Twin pregnancies constitute 2–4% of all births, and their incidence has been rising for the last three decades, especially in the developed countries (Ananth & Chauhan, Reference Ananth and Chauhan2012). In Poland in 2014, 2.6% of all births were twin (Central Statistical Office, 2015). Due to an increased use of assisted reproduction techniques (ART) and delayed childbearing, which are associated with a high risk of multiple births (Kupka et al., Reference Kupka, Ferraretti, de Mouzon, Erb, D'Hooghe, Castilla and Goossens2014), the increasing trend in twin pregnancy prevalence is likely to be expected.
Twin gestation is a risk factor for hypertensive disorders of pregnancy. Reported rates of those disorders in twins differ from 13% to 37% according to the literature (Long & Oats, Reference Long and Oats1987; Ros et al., Reference Ros, Cnattingius and Lipworth1998; Santema et al., Reference Santema, Koppelaar and Wallenburg1995). According to Sibai et al. (Reference Sibai, Hauth, Caritis, Lindheimer, MacPherson, Klebanoff and McNellis2000), women with twins have relative risk of 2.04 for developing GH. A systematic review of controlled studies published between 1966 and 2002 revealed that twin pregnancy is associated with 2.93 relative risk of developing PE (Duckitt & Harrington, Reference Duckitt and Harrington2005). The relationship between PE and chorionicity is unclear, and published data is conflicting. There are studies indicating that dichorionic pregnancy (DCP) may be at higher (Sarno et al., Reference Sarno, Maruotti, Donadono, Saccone and Martinelli2014; Singh et al. Reference Singh, Singh, Surapaneni and Nirmalan2014, Sparks et al., Reference Sparks, Cheng, Phan and Caughey2013) or lower risk of PE than monochorionic pregnancy (MCP; Campbell & MacGillivray, Reference Campbell and MacGillivray1999; Campbell & Templeton, Reference Campbell and Templeton2004). Other studies (Carter et al., Reference Carter, Bishop, Goetzinger, Tuuli and Cahill2015; Leduc et al., Reference Leduc, Takser and Rinfret2005; Savvidou et al., Reference Savvidou, Karanastasi, Skentou, Geerts and Nicolaides2001) provide information about the lack of similar association.
The aim of the study was to analyze the relationship between chorionicity of twin pregnancy and the risk of both hypertensive disorders of pregnancy: GH and PE.
Materials and Methods
A historical cohort study of women in twin pregnancies who delivered at the 1st Department of Obstetrics and Gynecology, Medical University of Warsaw (tertiary university perinatal department) between January 2009 and December 2014 was conducted.
All patients with proper chorionicity assessment who delivered after completed 22 weeks of gestation were included in the study. All the pregnancies were dated according to the last menstrual period or the date of embryo transfer in ART and verified by the crown-rump length measured in the first trimester (in case of crown-rump length discordance, this measurement was taken from the larger twin). Chorionicity was assessed by the ultrasound scan performed in the first trimester of pregnancy on the basis of the number of gestational sacs, embryos, amniotic sacs, membrane thickness, and λ-sign or τ-sign. If there were no reliable sonography results, twins of a different gender were considered dichorionic.
The presence of PE and GH was assessed according to the ACOG guidelines (ACOG Task Force on Hypertension in Pregnancy, 2013). PE was established by the new onset of hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg on two occasions at least 4 hours apart after 20 weeks of gestation) in a previously normotensive woman, together with proteinuria 0.3 g/24 h or protein/creatinine ratio >0.3. If there was no proteinuria, PE was diagnosed by the onset of hypertension as above with at least one sign of systemic findings: thrombocytopenia (platelet count <100.000/microliter), renal insufficiency (serum creatinine >1.1 mg/dL or doubling of creatinine in the absence of other renal disease), impaired liver function (liver transaminases at least twice the normal concentrations), pulmonary odema, and new onset of cerebral or visual symptoms. The presence of any of the following features classified PE as severe: systolic blood pressure ≥160 mmHg or diastolic blood pressure ≥110 mmHg on two occasions at least 4 hours apart while the patient was at bed rest (or under hypertensive therapy), thrombocytopenia (platelet count <100.000/microliter), impaired liver function (liver transaminases at least twice the normal concentrations and/or severe persistent right upper quadrant or epigastric pain unresponsive to analgesic therapy), progressive renal insufficiency (serum creatinine >1.1 mg/dL or doubling of creatinine in the absence of other renal disease), pulmonary odema, and new onset of cerebral or visual symptoms. Early onset PE was defined as PE that developed before 34 weeks of gestation, whereas late onset PE developed at or after 34 gestational weeks. The presence of GH was established by the new onset of hypertension (the same diagnostic criteria of hypertension as in PE) without proteinuria or systemic features. Patients with chronic hypertension and glomerulonephritis were excluded from the study due to the difficulties in interpretation of both possible high blood pressure values and proteinuria during pregnancy.
