Twin pregnancies are increasing in incidence in developed countries, partly due to the increased use of artificial reproductive technologies and, to a lesser extent, due to the increase in mean maternal age (Blondel et al., Reference Blondel, Kogan, Alexander, Dattani, Kramer, Macfarlane and Wen2002). Twin pregnancies have higher perinatal mortality and morbidity rates than singletons (Amaru et al., Reference Amaru, Bush, Berkowitz, Lapinski and Gaddipati2004; Hawrylyshyn et al., Reference Hawrylyshyn, Barkin, Bernstein and Papsin1982; Spellacy et al., Reference Spellacy, Handler and Ferre1990) and this is largely associated with the increased rates of preterm birth and delivery of small for gestational age (SGA) babies (Ghai & Vidyasagar, Reference Ghai and Vidyasagar1988; Kiely, Reference Kiely1990). Reports on the risk of adverse perinatal outcomes attributable to intrauterine growth restriction (IUGR) in twins have been conflicting, and there is no consensus about the contribution of prematurity to morbidity and mortality (Baker et al., Reference Baker, Beach, Craigo, Harvey-Wilkes and D'Alton1997; Buekens & Wilcox, Reference Buekens and Wilcox1993; Kilpatrick et al., Reference Kilpatrick, Jackson and Croughan-Minihane1996; Yinon et al., Reference Yinon, Mazkereth, Rosentzweig, Jarus-Hakak, Schiff and Simchen2005).
The aim of this study was to evaluate the perinatal outcomes of an IUGR pre-term twins group compared with a matched group of normal growth twins of the same gestational age in order to assess whether prematurity or fetal condition is the main risk factor associated with higher morbidity and mortality.
Subjects and Methods
Perinatal outcomes in a group of 108 IUGR preterm twins (from 24th to 36th week of gestation) were evaluated and compared with a group of 103 normal growth twins, matched for gestational age at delivery, included as the next normal growth twin born. All deliveries were attended at the Virgen de las Nieves University Hospital, Granada, Spain, between January 2003 and June 2011. The local ethics committee approved the study design.
We defined IUGR as birth weight below the 10th centile for gestational age, according to specific growth charts for twins (Voigt et al., Reference Voigt, Schneider and Jährig1996). Pregnancies with major fetal anomalies, aneuploidies, or findings consistent with twin-to-twin transfusion syndrome were excluded.
Maternal characteristics (age and parity), pregnancy data (chorionicity, antenatal steroid therapy, and gestational age at delivery), and delivery data (route of delivery, birth order, and birth weight) were compared between the two groups in order to consider possible confounding variables. Gestational age was assessed during the first trimester of pregnancy by considering the date of the last menstrual period, the date of ovum pick-up in in vitro fertilization cases and first trimester ultrasound biometry. Chorionicity was also assessed by first trimester ultrasound and confirmed with post-delivery placental examination.
The primary outcome for the study was perinatal mortality rate (PMR), defined as stillbirths or neonatal deaths occurring within 4 weeks of delivery. Secondary perinatal outcomes evaluated included rates of low Apgar score (below 5 at 1 minute or below 7 at 5 minutes), admission to the neonatal intensive care unit (NICU), respiratory distress syndrome (RDS), intraventricular hemorrhage (IVH), clinical sepsis, jaundice (serum bilirubin > 15 mg/dl), hematologic disorder (anemia as serum hemoglobin < 10 g/dl, thrombocytopenia as platelet count < 150,000/μl, or polycythemia as hematocrit > 60%), metabolic disorder (hypoglycemia as serum glucose < 40 mg/dl, hypocalcaemia as serum ionic calcium < 1 mmol/L), and neurologic sequelae (as any kind of psychomotor impairment or sensorial deficit). Furthermore, the rate of survival with any neonatal complication or sequelae was studied.
Sample size was determined assuming a 30% incidence of at least one perinatal death or neonatal morbidity in IUGR preterm twins and 10% in normal preterm twins, and a 10% incidence of lost cases. As such, a global sample of at least 180 fetuses provides 90% power for a two-sided test and a type 1 error of 0.05.
Statistical analysis was performed using SPSS version 19. Continuous variables were compared using Student's t test and dichotomous variables using the χ2 Pearson test with continuity correction and Fisher's exact test in cases in which expected frequencies less than 5 were more than 20%. Odds ratios (OR), as estimate of the relative risk of an event, and 95% confidence intervals (CI) are reported for each mortality rate. A 95% CI from which 1.0 is excluded indicated an OR statistically significant. A p value of <.05 was considered significant in all tests.
Results
Summary maternal, pregnancy, and delivery descriptive data are displayed in Table 1. Groups were similar with regard to maternal age, parity, gestational age at delivery, chorionicity, antenatal steroid administration, birth order, fetal gender, or cesarean section rates. As expected, the groups were different with respect to mean birth-weight, with the IUGR group being lighter.
TABLE 1 Maternal and Delivery Data

aMean +SD; bproportion ns (not significant). IUGR = intrauterine growth restriction.
