Data from population-based surveys have confirmed previous observations reporting an excess of congenital anomalies — nearly twice as many — in monochorionic (MC) compared to dichorionic (DC) twins and to singletons (5–6% in the former, compared to 2–3% in the latter; Glinianaia et al., Reference Glinianaia, Rankin and Wright2008; Hall, Reference Hall2003). Together with the more common complications of MC pregnancies, namely twin-to-twin transfusion syndrome (TTTS) and selective intrauterine growth restriction (sIUGR), congenital anomalies also contribute to the increased rate of perinatal morbidity and mortality observed in MC pregnancies (Alexander et al., Reference Alexander, Ramus, Susan, Cox and Gilstrap1997; Pharoah & Dundar, Reference Pharoah and Dundar2009; Wood et al., Reference Wood, Tang, Ross and Sauve2014). MC twins, resulting from a single fertilized egg giving rise to two separate embryos, are monozygotic (MZ) and considered genetically identical. For this reason, when congenital anomalies are present they are expected to affect both twins. However, many studies have reported that MZ twins are not genetically identical, and may be discordant for chromosomal anomalies, Mendelian disorders, and other important phenotypes, including structural anomalies (Machin, Reference Machin2009; Silva et al., Reference Silva, Martins, Matias and Blickstein2011). These findings have given rise to intense debate about the genetic, epigenetic, and environmental origins of the discordance, a debate that goes beyond the scope of the present study (Castillo-Fernandez et al., Reference Castillo-Fernandez, Spector and Bell2014; Zwijnenburg et al., Reference Zwijnenburg, Meijers-Heijboer and Boomsma2010). Obstetricians involved in the surveillance of MC twins are well aware that these fetuses are not only at increased risk of congenital structural anomalies, but that these anomalies can also occur discordantly and require a careful and complex clinical management (Gratacos et al., Reference Gratacos, Ortiz and Martinez2012; Khalil et al., Reference Khalil, Rodgers, Baschat, Bhide, Gratacos, Hecher and Ville2016).
Although there have been numerous studies describing MC discordancies for abnormal karyotype and for cardiac and non-cardiac malformations (often comprising of minor findings such as single umbilical artery, choroid plexus cyst, and pyelectasis), these are limited to single case reports or to small series complicated or not by TTTS (Sperling et al., Reference Sperling, Kiil, Larsen, Brocks, Wojdemann, Qvist and Tabor2007; Springer et al., Reference Springer, Mlczoch, Krampl-Bettelheim, Mailath-Pokorny, Ulm, Worda and Worda2014). The primary aim of this study was to describe the spectrum of major discordant structural anomalies observed in a large cohort of MC pregnancies, coded using a standardized system to make classification as objective as possible. The secondary objective was to describe the management of these pregnancies and the perinatal outcome of both anomalous and normal MC twins.
Materials and Methods
Case Selection
The study complied with our Institution's research guidelines for clinical observational and retrospective studies.