Body mass index (BMI) was calculated by defining the body mass by the square of the body height. Preterm delivery was defined as birth before completed 37 weeks of gestation. Gestational diabetes mellitus (GDM) was diagnosed according to the recommendations of Polish Gynecologic Society (Wender-Ożegowska et al., Reference Wender-Ożegowska, Bomba-Opoń, Brązert, Celewicz, Czajkowski, Karowicz-Blińska and Zawiejska2014). Intrauterine fetal demise was defined as fetal death occurring at or after 22 completed gestational weeks. Intrauterine growth restriction (IUGR) was defined as estimated weight below the 10th percentile for the gestational age. Low birth weight (LBW) was defined as birth weight below 2,500 g, very low birth weight (VLBW) as below 1,500 g and extremely low birth weight (ELBW) as below 1,000 g. Low 1st and 5th minute Apgar scores were defined as 7 points or below.
The cohort was divided into two groups according to chorionicity: MCP twin pregnancy group and DCP twin pregnancy group. Maternal characteristics, GH/PE occurrence and severity, and gestational age at delivery were compared in both analyzed groups. The study outcome was the development of GH or PE in the MCP and DCP groups. The comparative analysis of patients with PE in both groups was also performed. It included maternal characteristics, selected pregnancy complications, and neonatal outcome.
Statistical analysis was performed using Statistica 12 (StatSoft Inc.). Data were presented as means ± standard deviations or numbers of subjects and percentages. A U-Mann–Whitney test was used for quantitative data comparison and Fisher's exact test was used for categorical data comparison with a p value < .05 considered significant. Eventually, a logistic regression mode for PE adjusted for potential confounding factors was created and odds ratios with 95% confidence intervals were reported.
Results
The cohort consisted of 315 women with twin pregnancies. Two patients were excluded due to chronic hypertension and one due to glomerulonephritis, leaving 312 women included in the study. Seventy-nine (25.3%) of pregnancies were MCP and 233 (74.7%) were DCP. The basic characteristics of the analyzed groups are presented in Table 1.
TABLE 1 Characteristics of Study Groups
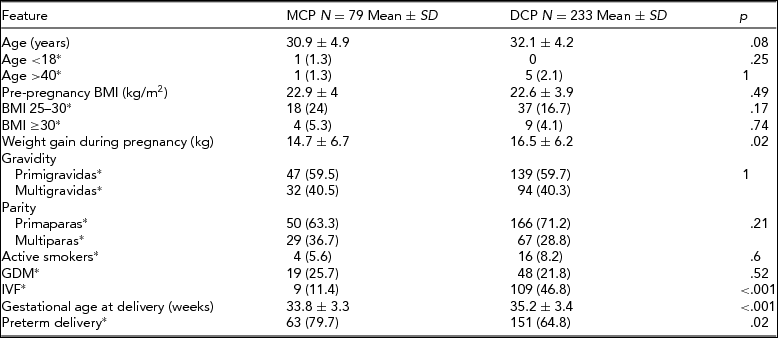
*number (%); SD = standard deviation; MCP = monochorionic twin pregnancy group; DCP = dichorionic twin pregnancy group; BMI = body mass index; GDM = gestational diabetes; IVF = in vitro fertilization.
The prevalence of GH and PE in both study groups is presented in Table 2. Hypertensive disorders in pregnancy were diagnosed significantly more often in DCP pregnancies (19.7% vs. 8.9%; p = .03), which was mostly due to the higher incidence of PE in that group (13.3% vs. 3.8%; p = .02). There were no statistical differences between mild and severe PE, or between early and late onset of PE in the MCP and DCP groups. There were only three cases of PE in the MCP group, all early onset and mild. The incidence of GH did not differ between the analyzed groups. Gestational hypertensive disorders in pregnancy were afterwards stratified by gestational age at delivery into groups <28 weeks, 29–31 weeks, 32–36 weeks, and ≥37 weeks of gestation. The proportion of DCP with PE increased with gestational age at delivery until 37 weeks, while for MCP, PE occurred only in the 32–36 weeks group. Gestational hypertensive disorders occurred in MCP and DCP pregnancies in the 32–36 weeks and ≥37 weeks group in similar proportions.
