Telomeres, which are non-coding DNA sequences capping the end of eukaryotic chromosomes, are known to play an important role in genome protection. In cell cultures, telomeres have been shown to shorten after each round of cell division (Allsopp et al., Reference Allsopp, Vaziri, Patterson, Goldstein, Younglai, Futcher and Harley1992) until they reach a critical length and the cell loses its ability to divide further. In this respect, telomere length has been used as an indicator of both replicative history and regenerative potential. Telomere erosion can also be counterbalanced by telomerase, an enzyme able to elongate telomeric ends, such as in highly proliferating stem cells (Blasco, Reference Blasco2005; Bodnar et al., Reference Bodnar, Ouellette, Frolkis, Holt, Chiu, Morin and Wright1998; Oeseburg et al., Reference Oeseburg, de Boer, van Gilst and van der Harst2010).
Aging is known to be associated with telomere shortening in some tissues, such as blood, whereas in other tissues, such as skeletal muscle, the question is still a matter of debate (Daniali et al., Reference Daniali, Benetos, Susser, Kark, Labat, Kimura and Aviv2013; Decary et al., Reference Decary, Mouly, Hamida, Sautet, Barbet and Butler-Browne1997; Ponsot et al., Reference Ponsot, Lexell and Kadi2008). The in vivo biology of telomeres remains imperfectly understood and may be governed by several other regulatory factors (de Lange, Reference de Lange2006; Ponsot et al., Reference Ponsot, Echaniz-Laguna, Delis and Kadi2012). Such complex interplay could partly explain why telomere length is not influenced by aging alone but also by environmental factors. For example, chronic life stress has been suggested to accelerate blood telomere shortening (Epel et al., Reference Epel, Blackburn, Lin, Dhabhar, Adler, Morrow and Cawthon2004), whereas physical training has been associated with longer telomeres in leukocytes (Cherkas et al., Reference Cherkas, Hunkin, Kato, Richards, Gardner, Surdulescu and Aviv2008; Du et al., Reference Du, Prescott, Kraft, Han, Giovannucci, Hankinson and De Vivo2012; LaRocca et al., Reference LaRocca, Seals and Pierce2010) and in skeletal muscle (Kadi et al., Reference Kadi, Ponsot, Piehl-Aulin, Mackey, Kjaer, Oskarsson and Holm2008).
Telomere length does not vary between the sexes at birth; however, during adulthood, women consistently exhibit longer telomeres in leukocytes (Aviv et al., Reference Aviv, Shay, Christensen and Wright2005; Benetos et al., Reference Benetos, Okuda, Lajemi, Kimura, Thomas, Skurnick and Aviv2001; Nordfjall, Eliasson, Stegmayr, Melander et al., Reference Nordfjall, Eliasson, Stegmayr, Melander, Nilsson and Roos2008). Estrogens may protect telomeres from oxidative stress and DNA damage by decreasing the amount of reactive oxygen species and influencing vascular biology (Aviv, Reference Aviv2002; Song et al., Reference Song, Kim, Jo, Hwang, Chae, Chung and Kim2009; Wong et al., Reference Wong, Yung, Leung, Tsang, Au, Chen and Huang2008), stimulating telomerase (Grasselli et al., Reference Grasselli, Nanni, Colussi, Aiello, Benvenuti, Ragone and Farsetti2008; Kyo et al., Reference Kyo, Takakura, Kanaya, Zhuo, Fujimoto, Nishio and Inoue1999; Misiti et al., Reference Misiti, Nanni, Fontemaggi, Cong, Wen, Hirte and Farsetti2000), and affecting telomerase activation through other indirect mechanisms (Rahimian et al., Reference Rahimian, Chan, Goel, Poburko and van Breemen2004; Vasa et al., Reference Vasa, Breitschopf, Zeiher and Dimmeler2000). Earlier studies have shown that longer exposure to endogenous estrogen, that is, longer duration of the reproductive years, is associated with longer telomere length in leukocytes (Gray et al., Reference Gray, Schiff, Fitzpatrick, Kimura, Aviv and Starr2014; Lee et al., Reference Lee, Im, Kim, Lee and Shim2005), peripheral mononuclear cells (Lin et al., Reference Lin, Kroenke, Epel, Kenna, Wolkowitz, Blackburn and Rasgon2011), and primordial germ cells (Aydos et al., Reference Aydos, Elhan and Tukun2005), and inversely associated with telomerase activity (Lin et al., Reference Lin, Kroenke, Epel, Kenna, Wolkowitz, Blackburn and Rasgon2011). Estrogen metabolism has also been suggested to be associated with telomere length through mechanisms linked to changes in body composition. After menopause, fat mass and, in particular, central obesity is increased, expressed as elevated insulin resistance and leptin levels (Aviv et al., Reference Aviv, Valdes, Gardner, Swaminathan, Kimura and Spector2006). Fat tissue is a source of leptin and adiponectin, both of which affect inflammation and insulin resistance. Inflammation enhances the turnover rate of leukocytes, and oxidative stress enhances the loss of telomere repeat per cell replication.
