Trisomy 21 is associated with increased risks of miscarriage, fetal growth restriction, preterm delivery, and fetal demise in both single and multiple pregnancies. It is the most common aneuploidy detected in singleton pregnancies.
The management of twin pregnancies discordant for fetal abnormality is dependent on the lethality of the anomalies. If the condition is lethal it is preferable to avoid the risks of selective reduction unless the condition has the potential to compromise the co-twin. Anencephaly, with the potential for polyhydramnios and subsequent preterm delivery, is the most common situation in which selective reduction is indicated for a lethal anomaly (Sebire et al., Reference Sebire, Sepulveda, Hughes, Noble and Nicolaides1997).
Pregnancies that are concordant for trisomy 21 are almost always terminated (Sebire et al., Reference Sebire, Sepulveda, Hughes, Noble and Nicolaides1997). There is limited data on the outcome of pregnancies discordant for trisomy 21 that continue. The purpose of this study is to review 10 years’ experience and review the clinical outcomes in these pregnancies.
Materials and Methods
The Royal Women's Hospital (RWH), Melbourne, is a tertiary referral center with a dedicated multiple pregnancy clinic providing care to over 150 sets of twins per annum. The majority of twin pregnancies are seen early enough in pregnancy to allow a detailed 12-week ultrasound examination. The frequency of subsequent ultrasounds is dependent on chorionicity. Monochorionic twins are assessed every two weeks from 16-week gestation and dichorionic twins every four weeks from 20-week gestation.
Aneuploidy screening is with either nuchal translucency alone or with combined first trimester maternal serum screening and nuchal translucency, depending on the gestation at which the patient is seen. Second trimester maternal serum screening is not utilized because of its very low sensitivity for trisomy 21 detection. Diagnostic testing is offered based on high-risk first trimester screening, fetal anomaly, or advanced maternal age.