This is a retrospective descriptive analysis of all consecutive MC pregnancies with major discordant structural anomalies referred after 14 weeks’ gestation to the ‘Umberto Nicolini’ Fetal Therapy Unit of the V. Buzzi Children's Hospital, University of Milan, Italy, between January 2004 and December 2015. All reported pregnancies were diagnosed as MC at first trimester ultrasound. Over the 12-year study period, 1,750 MC pregnancies were referred to our unit, including 485 complicated with TTTS. In total, 168 of these pregnancies were managed for major structural anomalies observed either in both twins (13 pregnancies) or in one (155 pregnancies). This cohort of 155 pregnancies is the object of the present study. All prenatal records were reviewed and the pregnancies were divided as follows: MC diamniotic (MC DA), MC triamniotic (MC TA), MC monoamniotic (MC MA), and DC triamniotic (DC TA). Pregnancies complicated with twin-reversed arterial perfusion (TRAP) sequence, conjoined twins, or intrauterine fetal death (IUFD) of one or both twins at referral were excluded from the study. Structural anomalies were defined as major when one MC twin presented at ultrasound with a lethal malformation, or when the detected anomaly required postnatal surgery or was associated with the increased risk of functional or neurological impairment. We also recorded when the discordant anomaly occurred in pregnancies complicated with sIUGR (defined as previously described; Rustico et al., Reference Rustico, Consonni, Lanna, Faiola, Schena, Scelsa and Ferrazzi2017), whether the affected twin was the small or the large one, or when it occurred in TTTS pregnancies, and whether the affected twin was the donor or the recipient. In this study, TTTS-related recipient twin cardiomyopathy (cardiomegaly, ventricular hypertrophy, atrio-ventricular valvular regurgitation, and right ventricle outflow tract obstruction; Manning & Archer, Reference Manning and Archer2016; Rustico et al., Reference Rustico, Lanna, Faiola, Schena, Dell'Avanzo, Mantegazza and Ferrazzi2012) was not considered as a discordant cardiac anomaly. Ultrasound findings such as single umbilical artery, choroid plexus cyst, pyelectasis, or stable borderline ventriculomegaly (less than 12 mm) were also not considered.
Case Diagnosis and Clinical Work-Up
A detailed anatomical ultrasound evaluation, including echocardiography, was carried out of the MC twins, as well as three-dimensional ultrasound examination of the fetal brain starting from the second trimester (GE Voluson 730 Expert, and GE E8, GE Healthcare, Zipf, Austria). We also carried out serial fetal biometry, including Doppler interrogation of the umbilical artery, ductus venosus, and middle cerebral artery. Whenever the major structural anomaly was identified in one twin in conjunction with normal ultrasound findings in the co-twin, fetal karyotyping (by amniotic fluid sampling of both amniotic sacs) was offered if not previously acquired (Gentilin et al., Reference Gentilin, Guerneri, Bianchi, Natacci, Colombo, Fogliani and Lalatta2008). Until 2010, conventional karyotyping was performed, subsequently replaced by array comparative genomic hybridization analysis (aCGH; Hilmann et al., Reference Hilmann, McMullan, Hall, Togneri, James, Maher and Kilby2013). In the presence of anomalies affecting the central nervous system (except for cases of anencephaly, encephalocele, holoprosencephaly, and spina bifida), of congenital pulmonary airway malformations (CPAM, formerly congenital cystic adenomatoid malformations or CCAM) and congenital diaphragmatic hernia (CDH), in utero magnetic resonance imaging (MRI) was also performed alongside ultrasound diagnosis (Conte et al., Reference Conte, Parazzini, Falanga, Cesaretti, Izzo, Rustico and Righini2016). All families received extensive counseling regarding the management options for discordant anomalies: selective feticide with bipolar cord coagulation (BCC; Lanna et al., Reference Lanna, Rustico, Dell'Avanzo, Schena, Faiola, Consonni and Ferrazzi2012), complete termination of the pregnancy (TOP), or conservative management. Issues addressed included the risk of co-twin loss and the increased risk of premature delivery after BCC, along with the option of conservative management. Patients with potentially correctable anomalies complicated with both TTTS were told about the option of fetoscopic laser surgery (FLS; Slaghekke et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, van Zwet, Weingertner and Oepkes2014). Autoptic examination was performed in all cases that ended in miscarriage or TOP, except for four cases of early termination.
All surviving infants, with and without prenatally detected major structural anomalies, were followed up with serial neurological examinations by a pediatric neurologist-psychiatrist, which is routine practice in Italy, for at least 24 months, with the exception of two cases lost some months after cardiac surgery (Marlow, Reference Marlow2013). Neonates/infants without standard karyotype/aCGH but with normal clinical assessment were considered to be normal.