It is reasonable to postulate that estrogen metabolism is related to telomere length and/or attrition rate by affecting the number of cell divisions and by protecting telomere sequences. Endogenous estrogens may, however, have different effects on telomeres than the exogenous estrogens (Lin et al., Reference Lin, Kroenke, Epel, Kenna, Wolkowitz, Blackburn and Rasgon2011) that are used in hormone replacement therapy (HRT). The couple of studies done so far have reported inconsistent results. Lin et al. (Reference Lin, Kroenke, Epel, Kenna, Wolkowitz, Blackburn and Rasgon2011) found no association between longer duration of HRT and either leukocyte telomere length (LTL) or telomerase activity, whereas Lee et al. (Reference Lee, Im, Kim, Lee and Shim2005) reported longer LTL in long-term HRT users compared to women who had never used HRT. In theory, HRT may have direct effects on telomeres, but it can also act as an anti-aging agent by maintaining healthier body composition and physical performance (Ronkainen et al., Reference Ronkainen, Kovanen, Alen, Pollanen, Palonen, Ankarberg-Lindgren and Sipila2009). We have shown previously that HRT promotes healthier inflammatory profile (Kangas et al., Reference Kangas, Pollanen, Rippo, Lanzarini, Prattichizzo, Niskala and Kovanen2014), and amount and distribution of body fat as well as better physical performance (Ronkainen et al., Reference Ronkainen, Kovanen, Alen, Pollanen, Palonen, Ankarberg-Lindgren and Sipila2009). Earlier studies have suggested that healthier body composition might protect telomere ends from accelerated shortening (Lee et al., Reference Lee, Martin, Firpo and Demerath2011; Njajou et al., Reference Njajou, Cawthon, Blackburn, Harris, Li, Sanders and Hsueh2012; Valdes et al., Reference Valdes, Andrew, Gardner, Kimura, Oelsner, Cherkas and Spector2005).
Telomere length varies between individuals and between different cells and tissues within the same individual. Leukocytes represent a constantly dividing cell population that presumably should show early signs of wear and tear as short telomeres. Adult muscle cells on the other hand are multinucleated post-mitotic cells that are not considered to divide and thereby consume telomeres after differentiation, but rather to describe an inherited telomere length. However, skeletal muscles also contain a population of quiescent muscle stem cells, called satellite cells, which can re-enter the cell cycle to initiate proliferation and donate nuclei to muscle fibers. Satellite cells are essential for muscle regeneration and are responsible for muscle growth (Alway et al., Reference Alway, Myers and Mohamed2014). A significant inverse relationship between age and skeletal muscle telomere length (SMTL) has been reported across the age span (Decary et al., Reference Decary, Mouly, Hamida, Sautet, Barbet and Butler-Browne1997; Wernig et al., Reference Wernig, Schafer, Knauf, Mundegar, Zweyer, Hogemeier and Zimmermann2005), potentially limiting the proliferative capacity of satellite cells. That may lead to satellite cell senescence, thereby contributing to a loss of skeletal muscle mass and consequently physical performance.
Theoretically, better preserved telomere length in satellite cells may partly explain the previously reported positive association between HRT and musculature (Pollanen et al., Reference Pollanen, Ronkainen, Suominen, Takala, Koskinen, Puolakka and Kovanen2007; Ronkainen et al., Reference Ronkainen, Kovanen, Alen, Pollanen, Palonen, Ankarberg-Lindgren and Sipila2009; Sipila et al., Reference Sipila, Taaffe, Cheng, Puolakka, Toivanen and Suominen2001). We hypothesized that HRT use protects telomeres from aging-associated shortening and that telomere attrition rate may be partially genetically determined. We used a design that controls for genotype and childhood environment (monozygotic twin pairs discordant for HRT) to investigate the possible association of long-term HRT use on telomere length in leukocytes and in skeletal muscle in order to define possible difference in protection of telomere length in mitotic (defined as relative LTL) and post-mitotic (defined as mean SMTL, SMTL, and minimum SMTL, which is assumed to represent satellite cell telomere length) cell lineages due to HRT. In addition, associations between LTL, mean, or minimum SMTL, and total body and thigh composition and physical performance were examined.