Perinatal mortality data are displayed in Table 2. A higher fetal mortality (102 per 1,000 vs. no cases) was observed in IUGR preterm twins versus normal growth ones of similar gestational age (OR 2.05, 95% CI 1.78–2.36). Overall perinatal mortality rate was also higher (162 per 1,000 vs. 29 per 1,000, OR 5.79, 95% CI 1.63–2.05). However, no significant differences were observed in neonatal mortality.
TABLE 2 Perinatal Mortality
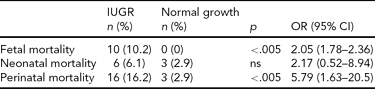
ns = not significant. IUGR = intrauterine growth restriction.
The data on neonatal morbidity in born-alive fetuses are displayed in Table 3. IUGR fetuses showed a lower incidence of RDS and a higher incidence of neurologic sequelae (Table 4). All the other conditions studied demonstrated no significant difference between IUGR preterm twins versus normal growth ones, neither any condition alone nor for survival rate without any morbidity in the neonatal period, nor low Apgar score or NICU admission rates.
TABLE 3 Neonatal Morbidity

ns = not significant.
IUGR = intrauterine growth restriction; IVH = intraventricular hemorrhage; NICU = neonatal intensive care unit; RDS = respiratory distress syndrome.
TABLE 4 Neurologic Sequelae

IUGR = intrauterine growth restriction.
Discussion
Perinatal outcomes in twins attributable to IUGR are not clear. IUGR, a term describing infants who have failed to reach their intrauterine growth potential secondary to a pathological cause (Muhlhausler et al., Reference Muhlhausler, Hancock, Bloomfield and Harding2011), is a well-established risk factor for fetal demise (Bryan, Reference Bryan1986; Grobman & Peaceman, Reference Grobman and Peaceman1998). However, some studies have reported reduced PMRs in low-birth-weight twins (Bleker et al., Reference Bleker, Breur and Huidekoper1979; McCarthy et al., Reference McCarthy, Sachs, Layde, Burton, Terry and Rochat1981; Williams et al., Reference Williams, Creasy, Cunningham, Hawes, Norris and Tashiro1982), and others find a similar outcome when adjusting for gestational age (Baker et al., Reference Baker, Beach, Craigo, Harvey-Wilkes and D'Alton1997; Buekens & Wilcox, Reference Buekens and Wilcox1993; Haimovich et al., Reference Haimovich, Ascher-Landsberg, Azem, Mandel, Mimouni and Many2011; Kilpatrick et al., Reference Kilpatrick, Jackson and Croughan-Minihane1996). Nevertheless, most studies that examined the effect of IUGR on morbidity and mortality in preterm infants were carried out among newborn populations from singleton pregnancies (McIntire et al., Reference McIntire, Bloom, Casey and Leveno1999; Tyson et al., Reference Tyson, Kennedy, Broyles and Rosenfeld1995), and very few have addressed the issue of twinning. Of these, most of them have focused on the fact of discordant twins (Amaru et al., Reference Amaru, Bush, Berkowitz, Lapinski and Gaddipati2004; Blickstein & Keith, Reference Blickstein and Keith2004; Demissie et al., Reference Demissie, Anath, Martin, Hanley, Macdorman and Rhoads2002; Fraser et al., Reference Fraser, Picard, Picard and Leiberman1994; Yinon et al., Reference Yinon, Mazkereth, Rosentzweig, Jarus-Hakak, Schiff and Simchen2005).
Moreover, twins are born lighter than singletons, with the normal distribution of birth weight for these infants shifted to the left of the normal singleton distribution (Alexander et al., Reference Alexander, Kogan, Martin and Papiernik1998). So, despite the use of singleton nomograms among various investigators, we believe, as others do (Yinon et al., Reference Yinon, Mazkereth, Rosentzweig, Jarus-Hakak, Schiff and Simchen2005), that to evaluate the intrauterine growth of twins it is more appropriate to use twin-specific growth curves, because these specific curves tend to show decreased growth velocity compared with singletons starting at 30–32 weeks, and its use avoids including small but appropriately grown twins.
In this study, using these specific charts, after matching for gestational age and excluding confounding variables, we found that perinatal mortality in IUGR twins was higher than in normal growth twins. Nevertheless, in our data as in other studies (Fraser et al., Reference Fraser, Picard, Picard and Leiberman1994), the increase in perinatal mortality is restricted to the risk of dying in utero, probably due to chronic deprivation of nutrients and oxygen related to a fetal pathologic condition. Neonatal mortality did not show significant differences between groups. This finding is consistent with the concept that the neonatal mortality in growth-restricted twins is mainly related to preterm delivery, rather than to an inherent problem with the IUGR twin fetus or neonate, as is similar in growth-restricted or not preterm fetuses.