Cases were identified by comprehensive search of the computerized ultrasound database, reviewing karyotype results from the genetics department, and by an exhaustive search of the discharge summary for every twin pregnancy managed at the hospital between January 2000 and December 2011.
Results
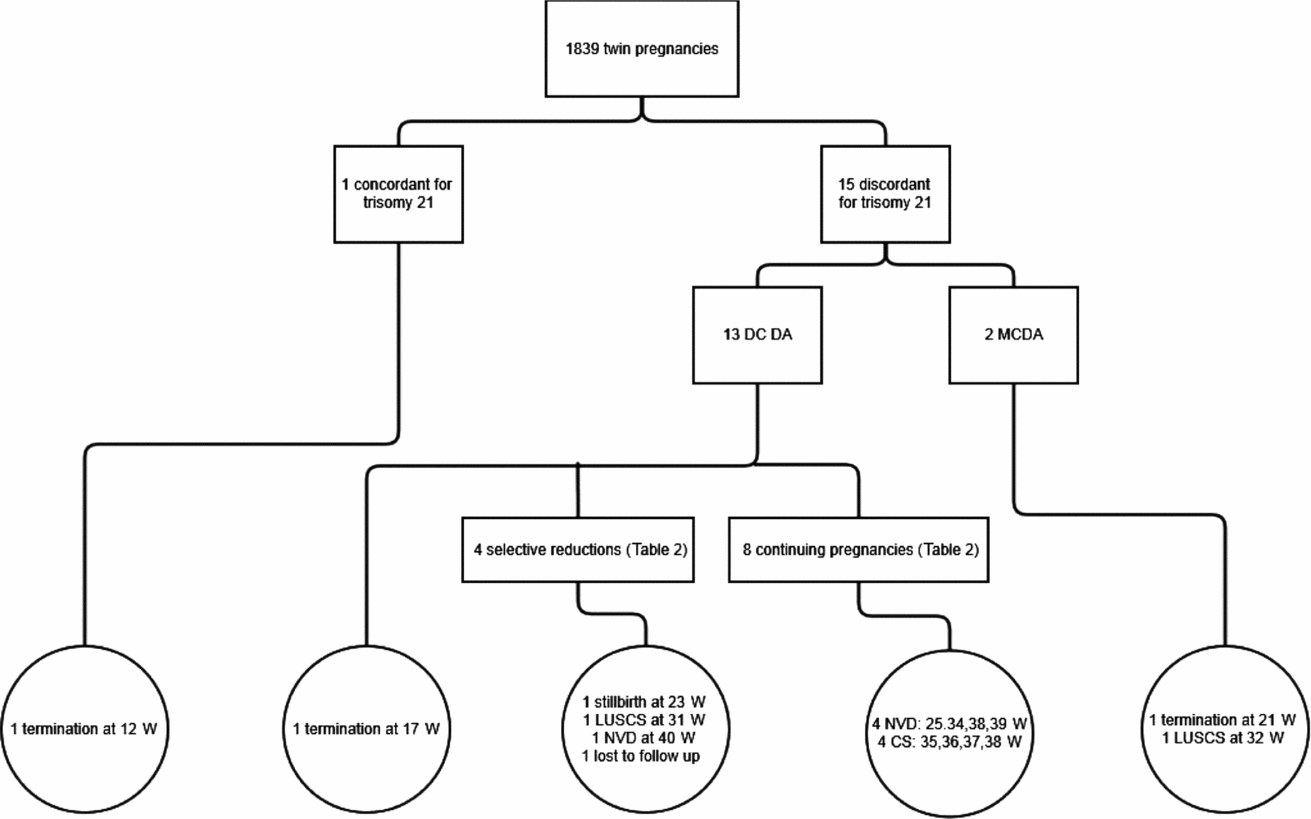
In the 12-year period, there were 1,839 twin pregnancies managed at RWH. Sixteen cases of trisomy 21 were identified. The course of these 16 pregnancies is detailed in Figure 1. One of these cases was a set of monochorionic twins with nuchal translucencies of 2.7 mm and 2.6 mm in a 40-year-old woman. This gave a trisomy 21 risk of 1 in 20 for each fetus. A chorionic villus sampling was performed and revealed trisomy 21. The entire pregnancy was terminated at 12+6 weeks by suction curettage.

FIGURE 1 Outcome of pregnancies diagnosed with trisomy 21.
Note: DC DA = dichorionic diamniotic twins; MC DA = monochorionic diamniotic twins; W = weeks’ gestation; LUSCS = lower uterine segment caesarean section; NVD = normal vaginal delivery; CS = caesarean section.
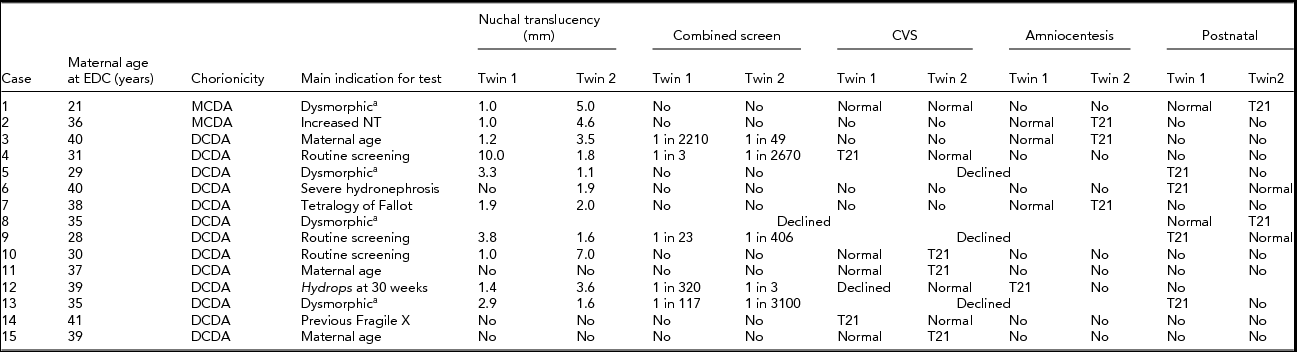
Fifteen cases of discordant twins remained available for evaluation. Table 1 details maternal age at the time of delivery, the timing of testing, and the method of diagnosis of trisomy 21. Results for nuchal translucency, combined first trimester maternal serum screening with nuchal translucency, chorion villus sampling, and amniocentesis results are detailed where available.
TABLE 1 Screening and Testing for Trisomy 21

aAt delivery.
Note: T21 = trisomy 21; CVS = chorion villus sampling; NT = nuchal translucency; DCDA = dichorionic diamniotic; MCDA = monochorionic diamniotic.