Data Analysis
To produce a standardized classification of our cases and make the description as objective as possible, each anomaly was coded and named retrospectively using the International Statistical Classification System of Diseases and Related Health Problems, tenth revision (ICD-10; World Health Organization [WHO], 2016). The classification of structural congenital anomalies is reported in Chapter XVII (Congenital Malformations, Deformations and Chromosomal Abnormalities), and contains a range of blocks of codes from Q00 to Q99.
Those anomalies familiar to prenatal ultrasonographers but not envisaged by the ICD-10 classification system, such as severe ventriculomegaly not due to obstruction of the ventricular system (for which there is a specific code), or vermian hypoplasia not classifiable as Dandy–Walker syndrome (which also has its own code), are shown in our tables in italics. Other anomalies not included (such as sacrococcigeal teratoma or romboencephalosynapsis) have been inserted in the blocks entitled ‘congenital malformation, and unspecified’. Anomalies affecting one system were reported separately from those affecting multiple systems. When multiple congenital anomalies were present, a detailed description of each major malformation was recorded, as suggested in the ICD-10 Manual for the Surveillance of Congenital Anomalies.
Results
Table 1 shows the antenatal characteristics at diagnosis of the 155 pregnancies (312 MC twins) with major discordant structural anomalies included in the study (139 affecting one system and 16 affecting multiple systems), followed by perinatal outcome. Median gestational age (GA) at diagnosis was 19.1 weeks (IQR 16.4–21.3), with 88% of cases identified before 22 weeks’ gestation. Karyotyping was performed in 94 (61%) cases. In the other cases, the women chose not to perform amniocentesis either because of advanced gestational age at diagnosis, out of personal choice, or to avoid the possible risks of complications for the co-twin.
TABLE 1 Antenatal Characteristics at Diagnosis and Perinatal Outcome in 155 MC Pregnancies (312 MC Twins) Complicated with Major Discordant Structural Anomaly

Note: MC = monochorionic, DC = dichorionic, IVF = in vitro fertilization, GA = gestational age, IUFD = intrauterine fetal death, sIUGR = selective intrauterine growth restriction, BCC = bipolar cord coagulation, TOP = termination of pregnancy. Values are indicated with number (n) and percentage in brackets, or with median and interquartile range (IQR) in brackets.
a n 119 pregnancies with at least one live birth monochorionic twin.
b n two monochorionic triamniotic pregnancies had two normal live birth co-twins.
Major discordant structural anomalies were associated in 41 (26%) cases with sIUGR, and in 8 (5%) cases with TTTS. IUFD of both twins occurred in 11 pregnancies (7%). In 5 cases (3%), single IUFD of the normal twin occurred and five anomalous co-twins were delivered. Overall, 55 (35%) pregnancies were managed by BCC of the anomalous twin. Co-twin loss subsequent to this procedure occurred in eight cases (5%). One pregnancy miscarried, while 16 (10%) were terminated. A total of 72 anomalous twins and 116 normal co-twins (two MC TA pregnancies had two normal co-twins) were delivered at a median GA of 34.6 weeks (IQR 31.0-36.3). A total of 22 twins (14%) with structural anomalies died in the neonatal/infant period, with an overall survival rate of 32% (50/155). Normal co-twin survival rate was 71% (111/157).
Table 2 is a detailed description of the number and type of major discordant structural anomalies affecting one system (139/155, 90%) coded according to the ICD-10 classification. Of these, 30 were lethal or potentially lethal. The anomalies occurring in one of the twins of MC DA (112), MC TA (2), and DC TA (6) pregnancies are listed in Group 1; the anomalies occurring in MC MA pregnancies (19) are listed in Group 2. Table 2 also shows the number of cases ending in IUFD, TOP, or miscarriage, and the number of cases managed with BCC or FLS. The postnatal outcome of the anomalous twins and of the normal co-twins (in brackets) is reported, and the major associated anomalies detected only after birth are also described.
