The first ‘classical’ twin study was published in 1922 by Walter Jablonski, the first ophthalmologist who used both monozygotic and dizygotic twins to examine the determining factors in the development of the refraction of the eye. He declared: ‘the smaller differences are seen in the majority of monozygotic cases, while larger differences occur in dizygotic cases’ (Jablonski, as cited in Liew et al., Reference Liew, Elsner, Spector and Hammond2005, p. 199). Since then, numerous articles have discussed the role of inheritance, the genetic predisposition, and the gene-environment interactions taking part in the formation of the refractive characteristics of the eye, confirming that genetic factors have major importance that account for 77–94% of the genetic variance in refraction (Lyhne et al., Reference Lyhne, Sjølie, Kyvik and Green2001).
The cornea is one of the most important refracting tissues of the eye, accounting for 43.25 dioptries of the total 58.60 dioptries of a healthy human eye. The cornea has five layers (epithelium, Bowman layer, stroma, Descemet membrane, and endothelium), which thickness can be measured by numerous devices, such as pachymetry (optical or ultrasound), anterior segment optical coherence tomography (AS-OCT), or Pentacam. The normal central corneal thickness (CCT) measures 0.49–0.56 mm, and there is a decrease of CCT with age in normal eyes (Aghaian et al., Reference Aghaian, Choe, Lin and Stamper2004; Kato, Reference Kato2014; Prasad et al., Reference Prasad, Fry and Hersh2011). Evidence in the recent literature has highlighted the importance of central corneal thickness in relation to several ocular and non-ocular conditions. Most notably, thinner CCT has been identified as a risk factor for open-angle glaucoma and it is related to corneal endothelial alterations (Kanski, Reference Kanski2003; Kaushik et al., Reference Kaushik, Pandav, Banger, Aggarwal and Gupta2012). CCT can affect the measurement of applanation tonometry and the decision to perform some keratorefractic surgical procedures.
Abnormal CCT is thought to be one of the major risk factors of glaucoma, one of the most common cause of irreversible blindness worldwide (Vithana et al., Reference Vithana, Aung, Khor, Cornes, Tay, Sim and Wong2011), which affects more than 60 million people. Studies have shown that individuals with thinner corneas are more likely to develop primary open-angle glaucoma (POAG; Gordon et al., Reference Gordon, Beiser, Brandt, Heuer, Higginbotham, Johnson and Kass2002; Shih et al., Reference Shih, Graff Zivin, Trokel and Tsai2004). Thin corneas are also important clinical features of other visually debilitating diseases such as keratoconus.
Despite having an extensive knowledge of the structure and function of the cornea, little is known about the pathways that determine CCT, such as the volume of the surrounding anterior chamber.
Anterior chamber is the space between the posterior part of the cornea (corneal endothelium) and the anterior surface of the iris and lens; its volume measures around 159.74 ± 43.64 mm3 (Figure 1). In ophthalmologic practice, three different parameters of the anterior chamber are measured and considered: the depth (ACD), the angle (ACA), and the volume (ACV). The size of the anterior chamber, which contains the aqueous humor, varies among people and it can also play a role in the refraction of the eye. In general, individuals with nearsightedness (myopia) have a deeper anterior chamber, while farsighted people (hyperopia) have a shallower chamber (Chang et al., Reference Chang, Tsai, Hu, Lin and Shih2001). Moreover, the depth and volume of the anterior chamber decreases with age and depends on gender, and these parameters are associated with the severity of refractive errors (Chang et al., Reference Chang, Tsai, Hu, Lin and Shih2001; Fontana & Brubaker, Reference Fontana and Brubaker1980).

FIGURE 1 Anatomy of the anterior segment of the eye.
It was already known that refractive disorders — that is, myopia and hypermetropia — are also highly heritable traits. The more severe the myopia, the thinner the cornea (this is why the measurement of CCT is necessary before a refractive surgery in clinical practice), which is related to the deeper anterior chamber.