Material and Methods
Study Design
This study is part of a larger study, ‘Sarcopenia — Skeletal Muscle Adaptation to Postmenopausal Hypogonadism and Effects of Hormone Replacement Therapy and Physical Activity in Older Women: A Genetic and Molecular Biological Study on E strogen-related Pathways’ (SAWEs). The recruitment process has been described in detail elsewhere (Ronkainen et al., Reference Ronkainen, Kovanen, Alen, Pollanen, Palonen, Ankarberg-Lindgren and Sipila2009). Briefly, 537 monozygotic (MZ) female twin pairs from selected age groups of the population-based Finnish Twin Cohort (Kaprio & Koskenvuo, Reference Kaprio and Koskenvuo2002) were invited to self-select themselves as discordant for the use of HRT. A total of 15 MZ pairs who responded to the invitation met the inclusion criteria and had no contraindications for participation in the measurements and muscle biopsy sampling, and were invited to the laboratory examinations. Four of these twin pairs were then excluded from the present analysis owing to the use of tibolone, which is not an estrogen-based HRT treatment. Zygosity was verified at the paternity testing laboratory (National Public Health Institute, Helsinki, Finland) using DNA extracted from a venous blood sample with a battery of 10 highly polymorphic gene markers. All laboratory measurements and data analyses were carried out blind to HRT status.
Mean duration of HRT among the users was 7.3 (SD 3.7, range 2–16) years, while their co-twins had never used HRT. Of the HRT users, five women used estradiol-only (1–2 mg) preparations and six a combined treatment comprising both estrogenic (1–2 mg) and progestogenic compounds. We have reported earlier that while the HRT users had higher levels of both total and free 17β-estradiol (E2) and total estrone (E1) than their non-user sisters, both co-twins were similar in their physical activity (PA) habits, daily energy intake, use of medication, and smoking behavior (Ronkainen et al., Reference Ronkainen, Kovanen, Alen, Pollanen, Palonen, Ankarberg-Lindgren and Sipila2009; Ronkainen et al., Reference Ronkainen, Pollanen, Alen, Pitkanen, Puolakka, Kujala and Kovanen2010).
The Ethics Committee of the Central Finland Health Care District approved the study and it was conducted according to the guidelines laid down by the World Medical Association in the Declaration of Helsinki (2000). Written informed consent was provided by the participants before the measurements.
Telomere Length Measurements
Relative LTL was determined from peripheral blood DNA by a quantitative real-time polymerase chain reaction (qPCR) based method, originally developed by Cawthon et al. (Reference Cawthon, Smith, O'Brien, Sivatchenko and Kerber2003), with minor modifications (Eerola et al., Reference Eerola, Kananen, Manninen, Hellstrom, Tienari and Hovatta2010; Kananen et al., Reference Kananen, Surakka, Pirkola, Suvisaari, Lonnqvist, Peltonen and Hovatta2010). Briefly, a separate qPCR reaction was performed with telomere sequence-specific primers and a single copy control gene, β-hemoglobin. Both the telomere and β-hemoglobin reactions were performed with a CFX 384 Real-Time PCR Detection System (Bio-Rad, Hercules, CA). Melt-curve analysis was carried out to ensure specific primer binding. A DNA dilution series (0.5, 1.0, 2.0, 5.0, 10, 20, and 30 ng) was used to create a standard curve (R 2 = 0.997 for the telomere and 0.998 for the β-hemoglobin run) and to perform absolute quantification of each sample. We used Bio-Rad CFX Manager v.1.6 software to perform quality control and to calculate the T/S (telomere to single-copy gene intensity) ratios for the samples to obtain the relative telomere length. The correlation coefficient of variation (CV) for repeat measures was 6.37% for the telomere reaction, 4.99% for the β-hemoglobin reaction, and 6.97 % for the ratio (T/S), as determined by running six control samples across six plates.