As in this study, several papers report lower RDS rates among SGA infants (Cifuentes et al., Reference Cifuentes, Bronstein, Phibbs, Phibbs, Schmitt and Carlo2002; Gluck & Kulovich, Reference Gluck and Kulovich1973; Horbar et al., Reference Horbar, Badger, Carpenter, Fanaroff, Kilpatrick, LaCorte and Soll2002; Procianoy et al., Reference Procianoy, Garcia-Prats, Adams, Silvers and Rudolph1980). One possible explanation that has been proposed for these phenomena is that the fetus reacts to distress, increasing the glucocorticoid production, which could enhance lung maturation (Cock et al., Reference Cock, Albuquerque, Joyce, Hooper and Harding2001; Laatikainen et al., Reference Laatikainen, Raisanen and Salminen1988; Perelman et al., Reference Perelman, Farrell, Engle and Kemnitz1985). In our data, the RDS rate of the IUGR group was significantly lower than normal growth twins, despite maternal steroid therapy being similar in both groups. Thus, we can conclude from these findings that IUGR confers a protective effect on lung maturation.
The rate of hyperbilirubinemia and jaundice was similar in the IUGR twins and the normal growth group. It has been hypothesized that delayed liver maturation caused by IUGR might contribute to an increased risk of this condition (Flecknell et al., Reference Flecknell, Wootton, John and Royston1981), and a recent study found an increased rate of hyperbilirubinemia in IUGR twins (Haimovich et al., Reference Haimovich, Ascher-Landsberg, Azem, Mandel, Mimouni and Many2011), but these results have not been confirmed in this study.
Reduced oxygen and nutrients supply leads to hypoglycemia, hypocalcaemia, thrombocytopenia, and polycythemia, among others, and has been recognized as a condition associated with IUGR (Haram et al., Reference Haram, Svendsen and Myking2007; Kramer et al., Reference Kramer, Olivier, McLean, Willis and Usher1990). Meanwhile, pre-mature babies are at an increased risk of anemia and hypoglycemia (Haimovich et al., Reference Haimovich, Ascher-Landsberg, Azem, Mandel, Mimouni and Many2011). No difference between the groups in the incidence of any metabolic or hematological disorders analyzed was identified. So we conclude that they are only related to prematurity and are not associated with IUGR in twins.
Twins are known to have a higher incidence of neurodevelopmental disorders compared with singletons (Kragt et al., Reference Kragt, Huisjes and Touwen1985), and premature birth, growth retardation, and birth asphyxia are important antecedents of these problems (Ghai & Vidyasagar, Reference Ghai and Vidyasagar1988). Numerous experimental studies in animals have shown that early undernutrition influences future cognition (Morgane et al., Reference Morgane, Austin-LaFrance, Bronzino, Tonkiss, Díaz-Cintra, Cintra, Kemper and Galler1993), and several investigations (Hawdon et al., Reference Hawdon, Hey, Kolvin and Fundudis1990; Hutton et al., Reference Hutton, Pharoah, Cooke and Stevenson1997; Robertson et al., Reference Robertson, Etches and Kyle1990) raise the hypothesis that infants who suffer growth restriction during the prenatal period, and hence are likely to be deprived of an optimal supply of nutritional substrates, are at risk of impaired neural and cognitive development. In our findings, the incidence of neurologic sequelae in the IUGR group was fourfold that of normal growth twins (p < .05). IVH increases with prematurity and, when significant (grade 3 or 4), can result in major neurologic impairments (Vohr et al., Reference Vohr, Coll, Flanagan and Oh1992); however, in this study, groups were not different in the incidence of this condition.
We also analyzed the presence of any significant diagnosis/morbidity in our study population. We found that 55.1% of IUGR twins had any morbidity, compared with 45.6% in normal growth fetuses. There were no significant differences between two groups. These figures are consistent with other investigators (Fraser et al., Reference Fraser, Picard, Picard and Leiberman1994; Ghai & Vidyasagar, Reference Ghai and Vidyasagar1988; Ho & Wu, Reference Ho and Wu1975) and point out that IUGR itself did not seem to be associated with overall neonatal morbidity.
Our study is limited by the size of the groups. Despite a size calculation being carried out, certain outcomes were relatively rare, and the study was underpowered enough to analyze meaningful differences between groups for these outcomes. However, we were able to evaluate the clinically important neonatal morbidity or perinatal death. On the contrary, data may be generalizable only to a similar clinical institution, that is, a tertiary care center that cares for high-risk and preterm fetuses and neonates.
In summary, this study provides data for better understanding of the effect of IUGR on morbidity and mortality rates in preterm twins. Understanding the effect of growth restriction on neonatal performance is of considerable value in terms of obstetric and neonatal decision-making processes, as well as for parental consultation. The importance of our results is that they attempt to establish the impact of growth restriction on perinatal outcome in preterm twins. We have shown that adverse perinatal outcomes of preterm twins complicated with IUGR are represented by a higher stillbirth rate, and this stresses the importance of antenatal recognition. However, once born alive, neonatal outcomes seem to be associated only with gestational age at birth, except for a protective effect on lung maturation and a higher incidence of long-term neurologic sequelae.






