Clinical Report
Three-dimensional (3D) facial analysis (3DFA) is being increasingly investigated for multiple applications, including for the monitoring of medical therapy of conditions associated with craniofacial anomaly (CFA) and dysmorphology (Baynam et al., Reference Baynam, Walters, Claes, Kung, LeSouef, Dawkins and Goldblatt2013; Kung et al., Reference Kung, Walters, Claes, Goldblatt, Souef and Baynam2013). Craniofacial anomalies are a diverse set of congenital and acquired conditions with a significant individual and societal burden; they are associated with a high incidence of speech, hearing, oral function, appearance, and social morbidity (Leopold & Rhodes, Reference Leopold and Rhodes2010; Mosey, Reference Mosey2004). The treatment of CFA, preferably monitored in a non-invasive manner, can ameliorate this morbidity. Congenital vascular anomalies affect 1.5% of the general population (Eifert et al., Reference Eifert, Villavicencio, Kao, Taute and Rich2000) and they may be (sub)cutaneous and facial. There are multiple treatment modalities available for these conditions and they include drugs that modify angiogenesis; these include mTOR pathway inhibitors such as rapamycin (Phung et al., Reference Phung, Ziv, Dabydeen, Eyiah-Mensah, Riveros, Perruzzi and Benjamin2006). More broadly, activating mutations in the mTOR, and interacting, pathways have been associated with developmental brain anomalies and neurocognitive disorders including tuberous sclerosis complex (TSC) (Crino, Reference Crino2011). Accordingly, topical rapamycin has been used for the treatment of facial angiofibromas in children with TSC (Foster et al., Reference Foster, Bint and Halbert2012) and a further inhibitor of the mTOR pathway, everolimus, has been successfully trialed for an internal organ complication of this condition (Jozwiak et al., Reference Jozwiak, Stein and Kotulska2012). In this report, we describe the first assessment by 3DFA of the treatment response of a head and neck lymphatic vascular malformation to an mTOR inhibitor. This provides further supportive evidence for the potential utility of 3DFA as a non-invasive, non-irradiating, and relatively inexpensive technique for monitoring some medical therapies. It might also provide further perspectives for the investigation of disease biology.
An 11-year-old girl presented with extensive predominantly microcystic lymphatic vascular malformation involving the left cheek, lower lip, tongue, floor of mouth, laryngeal inlet, and extending inferiorly to the level of the thoracic inlet. In infancy and early childhood, she had a series of intralesional OK432 injections with moderate improvement. However, she continued to have significant episodes of hemorrhage from the tongue and was suffering socially from her abnormal facial appearance. After an encouraging publication by Hammill et al. (Reference Hammill, Wentzel, Gupta, Nelson, Lucky, Elluru and Adams2011) on the use of oral mTOR inhibitors for lymphatic malformations, she was commenced on oral sirolimus 0.8 mg/m2 at twelve hourly intervals. Her initial dose of 1 mg bd was gradually adjusted to 2 mg bd to obtain a trough sirolimus level of 10–15 ng/mL. Her full blood picture, electrolytes, liver function, fasting lipids, blood pressure, and growth were closely monitored. 3DFA was performed at baseline, 3 months and 6 months. Subtle improvement was noted clinically within the first 3 months and was demonstrated at the 3-month 3DFA. A repeat MRI scan was performed after 6 months of the treatment, which showed a corresponding reduction in the size of the larger cysts.
Materials and Methods
Facial scans were performed using the 3dMD Facial system (3dMD Pty Ltd, Atlanta, Georgia, USA) with the subject sitting with her head held in a natural head position. Scans were performed prior to the commencement of treatment (T0), and 3 (T1) and 6 months after the commencement of treatment (T2). The raw scan data are cleaned of superfluous surfaces prior to processing with a purpose-built anthropometric mask (AM; Claes et al., Reference Claes, Walters and Clement2012b). The AM is comprised of a spatially dense set of quasi-landmarks; this mask is fitted to the cleaned raw scan data using a set of nested fitting algorithms. This effectively anneals the floating AM to the target 3D scan to establish a manifold representing a subject's face that comprises a discrete set of spatially dense corresponding 3D quasi-landmarks, which can be used in geometric morphometric analysis. Subsequently, facial discordance can be objectively measured with a robust superimposition of processed scans of a subject's face at different treatment time points. The proportion of the face that is discordant can be summarized as percentage scores of relative significant discordance (RSD%), where thresholds can be either arbitrarily set in terms of magnitude (millimeters) or on statistical confidence limits based on the distribution of the found discrepancies. These statistical outcomes can be visualized by the production of a facial histogram illustrating regional facial discordances. As a measure of the severity of the discordance, the distribution of discrepancies can be summarized in root mean squared error (RMSE) score (Claes et al., Reference Claes, Walters and Clement2012b).
In addition to quantifying facial discordance between time points, a measure of facial harmony was made at baseline and at conclusion of the current treatment cycle utilizing dysmorphometrics. In this case, the quasi-landmark set of the mapped patient scan is subject to a robust statistical superimposition to a quasi-landmark variance/covariance model (Claes et al., Reference Claes, Daniel, Walters, Clement, Vandermeulen and Suetens2012a) derived from a reference set of facial scans from healthy subjects. This superimposition simultaneously detects the harmonious (within range) and disharmonious (outside range) quasi-landmarks. The outside range quasi-landmarks can be substituted by harmonious quasi-landmarks that are determined by the covariance matrix of the reference model. This enables the production of a harmonious counterpart to the patient face, termed a ‘normal equivalent’ (NE), providing a patient-specific control (Claes et al., Reference Claes, Walters, Gillett, Vandermeulen, Clement and Suetens2013).
Results
The facial harmony assessments (dysmorphometrics; normal equivalent/patient presentation superimpositions) clearly demarcated facial swellings associated with the lymphatic vascular malformation (Figure 1). Consistent with the clinical presentation (Figure 2), the distance dysmorphogram demarcated the extent of the swelling in the left cheek and lower lip, in particular.