Mean and minimum SMTL was determined from a muscle biopsy sample obtained from the mid part of the vastus lateralis, defined as the midpoint between the greater trochanter and the lateral joint line of the knee. Following the removal of all visible residues of fat and connective tissue, the biopsy samples were frozen in liquid nitrogen and stored at -80°C until use. A southern blot protocol specifically adapted for the study of skeletal muscle was used in the analysis (Decary et al., Reference Decary, Mouly, Hamida, Sautet, Barbet and Butler-Browne1997; Ponsot et al., Reference Ponsot, Lexell and Kadi2008). Total genomic DNA was digested with the restriction enzyme HinfI (New England Biolabs, Ipswich, MA, USA), and telomeric terminal restriction fragments were generated. Digested DNA, together with two DNA ladders (1 kb plus and high molecular weight; Invitrogen Life technologies, Paisley, UK), were resolved using electrophoresis in 0.7% agarose gels. The telomeric terminal restriction fragments were detected by hybridization to a 32P-labelled (TTAGGG)4. The signals were analyzed using a computer-assisted system (Scion image 4.03 software; Scion Corporation, Bethesda, MD, USA) and modeled using an improved signal analysis model for the assessment of telomere restriction fragment length (Ponsot et al., Reference Ponsot, Lexell and Kadi2008). Mean SMTL comprises telomeres from all cells, of which the majority contain post-mitotic myonuclei, which prior to differentiation into muscle fiber cells had undergone only a few mitoses. In contrast, minimum SMTL shows the length of the shortest telomeres in the sample. Hypothetically, the shortest telomeres are found in satellite cells and in myonuclei that have been added to the myofibers during the most recent mitotic cycles (Ponsot et al., Reference Ponsot, Lexell and Kadi2008). SMTL was successfully determined in 10 twin pairs. The CV of telomere length measurements in skeletal muscle has been below 5% for both mean and minimum length (Ponsot & Kadi, Reference Ponsot and Kadi2008).
Total Body and Thigh Composition
Body mass index was calculated as a function of measured body weight and height (kg/m2). Percentage body fat and total body fat-free mass (FFM) were measured by a multi-frequency bioelectrical impedance method (InBody [720], Biospace, Seoul, Korea) after overnight fast. In our laboratory, the CV for two consecutive measurements of percentage of body fat has been 0.6%.
Thigh composition was assessed from computed tomography scans (Siemens Somatom Emotion scanner, Siemens AG, Erlangen, Germany) obtained from the same site as the muscle biopsy. Thigh muscle compartment cross-sectional area, thigh fat areas (total fat area, subcutaneous fat, and fat infiltrated into muscle tissue), and relative proportion of fat (%) were analyzed using software developed at the University of Jyväskylä for cross-sectional computed tomography image analysis (Geanie 2.1, Commit; Ltd., Espoo, Finland), as described earlier (Ronkainen et al., Reference Ronkainen, Kovanen, Alen, Pollanen, Palonen, Ankarberg-Lindgren and Sipila2009). In our laboratory, the CV between two consecutive measurements was 1 to 2% for muscle area (Sipila et al., Reference Sipila, Taaffe, Cheng, Puolakka, Toivanen and Suominen2001).
Physical Performance
Vertical jumping height (cm) was calculated from flight time during a countermovement jump and measured on a contact mat (Bosco et al., Reference Bosco, Luhtanen and Komi1983). Three maximal efforts were conducted and the best result was used in the analysis. In our laboratory, the CV between two consecutive measurements has been 5% (Sipila et al., Reference Sipila, Taaffe, Cheng, Puolakka, Toivanen and Suominen2001). Vertical jumping height can be used as an estimate of lower body muscle power, that is, the ability of the neuromuscular system to produce the greatest possible force as rapidly as possible.
Hand grip strength (kg) was measured using an isometric dynamometer (Good Strength IGS01, Metitur Oy, Jyväskylä, Finland). Subjects were encouraged verbally to produce maximal contraction. Hand grip strength was recorded in kilograms to within accuracy of 0.1 kg and the best attempt of three trials was used in the analysis.
Physical Activity
PA was assessed using a modified version of the seven-point Grimby scale. The categories used in questionnaire were (1) inactive, (2) light activity 1–2 times per week, (3) light activity several times per week, (4) moderate activity 1–2 times per week, (5) moderate activity several times per week, (6) high activity several times per week, and (7) competitive sports several times per week. For the analysis, categories 1 and 2, 3 and 4, and 5 to 7 were combined to describe inactive, moderate, and vigorous activity groups.
