable the optimal precision of measurement of the two variants of resistance to SU.
Table 1. Summary of resistance groups
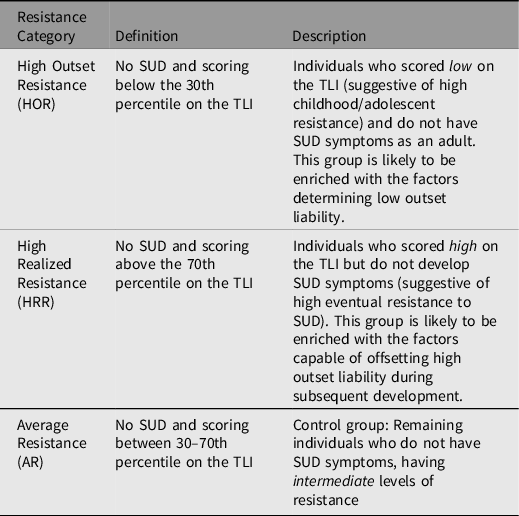
Note: SUD, substance use disorder; TLI, Transmissible Liability Index.
Having these two tools and targeting the non-development of SU/addiction as the end phenotype, we aim to identify novel factors that have the highest probability of enhancing the two variants of resistance to SU. This will be addressed using two approaches. The first one will focus on the identification of putative resistance factors (PReF) using a mixed-methods approach known as Group Concept Mapping (GCM; Rosas & Kane, Reference Rosas and Kane2012). From the Mid-Atlantic Twin Registry, we will recruit participants who are never, ever-attempted, current, and former illicit drug users to identify and prioritize factors they consider to be most impactful on their decisions to either not engage in or reduce SU, applying concept mapping. Prior to implementing the group concept mapping protocol, we will test it with a pilot subset recruited into each of the four groups according to their level of SU: (1) have engaged in no lifetime illicit SU; (2) have attempted any illicit SU but did not continue (have not used in the last 2 years); (3) have been abstinent from all illicit substances for more than three months, a timeframe that would indicate entry into early remission; and (4) currently engaged in illicit SU (three months or less ago).
Participants will individually engage in GCM using an online platform, groupwisdomTM (The Concept System, 2021), in three steps: (1) Prompted Brainstorming/Idea Generation of Resistance Factors, production of statements that identify the specific reasons a participant has not engaged in or has limited SU; (2) Structuring of Resistance Factors through Concept Sorting and Rating, review of the statements reported across all participants and sorting into groups according to common themes that are important to the participant, as well as rating each statement to indicate the importance of every factor in the context of every participant; and (3) Results Dissemination, a prompted discussion of the results produced from the analysis of concept sorting and rating data to establish the degree to which summarized results (e.g., the common themes produced across all participants as well as the statements that contributed to the themes) reflect the participants’ experiences. Dissemination will occur via video teleconferencing with research team facilitation. Results from the GCM approach will identify a set of factors for future use in self-report surveys.
The second PReF identification approach will focus on secondary data analysis of existing longitudinal data. We will identify factors associated with resistance using data mining (supervised learning and multidimensional scaling [Breiman et al., Reference Breiman, Friedman, Stone and Olshen1984]) in these data as well as newly collected measures and test the degree to which these factors influence the genetic and environmental variances across levels of exposure, while also accounting for genetic confounding using twin models of resistance indices. We expect to detect resistance factors with greatest influence, generalizable to the population at specific age periods.
The twin data provide a unique opportunity to account for genetic influences that may interact with or contribute to ‘environmental’ variables (Button et al., Reference Button, Corley, Rhee, Hewitt, Young and Stallings2007; Guo, Reference Guo2006; Vanyukov, Reference Vanyukov and Fishbein2004; Walden et al., Reference Walden, McGue, Lacono, Burt and Elkins2004). We will detail the effects of these factors on the development of resistance to SU across adolescence through adulthood using latent growth mixture modeling (LGMM). LGMM utilizes latent factors to estimate the fixed (group level) and random (individual level) components of individual differences in developmental trajectories (Muthén & Muthén, Reference Muthén and Muthén1998–2011; Raudenbush & Bryk, Reference Raudenbush and Bryk2002). We will use data from the VTSABD and MTFS to apply standard twin models (Maes, Reference Maes and Purcell2005) to childhood/adolescent indices of resistance. These models will evaluate whether phenotypic variance components — additive genetic, A, dominance genetic, D, common environment, C, and unique environment, E — are similar in adolescent females and males in magnitude and nature (quantitative and qualitative sex differences) and whether they are consistent across age groups in adolescents and young adults (Neale & Cardon, Reference Neale and Cardon1992). Next, we will incorporate putative resistance factors (PReF), identified through mining of existing data, to evaluate main effects on the adolescent resistance phenotype, while simultaneously estimating variance components (latent factors). In addition to main effects, PReF may also interact with each of latent factors, thus moderating their contribution (van der Sluis et al., Reference van der Sluis, Dolan, Neale and Posthuma2008). We will extend analyses to early middle adulthood using PReF identified from concept mapping.
To account for the possibility that the same genetic or environmental factors contribute to variance of the PReF as to variance in the resistance phenotypes, we will extend the analyses to bivariate modeling, allowing moderation of the parameters accounting for covariation between resistance phenotypes and PReF as well as moderation of parameters specific to the resistance phenotypes (Purcell, Reference Purcell2002). These bivariate analyses will be applied to the continuous measures of resistance. These models will be expanded to investigate how resistance phenotypes are related to SU, or whether the same latent factors contribute to both. Variance due to sample differences will be taken into account in a manner similar to prior mega-analyses using raw data of smoking initiation across 11 twin samples (Maes et al., Reference Maes, Prom-Wormley, Eaves, Rhee, Hewitt, Young, Corley, McGue, Iacono, Legrand, Samek, Murrelle, Silberg, Miles, Schieken, Beunen, Thomis, Rose, Dick and Neale2017).
Conclusions
SU with its high morbidity and mortality remains an intractable problem despite the enormous resources expended on the various aspects of the ‘War on Drugs’. Whereas the society focuses on the interdiction of drug supply, including restrictions on prescribing addictive substances, it also inconsistently and selectively removes moral and legal obstacles to SU. Research targets mainly the disorder while prevention remains insufficiently effective to substantially lower the prevalence of problem use. Research translation has not resulted in measures that are capable of precluding or countering the consecutive drug ‘epidemics’. The ‘risk factors’, the main focus of research, are difficult to impossible to utilize in practice. An alternative to current research approaches, implemented in an NIH-funded project, enabling tangible changes with high potential for success, and extendable beyond the SU problem to other psychiatric and medical conditions, is proposed in this article. With its focus on resistance to SU, this study makes a novel use of advantages of the twin design, taking twin research from the traditional evaluation of phenotypic variance components to the identification of actionable factors raising resistance.
Financial support
This work is supported by the National Institutes of Health grant R01DA054313.
Conflict of interest
The authors declare no competing interests.