Two of the 15 pregnancies were monochorionic diamniotic (MCDA) twins and 13 were dichorionic diamniotic (DCDA) twins.
The first MCDA twin pregnancy was in a 21-year-old gravida 3 para 2. Discordant nuchal translucencies of 1.0 mm and 5.0 mm were identified at 12-week gestation. A CVS was performed and revealed a karyotype of 46,XY. Fortnightly, ultrasounds were performed with the risk of twin-twin transfusion syndrome (TTTS) being considered significant. No sign of TTTS developed and the pregnancy remained uncomplicated until the patient presented in premature labor at 32-week gestation. Steroids were administered but labor suppression failed and an emergency cesarean section was performed at 32+1 weeks gestation for suspected fetal compromise. The twins were born with birth weights of 2,202 grams and 1,745 grams respectively. The smaller second twin appeared dysmorphic. Blood samples were taken from both twins and showed 15% mosaicism for trisomy 21 in each baby. This was a consequence of their shared monochorionic circulation. Cheek and skin biopsies confirmed 47,XY,+21 in the second twin only. The first twin's karyotype was 46,XY. Both twins were admitted to the neonatal nursery. They had an uneventful stay and other than dysmorphic features; twin 2 had no other features of trisomy 21. Extensive genetic counseling confirmed post-zygotic non-dysjunction as the cause for discordancy in this monozygotic pair. Placental histology confirmed MCDA twin placentation.
The second MCDA twin pregnancy was in a 37-year-old primigravid patient with discordant nuchal translucencies of 1.0 mm and 4.6 mm at 13+6 weeks gestation. Because of the experience described above, amniocentesis was advised but was declined. Combined first trimester serum screening and nuchal translucency measurements gave 1:3,750 and 1:35 risks of trisomy 21 respectively. The patient was advised of the risks of fetuses being discordant for aneuploidy, the high risk of developing TTTS, and a small risk of discordant structural anomalies. Serial ultrasounds were performed and, at 19-week gestation, twin 2, the fetus with the increased nuchal translucency, had severe ventriculomegaly of 15 mm with a dilated third ventricle but no signs of TTTS. Amniocentesis was agreed to at that time and revealed 46,XY and 47,XY+21 respectively. While awaiting the results of the full karyotype, the patient presented with polyhydramnios of the affected twin with normal liquor volume in the other twin and no other signs of TTTS. At that stage she was found to be 2 cm dilated and bleeding. After some consideration she proceeded to termination of pregnancy with prostaglandins. This was performed at 21-week gestation. She was delivered of stillborn male fetuses with birth weights of 310 grams (normal karyotype) and 365 grams (trisomy 21 affected fetus). Histology confirmed MCDA placentation. Autopsies were declined.
Thirteen DCDA twin pregnancies were analyzed. The mean maternal age at the time of delivery in this group was 35.5 years (SD 1.3) with a range of 28 to 41 years.
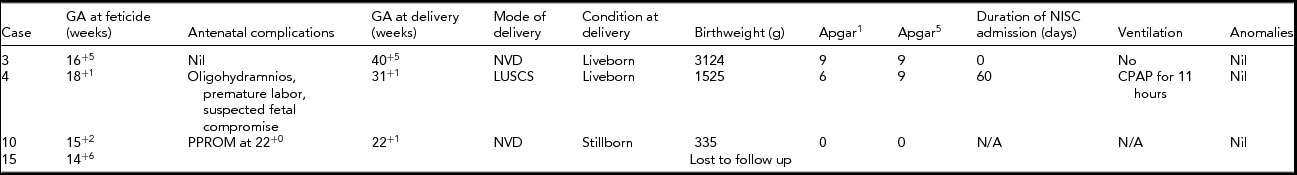
Four selective terminations were performed between 14- and 18-week gestation. The outcomes of these pregnancies are detailed in Table 2. One patient had a selective termination at 14+6 weeks gestation. She returned to Hong Kong for continuing pregnancy care immediately after the termination and was lost to follow-up.
TABLE 2 Outcome for the Non-Affected Twin After Feticide

GA = gestational age; NVD = normal vaginal delivery; LUSCS = lower uterine segment caesarean section; NISC = Neonatal Intensive and Special Care Nursery; CPAP = continuous positive airway pressure; PPROM = preterm prelabor rupture of membranes.