Statistics
Owing to the relatively small number of observations, the statistical significance of the differences between the means of the HRT users and non-users was tested with Wilcoxon's signed rank test. Data are shown as means and standard deviations, unless otherwise stated. Associations between age and leukocyte and skeletal muscle telomeres were analyzed using standardized regression coefficients to represent bivariate correlations, and p values were adjusted for twin dependency. Associations between telomere length and body composition, and telomere length and physical performance were tested with linear regression analysis. In regression models, within-pair dependency of twin individuals was taken into account using the cluster option in analysis. Possible confounders — age, level of PA, and HRT (user or non-user) — were entered into the model one at a time. Intraclass correlation coefficients (ICC) were computed for the twin pairs to estimate the level of within-pair similarity. The level of significance was set at p ≤ .05. Data analyses were carried out with IBM SPSS Statistics 22 (SPSS Inc., Chicago, IL) and Stata version 14 (Stata Corp, College Station, TX, USA).
Results
Participants’ Characteristics and Telomere Length
The mean age of the participants was 57.6 (SD 1.8, range, 55–62 years). Mean LTL, mean SMTL, and minimum SMTL did not differ between the HRT users and their co-twins (Table 1). On average, the HRT users had lower percentage body fat than non-user sisters, while no differences between the groups were observed in FFM. Moreover, no differences were observed between HRT users and non-users in thigh muscle area or amount of fat infiltrated into the muscle compartment. However, thigh fat area, relative proportion of fat, and subcutaneous fat were smaller in users than non-users. HRT users had better vertical jumping height, indicating higher muscle power, than their co-twins, but no difference was observed in hand grip strength.
TABLE 1 Telomere Length and Physical Characteristics of Monozygotic Twin Pairs Discordant for Hormone Replacement Therapy

Data are means and standard deviations. HRT = hormone replacement therapy. *n = 10 HRT users and 10 HRT non-users. Bold type indicates statistically significant difference between HRT users and non-users. The data presented have been partially reported in a previous publication (Ronkainen et al., Reference Ronkainen, Kovanen, Alen, Pollanen, Palonen, Ankarberg-Lindgren and Sipila2009).
Associations Between Leukocyte and Skeletal Muscle Telomere Length and Age
No significant relationship was found between LTL and mean (r = 0.114, p = .12) and minimum SMTL (r = 0.172, p = .47). Mean and minimum SMTLs were not associated (r = 0.220, p = .38). Age was not associated with LTL (r = -0.070, p = .69), minimum SMTL (r = -0.43, p = .12), or mean SMTL (r = -0.40, p = .097).
Within-pair analysis showed that LTL of HRT users correlated significantly with the corresponding values of non-users (r = 0.69, p = .020, Figure 1A). Pairwise correlations were even higher in twin pairs for mean (r = 0.74, p = .014) and minimum (r = 0.88, p = .001) SMTL; see Figure 1A, B, and C.

FIGURE 1 Within-pair correlations between HRT users and HRT non-users in (A) relative leukocyte telomere length (LTL, n = 11 twin pairs), (B) mean skeletal muscle telomere length (SMTL, n = 10 twin pairs), and (C) minimum skeletal muscle telomere length (n = 10 twin pairs).
Associations Between Leukocyte Telomere Length (LTL), Total Body and Thigh Composition, and Physical Performance
In all participants, longer LTL was associated with lower BMI, percentage body fat, and relative proportion of fat in the thigh (Table 2, Figure 2A and B). Borderline significant association between longer LTL and lower thigh fat area, subcutaneous fat, and infiltrated fat were observed. Significant associations were not observed between LTL and FFM or physical performance. Linear regression revealed that BMI, percentage body fat, thigh fat area, relative proportion of fat, and subcutaneous fat were significant independent predictors of LTL after adjustment for age, PA level, and HRT use.
TABLE 2 Associations between Relative Leukocyte Telomere Length and Estimates of Total Body and Thigh Composition and Physical Performance in All Subjects (n = 22)

All p values are assessed with linear regression. *Model fit statistics are shown for the covariate and adjusting covariates listed in column one. SE = standard error; PA = physical activity; HRT = hormone replacement therapy. Bold type indicates statistical significance at the level of p < .05.
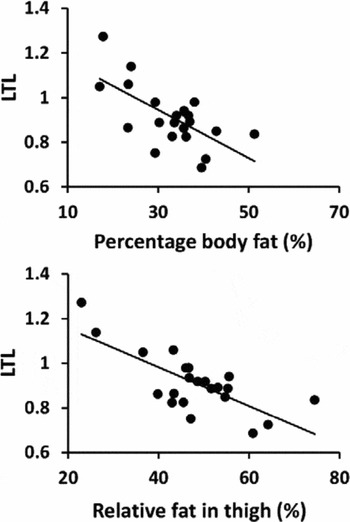
FIGURE 2 Association between relative leukocyte telomere length (LTL) and (A) percentage body fat (%), and (B) relative proportion of fat in thigh (%) in all subjects (n = 22 individuals).