FIGURE 1 Frontal, profile and bird's eye views of the 3D distance dysmorphogram (scale bar 0–5 mm) at T0 pre-treatment. Note. Dysmorphology is clearly demarcated with distinctive boundaries that may be reflective of underlying compartmentalization of the face by facial retaining ligaments.

FIGURE 2 Profile views of texture map (digital photograph component of 3D image) of T0 pre-treatment (A) and T2 post-treatment review (B). Note. Subtle differences can be observed with reduction in the swelling of the lips and improved facial definition.
While the minor amelioration of the anomaly was appreciated clinically, the detail of the changes was revealed in the superimpositions of mapped faces at sequential time points in the treatment cycle (Figure 3). The initial post-treatment review is illustrated as distance maps of quasi-landmark discordances (Figure 3A); the reduction of swelling was highly localized in the region of the lips and left nasolabial folds. Further reductions were recorded at the second review time point; however, there was a wider facial distribution of these reductions. These outcomes were also reflected in the summary statistics (Table 1) of quasi-landmark discordances with comparative increases in the relative discordance ratio (RSD%) and RMSE.

FIGURE 3 Frontal, bird's eye and profile views of distance maps of quasi-landmark discordance of the superimposition of pre-treatment and review time points (A. T0–T1; B. T1–T2; C. T0–T2). Note. There are highly localized differences between pre-treatment and 3 months post-treatment (T1). More global changes were observed between T1 and T2 (6 months).
TABLE 1 Summary Statistics of Quasi-Landmark Discordance Distributions Following the Robust Superimposition of Scans at Sequential Time Points
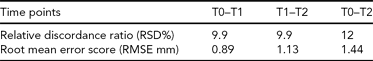
Note: TO (baseline/pre-treatment)–T1 (3 months), T1–T2 (6 months), and T0–T2. The overall proportion of face that is discordant (RSD%) for T0–T1 and T1–T2 is similar at 9.9%. However, visual inspection of the superimposed scans reveals that different facial regions are involved. The RSD% at T0–T2 is 12%. The reduction in severity of the craniofacial anomaly is consistent with the sequential increase in RMSE of the superimposed scans.
Discussion
We describe the first instance of 3DFA assessment of the response of a head and neck lymphatic vascular malformation to mTOR inhibitor therapy. Specifically, using methods that harmoniously assess facial form in an individualized manner, we objectively demonstrate the extent and the reduction in the degree of facial anomaly (dysmorphology) localized to the clinical site of the malformation, in concordance with subjective expert clinical assessment. Notably, while there was a clear subjective improvement of the lesion, as assessed by the treating physician and this child's parent, the degree of improvement was felt to be modest. This might support that the resolution of our approach will make it suitable for ascertaining milder, as well as more marked, degrees of therapeutic response. Also, the apparent, and almost circumscribed, distribution of the alteration of facial surface morphology may provide new knowledge about the architecture of lymphovascular compartments.
A proof of concept study for the use 3DFA for non-invasive treatment monitoring has been reported (Kung et al., Reference Kung, Walters, Claes, Goldblatt, Souef and Baynam2013). This study's findings give further grounds to support additional investigation of this technique. If the prospective utility of this approach is validated, then in selected circumstances it might be considered as a supplement to or, at least a partial, replacement for other more costly imaging modalities. The portability of the imaging equipment might also facilitate assessments in regional or remote areas. In addition to monitoring apparently isolated facial vascular anomalies, first-tier candidates for this approach include genetic metabolic conditions with currently available therapies. Second-tier applications may include as a secondary outcome measure in clinical trials of novel or repurposed drugs for conditions associated with facial dysmorphology. It is notable that the mTOR pathway interacts with the RAS-MAPK pathway (Cargnello & Roux, Reference Cargnello and Roux2011) and that the RASopathies are a group of conditions that are associated with mutations in this network. Clinical trials, using repurposed oncotherapeutics, are being considered for this class of disorders; however, the lack of objective endpoints remains a challenge. 3DFA might offer one approach.
A further consideration is that the disruption of the mTOR pathway has been associated with neurocognitive disorders including autism (Crino, Reference Crino2011) and 3DFA has demonstrated patterns of facial (and brain) variation in autistic spectrum disorders (Hammond et al., Reference Hammond, Forster-Gibson, Chudley, Allanson, Hutton, Farrell and Lewis2008). It is possible that complementary insights into neurocognitive biology may be provided by 3DFA of the mTOR therapy response of both vascular anomalies and neurobehavioral phenotypes. Also, analogous to face–brain studies in autism, further perspectives may be obtained by paired 3DFA–neuroimaging studies.
In conclusion, this report supports the treatment monitoring potential of 3DF and the assessment of its utility will require further study.
Acknowledgments
We thank the child and her parents for allowing us to use her data to publish this report and acknowledge RD-Connect as a 3D facial analysis facilitating collaboration. Foundations for this work were provided via grants from Genzyme corporation and the Princess Margaret Hospital for Children Foundation.






