On July 22, 2011, a deranged man killed eight persons in a bomb attack in Oslo and, 2 hours later, began shooting children and staff at a youth camp on an island near the city. Over the ensuing 90 minutes he killed 69 staff and young people. After the initial killings on the island, the perpetrator reportedly waited for survivors to attempt escape so that he could target them as they swam. Police could not reach the island when the shooting began due to the lack of helicopters and vessels. Hundreds of other people were injured in the attacks, scores of them seriously.
The Oslo Massacre elicited strong emotional responses, including fear, heightened threat perception, and grief in the general population (Nordanger et al., Reference Nordanger, Hysing, Posserud, Lundervold, Jakobsen, Olff and Stormark2013; Thoresen et al., Reference Thoresen, Aakvaag, Wentzel-Larsen, Dyb and Hjemdal2012). Indeed, one in four Norwegians reportedly knew someone bereaved by the attacks (Thoresen et al., Reference Thoresen, Aakvaag, Wentzel-Larsen, Dyb and Hjemdal2012). Whether grief arose solely among those closely tied to the deceased or in the larger population, which included witnesses to the pain of those with close ties, the fraction of Norwegians grieving the death of their young surely reached very high levels in late summer of 2011.
Dating at least to the work of Bruce (Reference Bruce1959), researchers have noted that in several non-human species, environmental threats to the survival of young, proximate conspecifics appear to induce spontaneous abortion in gravid females, both under laboratory conditions and in the wild (Becker & Hurst, Reference Becker, Hurst, Hurst, Beynon, Roberts and Wyatt2008; Cheney & Seyfarth, Reference Cheney and Seyfarth2009; Labov, Reference Labov1981; Roberts et al., Reference Roberts, Lu, Bergman and Beehner2012; Rulicke et al., Reference Rulicke, Guncz and Wedekind2006). Labov (Reference Labov1981) infers that this ‘Bruce Effect’ functions as an adaptive strategy to limit female investment in offspring likely to die in environments prevailing at birth. In non-human animals, the Bruce Effect may provide a female counterstrategy to infanticide, or may be an adaptive strategy to limit investment in gestations that face a high risk of death (Labov, Reference Labov1981). Mechanisms associated with the Bruce Effect likely include the endocrine stress response (Beehner et al., Reference Beehner, Bergman, Cheney, Seyfarth and Whitten2005; Cheney & Seyfarth, Reference Cheney and Seyfarth2009), suggesting that the Bruce Effect may be part of a generalized female reproductive response to environments that threaten offspring.
Theory (Haig, Reference Haig and Stearns1999; Schooling, Reference Schooling2014; Stearns, Reference Stearns and Stearns1987; Trivers & Willard, Reference Trivers and Willard1973; Wells, Reference Wells2000) and empirical work in human populations (Bruckner et al., Reference Bruckner, Helle, Bolund and Lummaa2015; Karasek et al., Reference Karasek, Goodman, Gemmill, Falconi, Hartig, Magganas and Catalano2015; Orzack et al., Reference Orzack, Stubblefield, Akmaev, Colls, Munné, Scholl and Zuckerman2015) suggest that natural selection has conserved endemic selection in utero that allows women to spontaneously abort gestations least likely to yield grandchildren. Acute stressors on a population appear, moreover, to induce epidemic selection in utero via the maternal stress response that reportedly raises the level of fetal fitness required for a gestation to continue (Catalano & Bruckner, Reference Catalano and Bruckner2006; Catalano et al., Reference Catalano, Bruckner and Smith2008; Reference Catalano, Saxton, Bruckner, Goldman and Anderson2009; Reference Catalano, Currier and Steinsaltz2015; Navara, Reference Navara2014).
In both humans (Nesse, Reference Nesse, Carr, Nesse and Wortman2005; Segerstrom & Miller, Reference Segerstrom and Miller2004; Winegard et al., Reference Winegard, Reynolds, Baumeister, Winegard and Maner2014) and other species (Bercovitch, Reference Bercovitch2013; Bosch et al., Reference Bosch, Nair, Ahern, Neumann and Young2008; Bradshaw et al., Reference Bradshaw, Schore, Brown, Poole and Moss2005; Cheney & Seyfarth, Reference Cheney and Seyfarth2009; Douglas-Hamilton et al., Reference Douglas-Hamilton, Bhalla, Wittemyer and Vollrath2006; Fashing & Nguyen, Reference Fashing and Nguyen2011), the death of proximate conspecifics appears to trigger the stress response. Indeed, bereavement among pregnant women increases the risk of spontaneous abortion (László et al., Reference László, Svensson, Li, Obel, Vestergaard, Olsen and Cnattingius2013).