Associations Between Mean and Minimum Skeletal Muscle Telomere Length, Total Body and Thigh Composition and Physical Performance
Mean SMTL was not associated with total body composition or vertical jumping height (Table 3). A borderline significant positive association was found between mean SMTL and grip strength. Participants with larger total body FFM and greater thigh muscle area had longer minimum SMTL, but measures of total body or thigh fat or physical performance were not associated with SMTL (Table 4, Figures 3A and B). Regression models showed that FFM and thigh muscle area were significant independent predictors of minimum SMTL after adjusting for age, PA level, and HRT use.
TABLE 3 Associations Between Mean Skeletal Muscle Telomere Length and Estimates of Total Body and Thigh Composition and Physical Performance in All Subjects (n = 20)

All p values are assessed with linear regression. *Model fit statistics are shown for the covariate and adjusting covariates listed in column one. SE = standard error; PA = physical activity; HRT = hormone replacement therapy. Bold type indicates statistical significance at the level of p < .05.
TABLE 4 Associations Between Minimum Skeletal Muscle Telomere Length and Estimates of Total Body and Thigh Composition and Physical Performance in All Subjects (n = 20)

All p values are assessed with linear regression. *Model fit statistics are shown for the covariate and adjusting covariates listed in column one. SE = standard error; PA = physical activity; HRT = hormone replacement therapy. Bold type indicates statistical significance at the level of p < .05.

FIGURE 3 Association between minimum skeletal muscle telomere length (SMTL) and (A) total body fat-free mass (kg) and (B) thigh muscle cross-sectional area (cm2) in all subjects (n = 20 individuals).
Discussion
The results showed that the use of the long-term, estrogen-based HRT was not significantly associated with either leukocyte or SMTL. This finding is strengthened by the observation that pairwise correlations in LTL and SMTL between the MZ twin sisters discordant for HRT were high, despite the HRT use and more favorable total and regional body composition and better muscle power observed in the HRT users (Ronkainen et al., Reference Ronkainen, Kovanen, Alen, Pollanen, Palonen, Ankarberg-Lindgren and Sipila2009). Our results also revealed that longer telomere lengths were associated with healthier body composition and less clearly with physical performance. LTL, a biomarker that has been associated with inflammation, stress reaction, cardiovascular diseases, and mortality (Cawthon et al., Reference Cawthon, Smith, O'Brien, Sivatchenko and Kerber2003; Demissie et al., Reference Demissie, Levy, Benjamin, Cupples, Gardner, Herbert and Aviv2006; Epel et al., Reference Epel, Blackburn, Lin, Dhabhar, Adler, Morrow and Cawthon2004; Haycock et al., Reference Haycock, Heydon, Kaptoge, Butterworth, Thompson and Willeit2014; Huzen et al., Reference Huzen, de Boer, van Veldhuisen, van Gilst and van der Harst2010) was related to lower measures of total body fat and thigh fat compartments. In contrast, the shortest telomeres present in the vastus lateralis muscle tissue, that is minimum SMTLs, were associated with total body FFM and thigh muscle area, but not body fatness.
A discordant twin study is a particularly suitable design for investigating the environmental exposures such as HRT during late adulthood, since it allows controlling for genetic factors and also most of the environmental exposures that occur during the first years of life. This is especially important with regard to telomeres, as it is known that telomere length is highly heritable (Broer et al., Reference Broer, Codd, Nyholt, Deelen, Mangino, Willemsen and Boomsma2013; Hjelmborg et al., Reference Hjelmborg, Dalgard, Moller, Steenstrup, Kimura, Christensen and Aviv2015) and the majority of the attrition occurs during the first two decades, when cells divide and differentiate rapidly due to growth and development (Aubert et al., Reference Aubert, Baerlocher, Vulto, Poon and Lansdorp2012; Zeichner et al., Reference Zeichner, Palumbo, Feng, Xiao, Gee, Sleasman and Dimitrov1999). Our results from a genetically controlled sample suggest that long-term HRT is not associated with telomere length in older age. There are several possible explanations for that observation. First, it might be that exogenous estrogens (HRT) do not have a similar protective effect on telomere attrition as endogenous estrogens. Second, it is possible and highly likely, based on ours and others’ findings, that telomere length and possibly also attrition rate are strongly genetically regulated. It might be that similar genetic factors regulate both natural hormonal aging and telomere length, and therefore, for example, menopause occurs at later age in subjects with longer telomeres and longer life expectancy. Third, it might be, that telomere attrition during late adulthood is so minor that even the effect of the average use of HRT use over 7 years is beyond our measurement limit (Chen et al., Reference Chen, Kimura, Kim, Cao, Srinivasan, Berenson and Aviv2011).