TABLE 2 Hypertensive Disorders in Pregnancy in Both Study Groups
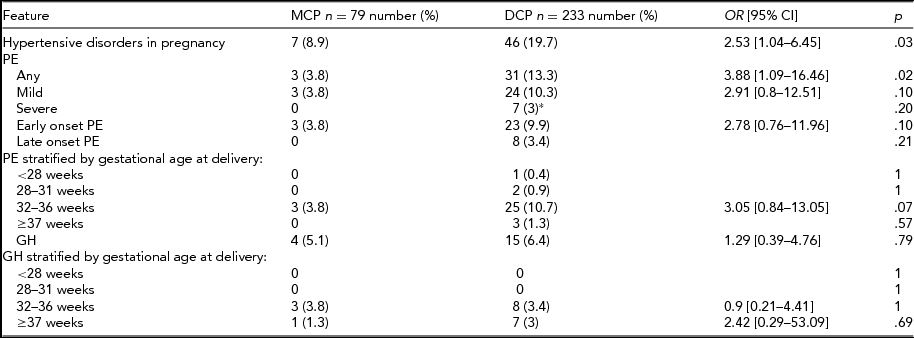
*Including two cases of HELLP syndrome (hemolysis, elevated liver enzymes, low platelet count); MCP = monochorionic twin pregnancy group; DCP = dichorionic twin pregnancy group; OR = odds ratio; 95% CI = 95% confidence interval; PE = preeclampsia; GH = gestational hypertension; weeks = gestational weeks.
Further analysis of pregnancy outcomes of patients with PE in MCP and DCP groups was also performed: 91.2% of patients with PE delivered preterm (3/3 in MCP and 28/31 in DCP; p = .8). However, we observed no differences in preterm deliveries ratios between MCP and DCP, and most of them were late preterm (2/3 in MCP vs. 21/31 in DCP; p = .8). There was one case of fetal demise in the DCP group due to IUGR and none in the MCP group.
Eventually, the multivariate logistic regression model, which included chorionicity and several confounding factors, was created. Among all analyzed factors only chorionicity and gestational age at delivery were associated with a significant increase or decrease of risk of PE. Results are presented in Table 3. After adjustment, dichorionicity was associated with almost 5-times higher risk of developing PE during pregnancy.
TABLE 3 Multivariate Logistic Regression Model for Pre-eclampsia Adjusted for Potential Confounding Factors
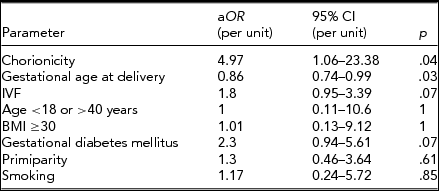
aOR = adjusted odds ratio; 95% CI = 95% confidence interval; IVF = in vitro fertilization; BMI = body mass index.
Discussion
The presented study revealed a significant difference in the prevalence of PE between DCP and MCP. There are only few published studies analyzing this association and their results are conflicting. A few studies indicated no relationship between chorionicity and PE (Carter et al., Reference Carter, Bishop, Goetzinger, Tuuli and Cahill2015; Leduc et al., Reference Leduc, Takser and Rinfret2005; Savvidou et al., Reference Savvidou, Karanastasi, Skentou, Geerts and Nicolaides2001). Two studies reported higher occurrence of PE in MCP than DCP (Campbell & MacGillivray, Reference Campbell and MacGillivray1999; Campbell & Templeton, Reference Campbell and Templeton2004). According to Campbell and Templeton (Reference Campbell and Templeton2004), the relative risk for developing PE in MCP was 1.38 [95% CI 1.04–1.82]. Recent publications indicated a higher incidence of PE in DCP. Sparks et al. (Reference Sparks, Cheng, Phan and Caughey2013) reported the ratio of PE in the group of 695 twin pregnancies to be significantly higher in DCP than in MCP (21.1% vs. 10.8%, p < .001). A similar association of PE and DCP was confirmed by Sarno et al. (Reference Sarno, Maruotti, Donadono, Saccone and Martinelli2014) one year later (30.4% vs. 12.8%, p = .02). Singh et al. (Reference Singh, Singh, Surapaneni and Nirmalan2014) also reported a higher incidence of PE in dichorionic and dizygotic twin pregnancies, but their study group was much smaller (208 women) and the results were not significant. In the presented study, the prevalence of PE was lower in both DCP and MCP twins than reported by Sparks et al. (Reference Sparks, Cheng, Phan and Caughey2013) and Sarno et al. (Reference Sarno, Maruotti, Donadono, Saccone and Martinelli2014). Our results were closer to those published by Singh et al. (Reference Singh, Singh, Surapaneni and Nirmalan2014: 13.2% vs. 4.9%). Sparks et al. (Reference Sparks, Cheng, Phan and Caughey2013) also noticed differences in the occurrence of mild PE, which affected significantly more women with DCP, while the incidence of severe PE was similar in both groups. The above was not confirmed in our study, but both differences may be due to a smaller sample size. Nevertheless, it is also important to notice the differences in the revised definition of severe PE, according to the ACOG guidelines (ACOG, 2013). In 2013, massive proteinuria (≥5 g) was eliminated from the diagnostic criteria of severe PE (ACOG; Task Force on Hypertension in Pregnancy, 2013).