The few historic data we have describing gestational fitness (i.e., the number of grandchildren eventually produced by a gestation) come from Northern Europe (Gabler & Voland, Reference Gabler and Voland1994) and Nordic countries (Lummaa et al., Reference Lummaa, Jokela and Haukioja2001) and show that gestations of male–male twins yielded the fewest grandchildren per gestation. Gestations of female–female twins, obversely, yielded the most grandchildren per gestation. The fitness difference between these gestations appeared due, in large part, to the likelihood of surviving to reproductive age. Male twins more likely died before reproductive age than all other infants. Male singletons had the next highest likelihood of death and the next lowest fitness. Although female twins more likely died than female singletons, the difference did not approach that between male twins and singletons, and enough female twins historically survived to ensure that gestations of female twins produced the most grandchildren per pregnancy.
Much observational literature has invoked stress-induced selection in utero to explain lower secondary sex ratios (i.e., ratio of male to female live births) following natural (Torche & Kleinhaus, Reference Torche and Kleinhaus2012) and manmade (Catalano et al., Reference Catalano, Bruckner, Gould, Eskenazi and Anderson2005) calamities, as well as societal disruption (Catalano, Reference Catalano2003). The secondary sex ratio presumably falls after such acute population stressors because selection in utero would abort male fetuses at lower levels of maternal stress than it would female fetuses, given the former's relatively low fitness if born and their relatively high need for maternal investment (Gaulin & Robbins, Reference Gaulin and Robbins1991; Powe et al., Reference Powe, Knott and Conklin-Brittain2010).
Taken as a whole, the literature summarized above suggests that natural selection may have conserved a Bruce Effect in humans that averts maternal investment in less fit offspring when infants and children in the population die at unexpectedly high rates. Because male twin gestations disproportionately populate the low end of the distribution of gestational fitness, they should suffer spontaneous abortion more frequently than would male singleton gestations when a population experiences the Bruce Effect. Female singleton gestations, however, rank below female twin gestations in fitness and, in such a population, should suffer spontaneous abortion more frequently than female twins.
Given the historic ranking of Nordic gestations on fitness, we hypothesize that if natural selection has conserved a Bruce Effect in humans, the monthly odds of a twin among Norwegian newborns exposed in gestation to the events of July 2011 will be lower than statistically expected among males and higher than expected among females. The timing of these associations should show that the decline of twins among male births occurs before the increase among female births because selection against less fit female fetuses occurs earlier in gestation than that against less fit males (Boklage, Reference Boklage2005; Orzack et al., Reference Orzack, Stubblefield, Akmaev, Colls, Munné, Scholl and Zuckerman2015).
Materials and Methods
We used monthly sex-specific counts of singleton and twin births registered in Norway for the 59 months beginning May 2007 and ending March 2012. We acquired the data from the Medical Birth Registry of Norway (Norwegian Institute of Public Health, n.d.).
Our test turns essentially on whether the observed odds of a twin among male births falls below, and the odds among female births rises above, their counterfactuals or values expected under the assumption that the events of July 2011 had not occurred. The typical approach to such tests assumes the counterfactual equals the statistically expected value of the observed odds and, in turn, that the statistically expected value equals the mean of the observations. Time series, however, often exhibit autocorrelation or trends, cycles, and the tendency to remain elevated or depressed, or to oscillate, after high or low values. The expected value of any observation in an autocorrelated series is not the mean of all observations but rather the value predicted by the best-fitting model of autocorrelation in the series.
We identified and modeled autocorrelation in the natural logarithms of the sex-specific monthly odds of a twin with Box–Jenkins methods (Box et al., Reference Box, Jenkins and Reinsel2008). The Box and Jenkins approach attributes autocorrelation to integration as well as to ‘autoregressive’ and ‘moving average’ parameters. Integration describes secular trends and seasonality. Autoregressive parameters best describes regression to the mean that persists for relatively long periods, while moving average parameters parsimoniously describe less persistent patterns. We transformed the sex-specific monthly odds of a twin among newborns to their natural logarithms to allow us to express any association with the Oslo massacre in the familiar effect on odds metric.
Following the conventions developed by Box and Jenkins (Box et al., Reference Box, Jenkins and Reinsel2008), we specified our test equation as a ‘transfer function’ that expands a model of autocorrelation to include variables measuring exposure to exogenous influences on the dependent variable. We specified our transfer function by adding a ‘massacre’ binary variable scored 1 for July 2011 and 0 otherwise to the best-fitting Box–Jenkins models for the natural logarithms of the sex-specific odds of a twin.