Few, and inconsistent, studies have confirmed the high heritability of investigated parameters of the anterior segment of the eye, such as CCT and ACD; however, no heritability of ACV has been reported (Alsbirk, Reference Alsbirk1977; He, Hur et al., Reference He, Hur, Zhang, Ding, Huang and Wang2008; He, Wang et al., Reference He, Wang, Zheng, Zhang, Yin, Huang, Mackey and Foster2008; Kim et al., Reference Kim, Zhao, Kim, Lim, Song, Guallar and Chung2013; Toh et al., Reference Toh, Liew, MacKinnon, Hewitt, Poulsen, Spector and Mackey2005; Zheng et al., Reference Zheng, Ge, Huang, Zhang, Liu, Hur and He2008). Furthermore, the Ghoangzou Twin Eye Study reported that 89% of additive genetic factors accounted for the variation of ACD in twin children, and shared genes are responsible for the significant phenotypic correlations between ACD and angle opening distance (He, Wang et al., Reference He, Wang, Zheng, Zhang, Yin, Huang, Mackey and Foster2008). However, to our knowledge, no study has been performed to investigate the correlation between the ACV and CCT.
Therefore, this work aimed at estimating the precise measurements of the influence of genetics, as well as shared and unshared environmental components of ACV and CCT and their association in a Hungarian twin cohort.
Methods
Study Population and Design
82 eyes of 41 monozygotic (MZ) and 28 eyes of 14 same-sex dizygotic (DZ) Caucasian twin pairs above 18 years of age were recruited from the Hungarian Twin Registry for an extensive ophthalmological examination at the Department of Ophthalmology, Semmelweis University, in 2009 and 2010 (Littvay et al., Reference Littvay, Metneki, Tarnoki and Tarnoki2013). We excluded opposite-sex DZ twin pairs to avoid bias of the heritability estimates in the presence of gender-specific or X-chromosome effects, pregnant subjects, twins with a history or signs of pathological changes, contact lens correction or previous refractive or cataract surgery. The twins did not suffer from glaucoma or elevated intraocular pressure. To determine zygosity, a multiple-choice, self-reported seven-part questionnaire was used (Heath et al., Reference Heath, Nyholt, Neuman, Madden, Bucholz, Todd and Martin2003). Risk factors, history of ophthalmologic diseases, and surgeries were all recorded. Risk factors included, for example, ocular hypertension, systemic high pressure, ‘eye rubbing’ (these conditions can affect the thickness of the cornea), contact lens wearing, lid closure problems (e.g., lagophthalmos due to facial paresis), and corneal inflammations, which can cause irregular corneal surface (Wong et al., Reference Wong, Wong, Foster, Crowston, Fong, Aung and SiMES2009). The study was approved by the Ethical Committee of Semmelweis University and conducted in full compliance with regulations of the Declaration of Helsinki. All participants gave informed consent.
Measurement of Ocular Parameters
Measurements were performed with a Pentacam HR rotating Scheimpflug camera (Oculus, Germany), a single imaging system that is a non-contact machine, to obtain objective measurements of the anterior segment of the eye (Zou et al., Reference Zou, Duan and Zhong2010). It includes a rotating Scheimpflug camera with a monochromatic slit light source. The device makes real-time images from the anterior segment of the eye, giving data with high repeatability and interoperator reproducibility (Lackner et al., Reference Lackner, Schmidinger, Pieh, Funovics and Skorpik2005). The image gives a complete representation of the anterior chamber, extending from the endothelium of the cornea to the posterior surface of the lens. The geometry of the anterior eye chamber is calculated in three dimensions. The Pentacam is a multifunction type device: it can also be used as corneatopograph and pachymeter. In addition, we could obtain information about the volume of anterior chamber: the device makes a 3D model of the eye and also provides lens densitometry. The Pentacam contains a rotating camera (the scanner), connected to a computer and higher angle of sight.
We selected the option of 25 images per scan; the real-time images of the subject's eye were taken by the same examiner. The measurements started automatically when classical alignment and focus of the eye were achieved. The results (CCT in μm, ACV in mm3) were automatically calculated by the device. Only measurements with a quality factor of 95% were considered valid and were included in the analysis. No eye drops were used before the examination. Twin pairs were examined under same lighting conditions and at the same time of day to avoid differences related to diurnal variations.