One DCDA twin pregnancy was terminated in its entirety at 17-week gestation. A CVS had been performed and confirmed trisomy 21 in one fetus. On presentation for selective reduction, a missed miscarriage of the non-affected fetus was diagnosed. The entire pregnancy was then terminated by prostaglandin induction of labor.
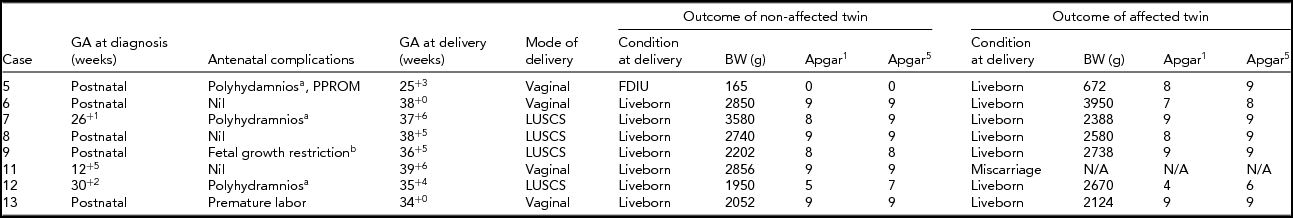
The outcomes of both the non-affected and the trisomy 21 affected twins are detailed in Table 3. In one case there was a full-term normal delivery after a miscarriage of the trisomy 21 fetus at 12+5 weeks. Antenatal complications, which were not limited to the affected fetus, are described.
TABLE 3 Outcomes for Non-Terminated DCDA Twin Pregnancies

aAffected twin.
bNon-affected twin.
Note: GA = gestational age; BW = birth weight; N/A = non-applicable; PPROM = preterm prelabor rupture of membranes; CS = caesarean section; FDIU = fetal death in utero.
One of the non-affected fetuses was an unexplained fetal death in utero (case 5). This pregnancy was complicated by polyhydramnios, preterm prelabor ruptured membranes, and chorioamnionitis in the trisomy 21 affected twin. Fetal demise was diagnosed on presentation in premature labor. Invasive prenatal diagnosis had not been performed. The fetus was macerated at delivery with a birth weight of 165 gram. Postmortem examination was declined and a karyotype was unfortunately not performed.
Case 6 was a DCDA twin pregnancy managed in a general antenatal clinic rather than the Multiple Pregnancy Clinic. An early ultrasound had been arranged by the patient's general practitioner. The crown lump lengths were 86 mm and 79 mm for twin 1 and twin 2 respectively. The crown lump length of twin 1 precluded nuchal translucency risk assessment. The nuchal translucency of twin 2 was 1.9 mm, giving a trisomy 21 risk of 1 in 407. The patient first attended the hospital at 24-week gestation. An ultrasound at that time demonstrated a 12 mm unilateral hydronephrosis for twin 1. This increased to 19 mm at 32 weeks and persisted after delivery. Despite the maternal age of 39 at the time of presentation, and the presence of fetal hydronephrosis, no additional prenatal testing was documented as being discussed with the patient. Down syndrome was diagnosed in twin 1 at delivery.
Respiratory support was required for only one of the non-affected twins (case 4). This pregnancy was complicated by oligohydramnios, premature labor, and suspected fetal compromise requiring an emergency cesarean section at 31+1 weeks after a selective termination had been performed at 18+1 weeks.
Three of the trisomy 21 twins required extended neonatal care: 41 days (case 5), 128 days (case 12), and 32 days (case 13).
None of the non-affected twins had fetal anomalies. Several of the twins with trisomy 21 had anomalies: an inguinal hernia and hydrocoele (case 5), severe unilateral hydronephrosis (case 6), tetralogy of Fallot (case 7), talipes equinovarus (case 9), and a complex cardiac condition with tricuspid regurgitation, patent ductus arteriosus, and chronic pulmonary hypertension leading to demise on day 128 (case 12).