Our result is in line with findings of Lin et al. (Reference Lin, Kroenke, Epel, Kenna, Wolkowitz, Blackburn and Rasgon2011), who reported no associations between HRT and peripheral mononuclear telomere length or telomerase activity, and Dalgård et al. (Reference Dalgard, Benetos, Verhulst, Labat, Kark, Christensen and Aviv2015), who investigated leukocyte telomeres. However, Lee et al. (Reference Lee, Im, Kim, Lee and Shim2005) reported higher telomere length in postmenopausal HRT users compared to non-users. The disparity between our results and those of Lee et al. might be related to selection bias. In the study by Lee et al., HRT users participated more often in regular exercise and used more vitamin supplements than non-users. In the present sample, not only genotype, but also PA habits, daily energy intake, use of medication, and smoking behavior were similar among the twin pairs discordant for HRT (Ronkainen et al., Reference Ronkainen, Kovanen, Alen, Pollanen, Palonen, Ankarberg-Lindgren and Sipila2009; Ronkainen et al., Reference Ronkainen, Pollanen, Alen, Pitkanen, Puolakka, Kujala and Kovanen2010). However, HRT users have been shown to have better mobility, greater muscle power, and more favorable body and muscle composition than non-users (Ronkainen et al., Reference Ronkainen, Kovanen, Alen, Pollanen, Palonen, Ankarberg-Lindgren and Sipila2009).
The novel finding of this study was that in MZ twin sisters, both mean and minimum SMTL showed a high correlation, indicating strong familiality, possibly due to major genetic effects. As expected, the correlation between twin sisters was higher in post-mitotic SMTL compared to length of the telomeres in more rapidly dividing leukocytes. However, a higher correlation was observed in minimum SMTL compared to LTL and mean SMTL, which suggest that telomere attrition rate may also be genetically regulated. So far, very few papers have compared telomeres measured in leukocytes and those in skeletal muscle cells. LTL and SMTL showed a correlation of 0.39 in the study by Ahmad et al. (Reference Ahmad, Heraclides, Sun, Elgzyri, Ronn, Ling and Hansson2012) and 0.71 in the study of Daniali et al. (Reference Daniali, Benetos, Susser, Kark, Labat, Kimura and Aviv2013); however, we found no association between LTL and mean and minimum SMTL. We hypothesize that this may be related to the different measurement techniques used (qPCR vs. southern blot) or to the fact that leukocytes and muscle cells are divergent in nature. Leukocytes divide more rapidly than other cell types and they have the lowest mean lifespan (Abramson & Melton, Reference Abramson and Melton2000). Muscle tissue regeneration, in turn, is slower and occurs only through satellite cell proliferation.
As expected, mean SMTL, which mainly consists of telomeres from myonuclei in muscle fibers that have undergone only few cell divisions, was not associated with body composition or physical performance. Consequently, we also estimated minimum SMTL, which describes the length of the shortest telomeres present in muscle tissue. Minimum SMTL hypothetically reflects length of telomeres in nuclei of satellite cells and in nuclei newly incorporated into muscle cells (Ponsot et al., Reference Ponsot, Lexell and Kadi2008). Satellite cell recruitment is an obligatory phenomenon both for skeletal muscle growth (Hawke & Garry, Reference Hawke and Garry2001) and regeneration. Satellite cell activation increases rapidly in muscles after exercise (Kadi et al., Reference Kadi, Schjerling, Andersen, Charifi, Madsen, Christensen and Andersen2004). Their proliferation introduces new myonuclei into muscle cells and contributes to muscle hypertrophy (Kadi & Thornell, Reference Kadi and Thornell2000). Hypothetically, longer minimum SMTLs may reflect greater capacity to generate new muscle cells due to inherited or environmental factors. In addition, absence of very short telomeres in muscle cells may indicate better muscle function (Hemann et al., Reference Hemann, Strong, Hao and Greider2001). Our results, which showed that longer minimum SMTLs were associated with higher FFM and greater muscle area in the thigh, support the hypothesis that minimum SMTL is a specific biomarker of skeletal muscle mass. The association observed between higher minimum SMTLs and increasing muscle mass is supported by Kadi et al. (Reference Kadi, Ponsot, Piehl-Aulin, Mackey, Kjaer, Oskarsson and Holm2008), who also found that minimum SMTLs tended to be longer in subjects with higher muscle mass. Whether length of the minimum SMTLs limits FFM at older age, or whether telomere shortening and decrease in FFM are simultaneously age-related, possibly genetically regulated (Arden & Spector, Reference Arden and Spector1997) processes, needs more research.