A recent study by Carter et al. (Reference Carter, Bishop, Goetzinger, Tuuli and Cahill2015) showed no differences in PE occurrence in MCP and DCP, which is opposite to our results. Their sample size was bigger (2,301 women); however, there were some differences between our study protocols, which may explain the inconsistency of the results: longer study period (Carter et al. collected data from 1990 to 2010), methods of data collection (Carter et al. lost 10% of patients in the follow-up between the USG scan and perinatal period) and differences in ethnicity of both groups.
Although we have corrected the risk of PE by considering several potential confounding factors, the association between PE and chorionicity remained significant. Analogous results were reported by Sparks et al. (Reference Sparks, Cheng, Phan and Caughey2013) and Sarno et al. (Reference Sarno, Maruotti, Donadono, Saccone and Martinelli2014). In our study, the DCP group were at almost 5 times higher risk of developing PE, which was similar to the risk of developing mild PE reported by Sparks et al. (Reference Sparks, Cheng, Phan and Caughey2013; 5.85, 95% CI 1.31–26.13). Gestational age at delivery was another significant factor influencing the risk of PE. More than half of twin pregnancies were delivered preterm, also due to hypertensive disorders, therefore an increasing gestational age influences the risk of diagnosing PE. The stratification by gestational age performed in the presented analysis revealed that most cases of PE were diagnosed in groups delivering ≥32 weeks of gestation, which was similar to results reported by Sparks et al. (Reference Sparks, Cheng, Phan and Caughey2013). It is important to notice that the majority of cases in our study developed early onset PE. It may be an important observation, as early onset and late onset PE are considered to be separate and pathophysiologically different conditions (Ihle et al., Reference Ihle, Long and Oats1987). Whether it is a consequence of a natural tendency for twins to resolve preterm, making it impossible for some of them to develop PE, or whether chorionicity affects the occurrence of early and late onset PE is not certain and remains an open question. Our study, due to a relatively small sample size, was not able to provide results about this.
We observed no differences in the prevalence of GH between MCP and DCP. To our knowledge, there is only one published study analyzing the occurrence of both PE and GH in twin pregnancies. Campbell and MacGillivray (Reference Campbell and MacGillivray1999) reported data on hypertensive disorders in twins with regard to placentation. The incidence of GH was similar in both groups (27.8% MCP vs. 25.6% DCP), while the incidence of PE was higher among MCP (20.5% vs. 14.4%). Dubé et al. (Reference Dubé, Dodds and Armson2002) reported the incidence of pregnancy-induced hypertension in MCP and DCP without analyzing PE and found no relationship with chorionicity. Our study is the first to analyze both hypertensive disorders in twin pregnancies according to modified ACOG diagnostic criteria. The lack of relationship between GH and chorionicity, confirmed also in our study, sustains suggested differences in etiopathogenesis of GH.
There are two main theories explaining the association between PE and twin placentation. The first one is the increased immunologic response and/or genetic incompatibility theory. Several studies revealed increased concentration of fetal nucleic acids as well as syncytiotrophoblast microparticles in the blood of women affected by PE (Goswami et al., Reference Goswami, Tannetta, Magee, Fuchisawa, Redman, Sargent and von Dadelszen2006; Hahn et al., Reference Hahn, Huppertz and Holzgreve2005). Other studies indicated the presence of maternal natural killer cell interactions with paternal antigens of leukocytes and cytotoxic T-cell response to those antigens as a mechanism leading to the development of PE (de Groot et al., Reference de Groot, van der Mast, Visser, De Kuiper, Weimar and Van Besouw2010; Stevenson et al., Reference Stevenson, Davison, Say, Ustuoplu, Liya, Abul-Einen and Toppozada1971). Women with DCP may be exposed to larger amount of microparticles shed by two placentas and to greater genetic variability, which may create more divergent immunological pathways for inappropriate immune response. If immunoincompatibility between the mother and two fetuses was responsible for the development of PE, higher occurrence of PE should be observed in dizygotic twin pregnancies in comparison to monozygotic ones. However, the increased zygosity has not been found to have a significant effect on PE development in twin pregnancies (Carroll et al., Reference Carroll, Tyfield, Reeve, Porter, Soothill and Kyle2005; Maxwell et al., Reference Maxwell, Lieberman, Norton, Cohen, Seely and Lee-Parritz2001; Savvidou et al., Reference Savvidou, Karanastasi, Skentou, Geerts and Nicolaides2001; Singh et al., Reference Singh, Singh, Surapaneni and Nirmalan2014). In our study group, we were not able to differentiate all monozygotic from dizygotic twins effectively.