Statistical Analysis
Descriptive analysis (mean, standard deviation, percentage for categorical variables) were carried out by SPSS Statistics 17 (SPSS Inc., Chicago, IL, USA). MZ and DZ subsamples were compared by independent samples t-tests. All parameters showed a normal distribution. Outliers were excluded from the analysis in order to have a normal distribution. The descriptive estimate of the genetic influence in MZ and DZ pairs was calculated using within-pair co-twin correlations adjusted for age and gender. Based on within-twin correlations between MZ and DZ twins, structural equation modeling (A-C-E model) was carried out by Mplus Version 7 (http://www.statmodel.com/; Muthen & Muthen, Reference Muthen and Muthen1998–2010) in order to break down the variance into additive genetic effects, and shared and unshared environment (Neale & Cardon, Reference Neale and Cardon1992). Empirical confidence intervals were calculated with a Bollen-Stine Bootstrap (Bollen & Stine, Reference Bollen and Stine1992). All inferential statistics were estimated using full information maximum likelihood. Furthermore, a bivariate Cholesky decomposition was carried out to derive the magnitude of covariation between the investigated phenotypes of interest and to estimate what proportion of this correlation is attributable to common underlying genetic and environmental factors. In order to estimate the amount of overlap between genes or environment that influence the two parameters, genetic and environmental correlations between those phenotypes were calculated. P values lower than .05 were considered significant.
Results
Descriptive Analysis
Subject characteristics are presented in Table 1. The DZ twins were significantly older compared with the MZ twins (p < .001). There were no other significant differences between the MZ and DZ twins in the investigated parameters. Age and gender corrections of the means were used in the ACE estimation. Age did not have a significant impact on CCT but it did have an impact on ACV (-0.103, 95% CI: -0.147, -0.06).
TABLE 1 Baseline Characteristics of Study Subjects
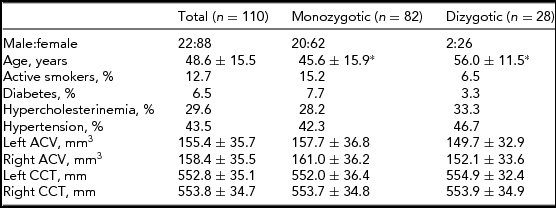
*p < .001; ACV = anterior chamber volume; CCT = central corneal thickness. Data are shown as mean ± standard deviation where appropriate.
Univariate Analysis
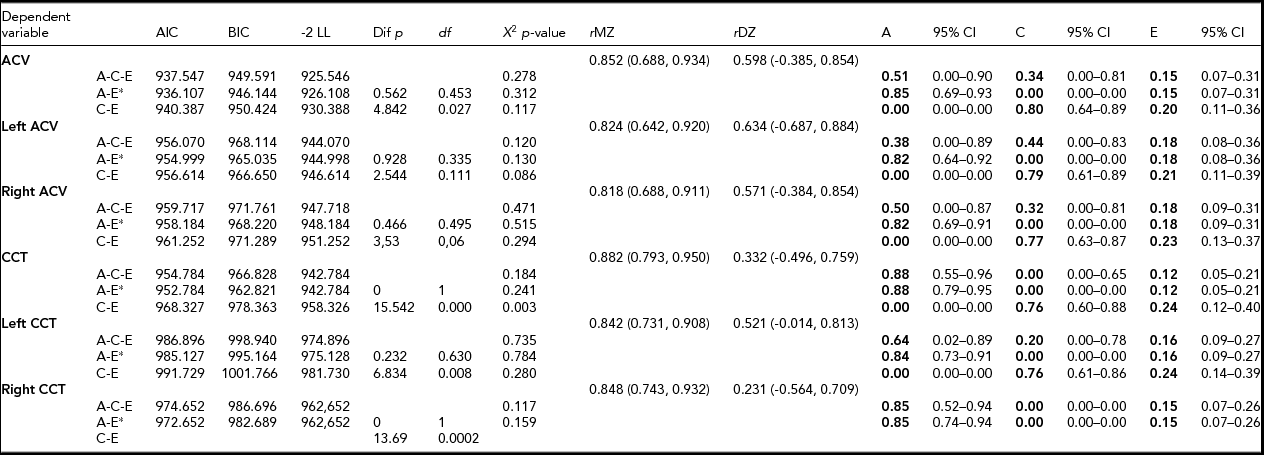
MZ co-twin correlations were higher than DZ co-twin correlations, indicating that age- and sex-adjusted heritability was 85% and 88% of ACV and CCT, respectively (Table 2). The AE model showed the best model fit. Unshared environmental factors were responsible for the minor part of the variance.
TABLE 2 Parameter Estimates for Additive Hereditary (A), Common Environment (C) and Unique Environmental Influences (E) by Structural Equation Modeling Adjusted for Age and Sex

CCT – central corneal thickness; ACV – anterior chamber volume; AIC – Akaike information criteria; BIC – Bayesian information criteria; LL – loglikelihood; Χ 2– chi square test p-value, based on model loglikelihood comparative model fit test; rMZ – saturated correlation between monozygotic twins; rDZ – saturated correlation between dizygotic twin. *best fitting model.