TABLE 2 Major Discordant Structural Anomalies Affecting One System (N 139) Classified According to the ICD-10
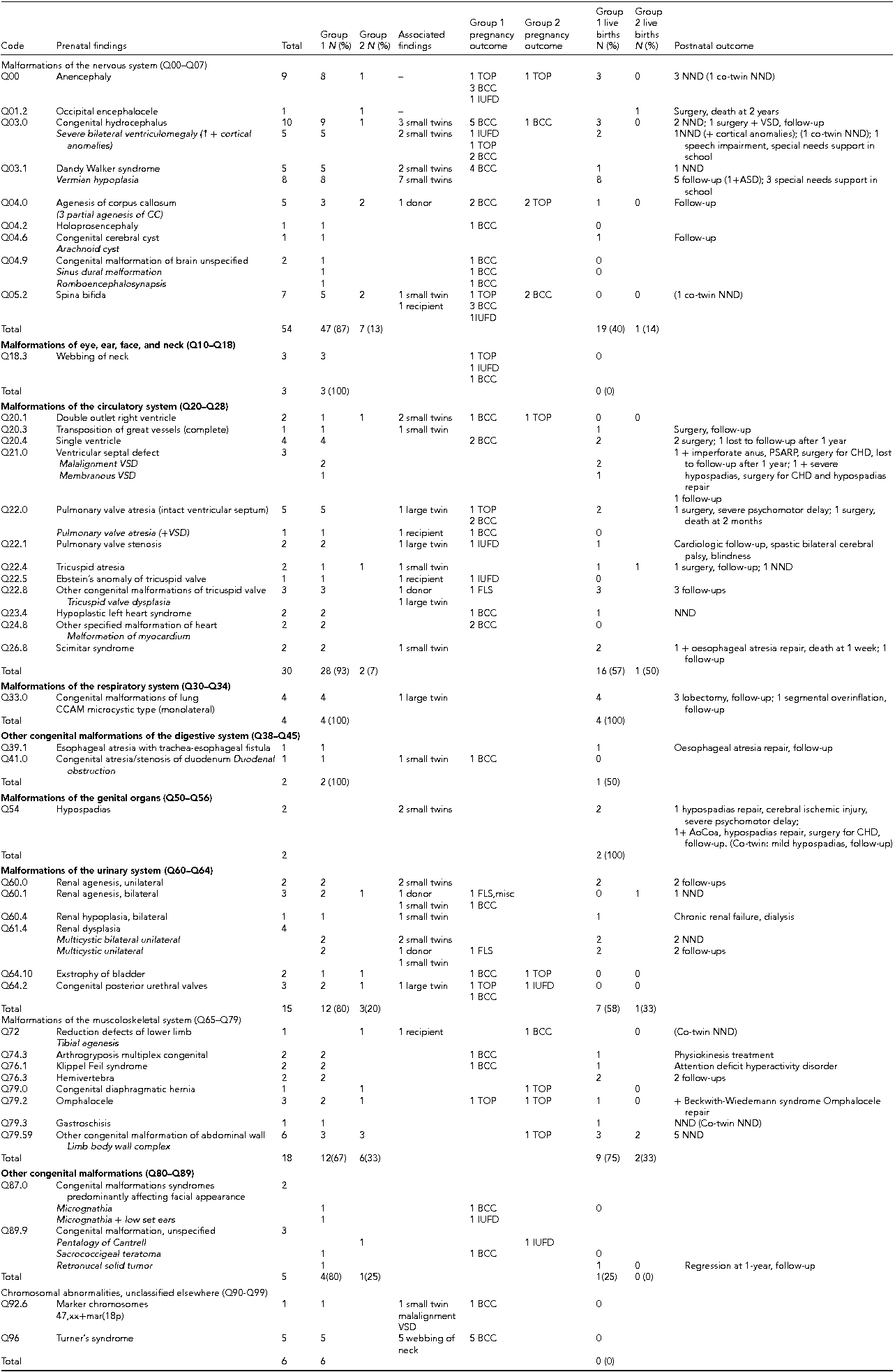
Note: Group 1 = number of anomalous twins in MC diamniotic, MC triamniotic, dichorionic triamniotic pregnancies; Group 2 = number of anomalous twins in MC mono amniotic pregnancies. Small twin/Large twin = anomalous twin of MC pregnancy with selective intrauterine growth restriction; D = donor; R = recipient: anomalous twin of MC pregnancy with TTTS; NND = neonatal death; TOP = termination of pregnancy; BCC = bipolar cord coagulation; IUFD = intrauterine fetal death; FLS = fetoscopic laser surgery; VSD = ventricular septal defect; PSARP = posterior sagittal anorectoplasty. Data are expressed as N (number) and percentage in brackets, and refer to the anomalous MC twins. Postnatal outcome of the normal co-twin is reported in brackets.