Discussion
Twin pregnancies complicated by trisomy 21 are challenging to manage. The decision to proceed with a selective termination of an affected twin is dependent on many factors. If the fetal abnormality is potentially lethal, parents will generally avoid selective termination to reduce the risks to the non-affected co-twin. However, there are circumstances in which a lethal condition in the affected twin can be potentially threatening to the co-twin. Anencephaly with associated polyhydramnios is the classic example (Sebire et al., Reference Sebire, Sepulveda, Hughes, Noble and Nicolaides1997).
When the condition is not necessarily lethal, classically with trisomy 21, decision-making is more complicated. Parents may elect to terminate the affected twin to avoid the chance of the child being liveborn with the condition. However, it is not always the parents’ wish to avoid a liveborn twin with trisomy 21. Many parents with twin pregnancies have conceived at an advanced maternal age or after extensive infertility treatment. They may either elect to continue with both twins regardless of the condition, or may wish to avoid any risk of losing either one or both of their twins.
In this cohort, in only four of the 15 pregnancies did the parents proceed with selective termination. The outcomes after selective termination in this group were varied. Excluding the patient lost to follow-up, we found that only one of the three selective terminations reached full term. It is not entirely clear that the preterm delivery in case 4 could be attributed to the procedure, but it is highly likely that the perinatal loss in case 10 was related.
The risks associated with selective termination are both operator and gestation dependent. In our cohort, selective termination was performed at a more advanced age than we would normally recommend. This occurred in all of these cases because of parents struggling to make the decision to proceed with selective termination.
The timing of selective termination is critical. Selective termination of monochorionic twins is likely to be associated with a better outcome if it is delayed until after 18-week gestation (Rossi & D'Addario, Reference Rossi and D'Addario2009). This relates to ability to visualize and access the vascular communications or the umbilical cord in these pregnancies.
In dichorionic twin pregnancies the lowest risk is generally associated with the administration of potassium chloride as early as possible. A loss rate for the co-twin is in the order of 5% when it is performed at around 12-week gestation but this can rise to as high as 15–20% if it is performed later in the second trimester (Wimalasundera, Reference Wimalasundera2010).
More controversially, delaying selective termination until well into the third trimester is potentially associated with significantly reduced risk to the normal twin, particularly when a viable gestation has been reached. The difficulty with this approach is two-fold. First, premature delivery may occur before there has been a chance to perform the selective termination. Second, the ethical and potentially legal issues associated with late termination of pregnancy can be significant.
The technique of selective termination is dependent on chorionicity. Monochorionic twins with their shared circulation are not candidates for intracardiac potassium chloride injection, which is the method of choice for dichorionic twins. Fetoscopically guided laser coagulation of the cord, ultrasound guided bipolar diathermy, and ultrasound guided radiofrequency ablation of the cord are all options for monochorionic twins. It is essential to completely interrupt the circulation to avoid damage to the normal co-twin via the vascular communications. The choice of technique varies with the condition, gestation, and operator experience, with more recent evidence suggesting radiofrequency ablation may be the preferred method (Nobili et al., Reference Nobili, Paramasivam and Kumar2013).
In those pregnancies where selective termination was declined because of concern over the risk of fetal loss or because of moral, religious, or ethical reasons, a range of antenatal complications was seen. Polyhydramnios occurred in three of the eight cases. Growth restriction was evident in several cases but just as likely to occur in the non-affected twin as the trisomy 21 affected twin.
This case review highlights the difficulties in prenatal diagnosis of twin pregnancies. Ten of the 16 diagnoses of trisomy 21 were flagged by first trimester screening. Three were diagnosed by a planned chorionic villus sampling: two for advanced maternal age and one for fragile X syndrome. One was diagnosed after amniocentesis for a fetal structural abnormality and the final two were diagnosed postnatally following normal first trimester screening. This highlights that the limitations of first trimester screening apply to twin pregnancies as well as singletons.
In only one of the three monochorionic twin pairs was there discordancy for trisomy 21 despite monozygosity. Even then, concordancy for trisomy 21 was not proven but was deemed highly likely given the nuchal translucency findings. Increased nuchal translucency may identify twin pregnancies at high risk for trisomy 21 but may also flag those with potential fetal structural anomalies. In monozygotic twins, cardiac malformations are significantly more frequent than dizygotic twins and may be reflected by an increased nuchal translucency in the first trimester. A discordant nuchal translucency measurement in monochorionic twins may flag a pregnancy at high risk of developing TTTS although it is of low positive predictive value (Kagan et al., Reference Kagan, Gazzoni, Sepulveda-Gonzalez, Sotiriadis and Nicolaides2007; Lewi et al., Reference Lewi, Gucciardo, van Mieghem, de Koninck, Beck, Medek and Deprest2010).