Cholesky Decomposition Analysis
A bivariate Cholesky decomposition model was performed in order to investigate a common genetic background of these traits. The AE model was statistically indistinguishable from the ACE model. The CE model fit was significantly worse. The correlation between ACV and CCT was negative and significant (r ph = -0.35, p < .05); the proportion of this attributed to additive genetic factors was 93.4% (95% CI: 41.8%, 106.1%). No significant role for environmental covariance was noted (Table 3, Figures 2 and 3).
TABLE 3 Cholesky Model Comparison


FIGURE 2 Cholesky decomposition AE model.
Note: Estimates are standardized. Cross-paths show proportion of the r = -0.35 explained by additive genetic and unique environmental effects. A and E estimates of the second phenotype include both covariance and residual variance components and therefore largely match the univariate estimates.

FIGURE 3 Anterior segment measurements (Pentacam, Oculus) of a 59-year-old monozygotic twins.
Note: A, left eye of first-born twin; B, right eye of first-born twin; C, left eye of second-born twin; D, right eye of second-born twin.
Discussion
To our knowledge, this was the first study that has been performed to estimate the genetic covariance of central corneal thickness and ACV, and the heritability of ACV. We demonstrated a strong additive genetic effect on both ACV and CCT, and that genetic factors accounted for the covariance in nearly 93%.
The heritability of these parameters has never been estimated in an Eastern European twin cohort. Toh et al. (Reference Toh, Liew, MacKinnon, Hewitt, Poulsen, Spector and Mackey2005) reported a higher heritability estimate (95%) of CCT compared to our findings in a large cohort. In contrast, a family study of Greenland Eskimos (Alsbirk, Reference Alsbirk1977) estimated lower heritability (60–70%). One reason for this may be that Alsbirk's study included both adults and children, while our study included only patients above 18 years. As part of the Guangzhou Twin Eye Study, Chinese children between the age of 8 and 16 were measured and a finding of 88% heritability in the boys and 91% in the girls was reported, which is comparable with our findings in adults (Zheng et al., Reference Zheng, Ge, Huang, Zhang, Liu, Hur and He2008).
Measurement of corneal thickness is important for the early identification of pathologic and ectasia-prone changes of the cornea before refractive surgery (American Academy of Ophthalmology, 1999; Kremer & Dufek, Reference Kremer and Dufek1995), and its high heritability can help identify the high-risk patients early, taking into account that CCT has become an endophenotype of major interest for the genetically complex disorder glaucoma. Although there is strong evidence of a genetic component for normal CCT variation, no genes have been identified for a long time (Dimasi et al., Reference Dimasi, Burdon and Craig2010). One of several genome-wide association study provided a genetic loci associated with CCT on chromosomes 9q34 and 16q24, which can be potentially related to open-angle glaucoma for association with CCT (Hoehn et al., Reference Hoehn, Zeller, Verhoeven, Grus, Adler, Wolfs and Mirshahi2012). Vitart et al. (Reference Vitart, Bencić, Hayward, Skunca Herman, Huffman, Campbell and Wright2010) reported 3 new loci associated with CCT (COL5A1, AKAP13, and AVGR8). Vithana et al. (Reference Vithana, Aung, Khor, Cornes, Tay, Sim and Wong2011) also identified novel genetic loci associated with CCT (COL8A2), and confirmed the involvement of a previously reported gene for CCT (ZNF469). Their findings implicate the involvement of collagen genes.
Although the heritability of anterior chamber depth was previously examined by several twin studies, heritability of the volume of anterior chamber, a more precise parameter, has never been published. We presumed that the magnitude of the genetic effects on ACV would be similar to the magnitude of genetic effects on ACD and ACA since ACV is derived from ACD and ACA. He, Wang et al. (Reference He, Wang, Zheng, Zhang, Yin, Huang, Mackey and Foster2008) reported an influence of 89% of additive genetic factors for ACD as measured by laser interferometry (He, Wang et al., Reference He, Wang, Zheng, Zhang, Yin, Huang, Mackey and Foster2008). The heritability of ACD was 83% in Koreans as measured by corneal topography and A-scan ultrasonography (Kim et al., Reference Kim, Zhao, Kim, Lim, Song, Guallar and Chung2013). Our heritability finding of 85% is of the same magnitude as the ACD reported in these studies. Our results seem to replicate the heritability estimates of the previous studies; however, we used only one non-contact automatic device to measure these parameters.