Q00 = 1 pregnancy MC TA; Q22.0 = 1 pregnancy MC TA; Q03.0 = Severe bilateral ventriculomegaly: ventricular size ≥15 mm; Q21.0 = VSD, ventricular septal defect 3.0 CCAM = congenital cystic adenomatoid malformation of the lung; Q60.1 = FLS, fetoscopic laser surgery; misc = miscarriage; Q79.2 = 2 large omphalocele, 1 small omphalocele.
The most frequent anomalies here were those of the nervous system (54 cases, 39%) and circulatory system (30 cases, 22%), followed by the musculoskeletal (13%) and urinary system (11%).
The family's choice to opt for BCC was more likely for anomalies of the nervous system (25/54 cases, 46%), or the circulatory system (9/30, 30%), or chromosomal anomalies (6/6, 100%), all of which were identified on the basis of anomalous ultrasound findings. Of the eight pregnancies with TTTS, four anomalies affected the donor (ICD codes Q04.0, Q22.8, Q60.1, and Q61.4) and four the recipient (codes Q05.2, Q22.0, Q22.5, and Q72). Of the 64 live births with discordant anomalies affecting one system (64/139, 46%), 20 died immediately after birth or within 2 months, due to the severity of the malformation (codes Q00, Q03.0, Q22.0, Q22.4, Q23.4, and Q79.59). One twin died aged 2 years (code Q01.2). None of the 19 MC MA anomalous twins survived: 14 were lost in utero (eight TOP, four BCC, two IUFD), and five died at birth.
Of the 43 one-system twins surviving infancy, 15 (35%) underwent surgery. Four children with anomalies of the nervous system (codes Q03.0 and Q03.1) needed educational support at school age. There were two children with heart anomalies (codes Q22.0 and Q22.1) born at 33 and 34 weeks, respectively: one had severe psychomotor delay and the other was affected with spastic bilateral cerebral palsy. Of the two cases with hypospadias (code Q54), one born at 30 weeks (birth weight 1485 g) suffered ischemic cerebral injury at birth and has severe psychomotor delay. The other was also diagnosed postnatally with mild aortic coarctation and underwent surgery at 3 months. The child with bilateral renal hypoplasia (code Q60.4) has chronic renal failure and is in dialysis at 6 years of age. The infant with Klippel Feil syndrome (code Q76.1) suffers from hyperactive attention deficit disorder, while the twin born with small omphalocele (code Q79.2) was found to have Beckwith–Wiedemann syndrome and is in specific follow-up. In this cohort of cases of congenital anomaly affecting one system only, severe neurodevelopmental disability was detected in 3 out of 43 surviving twins (7%).
Table 3 describes the 16 cases (16/155, 10%) of major discordant structural anomalies affecting multiple systems, occurring in 15 MC DA and in 1 DC TA pregnancy. All 16 had normal conventional karyotyping/aCGH, performed prenatally in 13 cases and postnatally in 3 cases. Of the eight live births (50%), one died in the neonatal period. The seven surviving twins had surgery or multiple surgeries. One of the seven (14%) who was delivered at 30 weeks suffers severe psychomotor delay.