The second theory explaining the increased risk of PE in DCP is the placental mass hypothesis. Larger placental mass may be related to an increased release of some molecules with anti-angiogenic activity. Soluble fms-like tyrosine kinase-1 (sFlt-1) was proved to play a crucial role in the pathogenesis of PE (Maynard et al., Reference Maynard, Min, Merchan, Lim, Li, Mondal and Karumanchi2003). The ratio of sFlt-1/PlGF was found to be a good predictor of PE (Bdolah et al., Reference Bdolah, Lam, Rajakumar, Vankatesha, Mutter, Sachs and Karumanchi2008; Dröge et al., Reference Dröge, Herraìz, Zeisler, Schlembach, Stepan, Küssel and Verlohren2015; Rana et al., Reference Rana, Hacker, Modest, Salahuddin, Lim, Verlohren and Karumanchi2012). Several studies showed increased concentrations of sFlt-1 in twin pregnancies affected by PE in comparison to those not affected (Dröge et al., Reference Dröge, Herraìz, Zeisler, Schlembach, Stepan, Küssel and Verlohren2015; Levine et al., Reference Levine, Maynard, Qian, Lim, England, Yu and Karumanchi2004; Rana et al., Reference Rana, Hacker, Modest, Salahuddin, Lim, Verlohren and Karumanchi2012) and higher concentrations in twin pregnancies in comparison to singletons (Bdolah et al., Reference Bdolah, Lam, Rajakumar, Vankatesha, Mutter, Sachs and Karumanchi2008). Soluble endoglin (sEng) is another anti-angiogenic factor found to synergize with sFlt-1 in the pathogenesis of maternal syndrome in PE (Venkatesha et al., Reference Venkatesha, Toporsian, Lam, Hanai, Mammoto, Kim and Karumanchi2006). Higher concentrations of sEng in twin pregnancies compared to singletons were also reported in the literature (Bdolah et al., Reference Bdolah, Lam, Rajakumar, Vankatesha, Mutter, Sachs and Karumanchi2008; Faupel-Badger et al., Reference Faupel-Badger, McElrath, Lauria, Houghton, Lim, Parry and Troisi2015). This could suggest the association between the increased placental mass and the risk of preeclampsia. However, no significant differences in placental mass between MCP and DCP were reported in the literature and for this reason the above theory seems to be less possible (Campbell & MacGillivray, Reference Campbell and MacGillivray1999; Gielen et al., Reference Gielen, Lindsey, Derom, Loos, Derom, Nijhuis and Vlietinck2006). A recent study by Faupel-Badger et al. (Faupel-Badger et al., Reference Faupel-Badger, McElrath, Lauria, Houghton, Lim, Parry and Troisi2015) also found no correlation between placental mass and sFlt-1, which suggests that greater mass does not explain more anti-angiogenic profile of twin pregnancies. Reduced placental perfusion and hypoxia were proved to increase sFlt-1 production (Nevo et al., Reference Nevo, Many, Xu, Kingdom, Piccoli, Zamudio and Caniggia2008). However, as sFlt-1 is a vasoconstrictor and may induce placental ischemia/hypoxia, it is still not clear whether possible placental hypoxia is the effect or the cause of increased anti-angiogenic factors release.
Our study is limited by its retrospective nature, a small sample size and selected study group from one tertiary university department. As chorionicity cannot be randomized, the presented study may be prone to confounding bias. We did not investigate a possible correlation between zygosity and hypertensive disorders in pregnancy because zygosity can be determined accurately only by the examination of DNA markers. Finally, as twin pregnancies are often delivered preterm, and monochorionic often earlier than dichorionic ones, it is possible that the rates of PE and GH could be different if MCP were continued longer. Nevertheless, after adjusting several confounding factors in multivariate logistic regression model, chorionicity was found to be an independent risk factor of PE.
Dichorionicity seems to be a risk factor for developing preeclampsia, while there is no association between GH and mode of placentation. Further studies on larger population are necessary to determine the relation of different hypertensive disorders in pregnancy and their severity with twin chorionicity.
Acknowledgments
We would like to gratefully thank Klaudia Wypych for helping in data collection.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.