ACA, lens vault, and ACV are the three most significant factors of angle width. ACA/ACV has been known as the most prominent contributor to angle width in both Chinese and Caucasians. This parameter is the most relevant to the risk for primary angle closure glaucoma (Foo et al., Reference Foo, Nongpiur, Allen, Perera, Friedman, He and Aung2012).
Accordingly, high-risk individuals for lower ACV could be successfully screened with the Pentacam, which tends to be useful for angle closure screening due to its powerful association with angle width (Foo et al., Reference Foo, Nongpiur, Allen, Perera, Friedman, He and Aung2012; Wang et al., Reference Wang, Qi, He, Wu and Lin2012). Benefits of such screening in the current practice are arguable, and further studies are needed to confirm this hypothesis. Since ACV had negligible influence on unique environmental factors (e.g., lifestyle habits; 15%) and is largely determined by genetic effects, the family risk-based assessment of ACV might help us to estimate the chance of an angle closure glaucomatic attack in people who have a higher familial risks for the development of this disease.
The estimated negative correlation between the two parameters, ACV and CCT, means that if CCT increases, the ACV decreases and vice versa. This relationship has been already investigated in the literature with mixed results. Similarly to our findings, an inverse correlation can be found between the anterior chamber depth (which is partly included in ACV) and CCT (Hashemi et al., Reference Hashemi, Yazdani, Mehravaran, KhabazKhoob, Mohammad, Parsafar and Fotouhi2009). In contrast, a Taiwanese study failed to demonstrate a relevant relationship between CCT and anterior chamber depth, suggesting the independent influence of CCT (Chen et al., Reference Chen, Liu, Tsai, Chen, Chou and Lee2009). Our results seem to confirm the findings of Hashemi et al. (Reference Hashemi, Yazdani, Mehravaran, KhabazKhoob, Mohammad, Parsafar and Fotouhi2009), and we were interested whether genetic components play a role in this relationship. Only the Ghoangzou Twin Eye Study has investigated this topic, confirming that a shared genetic background exists among ACD, axial length, and angle-opening distance in children (He, Hur et al., Reference He, Hur, Zhang, Ding, Huang and Wang2008); however, the relationship between ACV and CCT has not been investigated. The strong genetic covariance between ACV and CCT highlights that we are able to estimate the potential risk of eye diseases compromising vision. Their close association to each other and also to the angle width is useful for angle closure screening in high-risk patients. Our results seem to underline the genetic covariation of the relationship of the investigated parameters this relationship, which underlies the importance of common genetic transmission of these traits. Since the development of the eye finishes very early in the third month in utero, it could also serve as a possible explanation why we observed a strong correlation between the investigated variables. Moreover, the different developmental stages between the members of a DZ twin pair compared with MZ twins could also play a potential role in our findings.
As CCT is important in corneal diseases such as dystrophies and keratoconus, it has to be taken into account in planning keratorefractive surgical procedures and interventions such as corneal collagen cross-linking or graft implantation. We also have to know its value for classical measurement of intraocular pressure for a patient with open-angle glaucoma. Similarly, ACV (because of its close association with anterior chamber depth) is also useful for surgical planning and follow-up with patients who have intraocular lens implantation, as well as risk assessment of angle-closure glaucoma attack. Whether we have a simple ultrasound pachymeter or a high-resolution Scheimpflug device, we are able to estimate the risk of numerous eye diseases — not just those listed above — and we can screen the relatives of the high-risk patients before any clinical signs of those diseases where studies have identified the specific genetic factors.
This study should be interpreted with its limitations. The relatively small number of participating twins may have led to statistical errors in the ACE analysis by increasing the E variance. In addition, the sample size did not allow us to test genetic-environmental interactions. Obviously, such a small sample study is quite underpowered. While the bootstrap of the AE model found significant genetic relationship, the confidence intervals are wise and the 99% confidence intervals were already insignificant. None of the covariance components were significant in the ACE model. However, this small sample size is not rare in cases of similar studies.
In conclusion, this study is the first to demonstrate the high heritability of ACV and its strong genetic covariance with central corneal thickness. The results highlight the possible role of the identification of the specific genetic factors and early screening for open-angle and also angle-closure glaucoma and corneal endothelial alterations in high-risk individuals.