TABLE 3 Major Discordant Structural Anomalies Affecting Multiple Systems (N 16)

Note: VSD = ventricular septal defect, CC = corpus callosum, CHD = congenital heart disease, MCK = multicystic kidney, OE = esophageal atresia, IUFD = intrauterine fetal death, BCC = bipolar cord coagulation, TOP = termination of pregnancy, PSARP = posterior sagittal anorectoplasty. Small twin = anomalous twin of monochorionic pregnancy with selective intrauterine growth restriction. Data are expressed as number (N) and percentage in brackets and refer to the anomalous twin. Postnatal outcome of the normal co-twin is reported in brackets.
Regarding the outcome of the normal MC co-twins, the overall survival rate was 71% (111/157), with generally satisfactory follow-up. The five neonatal deaths occurred in twins delivered before 27 weeks. Of the seven independent twins delivered, two died because of necrotizing enterocolitis in course of sepsis, while the other five are doing well.
Discussion
This retrospective study was designed to provide a detailed description of the spectrum of major discordant structural anomalies observed prenatally in a large cohort of MC twins, including the management of these pregnancies and the outcome of the twins. Any analysis of the genetic mechanisms allegedly responsible for these discordances was beyond the scope of the study, as was any estimate of prevalence, since the entrance requirement for the study was the discordant anomaly.
Careful and accurate identification and classification of congenital anomalies is crucial, not only to establish the prevalence of malformations, but also to minimize the subjectivity of reports, make inter-center comparisons possible, and simplify data analysis. However, classifying the entire range of congenital anomalies represents an enormous challenge that only increases in the presence of rare and complex malformations or anomalies involving multiple systems. For the purposes of this study, we decided to adopt the ICD-10 system, currently used worldwide for postnatal disease classification, despite its reported limitations — that is, the lack of accuracy for coding certain anomalies (mainly those of the circulatory and nervous systems), and lack of inclusion of all malformations (Metcalfe et al., Reference Metcalfe, Sibbald, Lowry, Tough and Bernier2014). Despite these limitations, the code sets did enable us to bring together a vast spectrum of different malformations observed prenatally and made interpreting our data much easier.
Despite its inherent limitations with our center being a third-level facility, this study shows that the most common severe anomalies involve the nervous, circulatory, musculoskeletal, and multiple systems. Other studies have described discordant anomalies in twins, but they were not subdivided for chorionicity (Fernandes et al., Reference Fernandes, Carvalho, Flosi and Baiao2016; Harper et al., Reference Harper, Odibo, Roehl, Longman, Macones and Cahill2012). The few articles that do divide for chorionicity describe fewer cases of discordant anomaly than we do here (Gul et al., Reference Gul, Cebeci, Aslan, Polat, Sozen and Ceylan2005; Linskens et al., Reference Linskens, Elburg, Oepkes, Vugt and Haak2011). One register-based study (Glinianaia et al., Reference Glinianaia, Rankin and Wright2008) and a recent report on MC twins with suspected discordant malformations (which makes no distinction between major abnormalities and minor ultrasound findings such as single umbilical artery, choroid plexus cyst, double renal pelvis, and amniotic band syndrome) confirmed that nervous system, circulatory system, and multiple anomalies are much more common (Peng et al., Reference Peng, Zhou, Xie, Zheng, Xie and Yang2016). In our series, only eight cases of major anomalies (5%) were complicated with TTTS (four affected the donor and four the recipient), since we excluded cases of recipient-twin cardiomyopathy from the study. Interestingly, in the retrospective cohort reported of TTTS pregnancies treated with laser surgery (Patel et al., Reference Patel, Randolph, Benirschke, Llanes, Yedigarova and Chmait2012), the prevalence of non-cardiac anomalies (15 major, 4 minor) in the 377 live births (5%) was higher in donors than in recipients (8.5% vs. 2.0%). In the series described by Kontopoulos et al. (Reference Kantopoulos, Quintero, Salihu, Bornick and Allen2008), the incidence of Dandy–Walker syndrome was 200 times higher in complicated MC twins than in singletons, and the affected twin was the smaller (donor or IUGR) in 8/10 cases. In our cohort, too, of the 41 pregnancies with associated sIUGR, the small twin was affected by a major anomaly in 36/41 cases (88%). This association of congenital structural malformations with intrauterine growth restriction (and the hypoxic environment arising from placental dysfunction) on the one hand, and with TTTS and impaired perfusion in the donor twin on the other, points to a role for hypoxia and/or uneven perfusion in determining congenital anomalies. Although experimental models are non-transferable to humans, studies with animals have shown that hypoxia during pregnancy can induce congenital malformations. The most commonly reported anomalies are limb reduction defects, cleft lip/palate, heart defects, death of neuronal population and cerebral anomalies, and hemorrhage in the external genitalia with subsequent hypospadias (Webster & Abela, Reference Webster and Abela2007).