Overall, cross-sectional studies offer little evidence about associations between LTL and physical performance (Gardner et al., Reference Gardner, Martin-Ruiz, Cooper, Hardy, Sayer and Cooper2013). Conflicting findings have been reported on the association between LTL and walking speed (Gardner et al., Reference Gardner, Martin-Ruiz, Cooper, Hardy, Sayer and Cooper2013; Harris et al., Reference Harris, Deary, MacIntyre, Lamb, Radhakrishnan, Starr and Shiels2006; Lee et al., Reference Lee, Bang, Ko, Kim and Lee2013) and LTL and grip strength (Der et al., Reference Der, Batty, Benzeval, Deary, Green, McGlynn and Shiels2012; Harris et al., Reference Harris, Deary, MacIntyre, Lamb, Radhakrishnan, Starr and Shiels2006). We have previously shown that LTL predicts the decline in PA and walking ability in older twin sisters (Sillanpaa et al., Reference Sillanpaa, Tormakangas, Rantanen, Kaprio and Sipila2016). Data on SMTL is even scarcer, as it is limited to studies that have used muscle biopsies. High-intensity training causes an increased demand on skeletal muscle to repair, regenerate, and remodel muscle tissue, which in turn shortens minimum telomere length in skeletal muscle. Rae et al. (Reference Rae, Vignaud, Butler-Browne, Thornell, Sinclair-Smith, Derman and Collins2010) reported an inverse relationship between minimum telomere length and long-term exposure to endurance exercise. Kadi et al. (Reference Kadi, Ponsot, Piehl-Aulin, Mackey, Kjaer, Oskarsson and Holm2008) observed an inverse correlation between minimum SMTL and performance in power lifting. Our results suggest no or only weak associations between telomeres and physical performance. LTL approached significance as an independent predictor of hand grip strength (p = .060), which is a performance-based measurement known to be highly related to aging, overall health, and mortality (Rantanen et al., Reference Rantanen, Volpato, Ferrucci, Heikkinen, Fried and Guralnik2003).
In conclusion, findings from our genetically controlled twin data suggest that long-term use of HRT has no or at most very small effects on LTL and SMTL in postmenopausal women. It is possible that natural aging, associated with attenuation in sex hormones as well as telomere attrition, are regulated through genetic factors. Previous findings that HRT use is associated with longer LTLs (Lee et al., Reference Lee, Im, Kim, Lee and Shim2005) are possibly explained by selection bias or confounding effects of HRT. HRT users seem to be more prone to adopt a healthier lifestyle (Lee et al., Reference Lee, Im, Kim, Lee and Shim2005), while the use of HRT might result in more favorable body composition and better physical performance. Based on the findings of the present study and earlier investigations (Lee et al., Reference Lee, Martin, Firpo and Demerath2011; Njajou et al., Reference Njajou, Hsueh, Blackburn, Newman, Wu and Li2009; Nordfjall, Eliasson, Stegmayr, Lundin et al., Reference Nordfjall, Eliasson, Stegmayr, Lundin, Roos and Nilsson2008), it is likely that a healthier body composition is associated with longer telomere lengths and that the associations may differ with respect to the cell types in which telomeres are determined.
Acknowledgments
The authors acknowledge Dr Iiris Hovatta and Ms Laura Kananen for their help in carrying out the telomere length analysis by qPCR. We also acknowledge support from the EC FP7 Collaborative Project MYOAGE (GA-223576). The Gerontology Research Center is a joint effort between the University of Jyväskylä and the University of Tampere. The SAWEs study was funded by the Academy of Finland and the Finnish Ministry of Culture and Education (V.K.: grant numbers 251316 and 89/672/2008). E.S. is supported by a post doc research grant from the Academy of Finland (grant number 260001). J.K. has been supported by the Academy of Finland (grants 265240, 263278).