In one case, the false reassurance received from a normal chorionic villus sampling in monochorionic twins resulted in the diagnosis of trisomy 21 being delayed until the postnatal period. Our institution has subsequently recommended amniocentesis for diagnostic testing in all monochorionic twin pregnancies. This creates the obvious problem of delaying diagnosis, with the associated increase in potential loss of the co-twin. Parents need time to come to terms with making a decision about termination but it is important that they are made aware that delaying the decision, and subsequently the timing of selective termination, increases the risk to the non-affected fetus. As seen in case 2, a second case of discordant trisomy 21 in monochorionic twins was identified by this approach of amniocentesis for monochorionic twins.
This highlights the fact that monozygotic twins are never ‘identical’ (Machin, Reference Machin2009; Umstad et al., Reference Umstad, Short, Wilson and Craig2012). Heterokaryotypia for Turner syndrome, Down syndrome, and Klinefelter syndrome are all rare but have been reported (Rustico et al., Reference Rustico, Baietti, Coviello, Orlandi and Nicolini2005). These probably arise as a consequence of post-zygotic non-dysjunction. Discordance for phenotypic expression of X-linked diseases including Fragile X syndrome and Duchene muscular dystrophy has been attributed to skewed X-chromosome inactivation (Goodship et al., Reference Goodship, Carter and Burn1996). It is for this reason that our institution adopts a fetus-specific risk for both monochorionic and dichorionic twin pregnancies rather than a pregnancy-specific risk. We recognize that this contrasts with recommendations from other groups (Audibert & Gagnon, Reference Audibert and Gagnon2011).
Consideration must also be given to the fact that almost one-third of dichorionic twins are monozygotic. In our hospital, zygosity is, regrettably, not routinely determined by postnatal DNA analysis for twins that are concordant for sex and blood group. In our series, when both of a dichorionic twin pair was liveborn, all were discordant for either sex or blood groups suggesting dizygosity.
Pregnancies that continued without selective termination of the trisomy 21 fetus were frequently complicated. Polyhydramnios affected three of eight pregnancies, four were delivered prior to 37 weeks, and one non-affected twin was a fetal death in utero. Only one of the trisomy 21 affected twins was significantly smaller than its non-affected co-twin, with three of the non-affected twins being contrastingly smaller than the trisomy 21 affected twin. This highlights the importance of careful growth scans on all twin pregnancies, not just those affected by fetal abnormalities. Grouping birth weights into percentile groups for the pregnancies proceeding without intervention saw two of the seven non-affected liveborn twins above the 50th percentile compared with three of the seven affected liveborn twins (Roberts & Lancaster, Reference Roberts and Lancaster1999). The majority of the non-affected twins were in the 25th to 50th percentile range: four of seven versus one of seven in the affected group. One of the non-affected and three of the affected twins were in the 10th to 25th percentile range.
The mean gestation at delivery of pregnancies proceeding without selective termination was 35.5 weeks. This is identical to non-affected, dichorionic twin pregnancies delivered in our institution. However, the complication rates of preterm delivery and death of the non-affected co-twin in our series was higher than in other published series (Alvarado et al., Reference Alvarado, Pacheco, Alderete, Luis, de la Cruz and Quintana2012; Nobili et al., Reference Nobili, Paramasivam and Kumar2013) possibly reflecting our smaller numbers.
Counseling of women with a twin affected by trisomy 21 involves an explanation of chorionicity and zygosity, discussion of the possibility of discordancy for aneuploidy even in monochorionic twins, risks of fetal loss after chorionic villus sampling or amniocentesis, options for selective termination and the techniques and risks involved, and the possibility of antenatal complications including fetal death, growth restriction, polyhydramnios, and preterm delivery.
In conclusion, it is important to realize that monochorionic twins may be discordant for either abnormality or aneuploidy. Pregnancies that continue with one of the twins affected by trisomy 21 are at risk of polyhydramnios and preterm delivery.