Since only 6% of women in our series underwent IVF, this small number of cases makes it impossible to assess a possible statistical correlation between IVF and specific anomalies.
A discordant abnormal karyotype in association with major structural anomalies was found in 6 of the 94 twins (6%) undergoing invasive prenatal diagnosis. In five of these six cases, the anomaly was Turner's syndrome, confirming previous observations indicating that monosomy X is the most frequent heterokaryotypia in MZ twins (Gringas & Chen, Reference Gringras and Chen2001). With all the limits arising from a population examined after 14 weeks’ gestation, which makes the loss of affected fetuses likely before referral, we found no cases of trisomy 21 or of discordant copy number variations that might have caused the phenotypic discordance, which is an interesting but still unresolved issue (Shi et al., Reference Shi, Li, Huang, Chen, Zhou and Fang2017; Veenma et al., Reference Veenma, Brosens, de Jong, van de Ven, Meeussen, Cohen-Overbeek and de Klein2012).
Family choices and pregnancy outcomes were strongly influenced by the kind of anomaly affecting the twin and the type of MC pregnancy (whether monoamnotic or diamniotic). More than one-third of women requested selective feticide, either to avoid the potential risk of in utero fetal death following conservative management (and the risk of demise or severe morbidity in the co-twin), or to avoid the risk of a live-birth twin with major defects. Eight of the 16 terminated pregnancies were monoamniotic, a condition that presents more technical difficulties and additional risks than a diamniotic pregnancy when performing selective feticide (Valsky et al., Reference Valsky, Martinez-Serrano, Sanz, Eixarch, Acosta, Martinez and Gratacos2011). Furthemore, the combination of a major structural anomaly (even when non-lethal and potentially correctable postnatally) with selective IUGR was perceived by some parents as being too much to bear, and swayed parental decision making in the favor of selective feticide or termination. Parents’ concerns about the anomalous twin, together with the inherent possible complications of MC pregnancies, such as spontaneous double or single fetal loss, or co-twin demise after cord occlusion, resulted in less than 50% of anomalous fetuses being born alive. All these aspects, together with the rarity of the discordant anomaly in MC twins, limit clinicians’ ability to inform parents of the potential course and outcome in a particular malformation. This limitation could probably be overcome by combining the data from large centers to compile larger cases series for specific anomaly.
In total, 12 out of 22 neonatal deaths (54%) were caused by lethal anomalies in conservative management, while the remainder was due to prematurity. Overall, of the 50 surviving infants, 22 (44%) underwent major surgery or multiple surgeries. Four survivors (8%, three of whom had surgery) now suffer from severe neurologic morbidity. The vast majority of normal co-twin survivors had a good outcome and are free from neurologic morbidity.
In conclusion, this study shows that a wide spectrum of major discordant structural anomalies can occur in MC pregnancies, and operators involved in the care of these pregnancies should be aware of this. Parents are faced with complex decisions regarding the fate of the anomalous twin and the course of the pregnancy. While around half of the survivors with structural anomalies did not undergo surgery or suffer severe neurologic morbidity, many of these infants are still in follow-up and will need long-term care from their families and from health services.