The literature describing selection in utero reports that, for poorly understood reasons, selection against less fit females appears concentrated early in gestation whereas that against males, although also highest in the early weeks, spreads more broadly over pregnancy, with a peak at the 18th to 22nd week. Based on this timing, we expected that twins became less common among male births sooner after the massacre and female twins became more common. We specified our binary bereavement variable such that we estimated responses among all nine monthly birth cohorts exposed in utero to the events of July 2011 (i.e., infants born from July 2011 through March 2012).
We tested our hypothesis in three steps. First, we used the Box–Jenkins methods to identify the best-fitting models of autocorrelation for the natural logarithms of the sex-specific odds of a twin among newborns. We used the 59 months beginning May 2007 and ending March 2012. This segment of data provided sufficient months (i.e., 50) to estimate autocorrelation in the series (Glass et al., Reference Glass, Willson and Gottman1975) and to identify responses, if any, in all monthly birth cohorts exposed in utero to the event. Second, we estimated the transfer functions formed by adding the binary exposure variable to the models identified in the first step. To assess possible responses in all the birth cohorts exposed in utero to the massacre, we specified the binary variable in the ‘synchronous’ configuration (i.e., births in the same month as the massacre) as well as in eight lagged configurations (i.e., eight monthly birth cohorts following the massacre). Third, we deleted any non-significant lags of the exposure variable from the results of step 2 and estimated the pared equations.
Results
The monthly sex-specific ratio of twins to singletons had a mean of 0.0344 (SD=0.0055) for males and a mean of 0.0343 (SD=0.0053) for females. Step 1 produced the following models, in which all estimated coefficients were at least twice their standard errors. The fitted values of these models include the counterfactuals for male and female twin ratios following the Oslo Massacre.
mtt and ftt are counts of male and female twins born in month t. mt an ft are total male and female births in month t. -3.3787 and -3.3859 are constants. 0.3540B 13 is a moving average parameter implying that the natural log of the odds of a male twin in month t predicted, in part, values at month t+13. 0.3584B 3 is an autoregressive parameter implying that the natural logs of the odds of a female twin in month t predicted, in part, values at month t + 3. a is the error term of the model at month 1. The error terms exhibit no autocorrelation and have an expected value of 0. These models imply that twin ratios of neither sex exhibited secular trends over the test period but that both showed ‘echoes’ of high or low values. The echo for males appeared 13 months later, suggesting a weak seasonal pattern in which a high or low value at month t predicted a similarly deviant, but diminished in absolute size, value about a year later. The diminished echo for the female twin ratio appeared much sooner — 3 months later. We have no post hoc explanation for the detected autocorrelation in these two series but must, for reasons noted above, use it to arrive at the counterfactuals for our test.
Tables 1 and 2 show the results of step 2 or the estimation of transfer functions formed by adding a binary bereavement variable scored 1 for July 2011 and 0 otherwise to the two models shown above. As noted above, we included eight ‘lags’ of the binary variable to estimate responses among all nine monthly birth cohorts exposed in utero to the massacre (i.e., infants born from July 2011 through March 2012). Tables 1 and 2 also show the results of step 3 in which we estimated pared equations formed by deleting any non-significant lags of the binary variable from the results of step 2. Consistent with our theory and hypothesis, the odds of a twin among males born in the early months after the massacre fell significantly below the expected value while the odds of a twin among female births rose significantly in later months.
TABLE 1 Estimated Transfer Functions of the Natural Logs of the Monthly Odds of a Twin Among Males Born in Norway From May 2007 Through March 2012
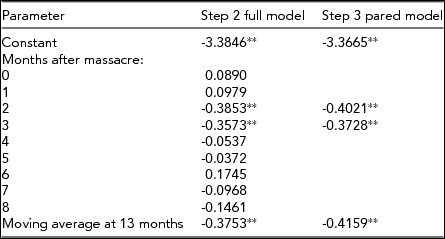
*p<.05; single-tailed test; **p<.01; single-tailed test.
TABLE 2 Estimated Transfer Functions of the Natural Logs of the Monthly Odds of a Twin Among Females Born in Norway From May 2007 Through March 2012

*p<.05; single-tailed test; **p<.01; single-tailed test.
Figure 1 shows the proportion of the monthly counterfactual values represented by observed values. The values for males born in September (0.896) and October (0.902) 2011 represented the two lowest values in the entire test period. Among females, only three values in the test period exceed that for February 2012 (1.071).

FIGURE 1 Proportion of the monthly counterfactual values represented by observed values for the log odds of male and female twin births in Norway, May 2007–March 2012.
We identified and adjusted for outliers in our dependent variable outside the nine test cohorts. Such outliers could have expanded the confidence intervals of the full models shown in Tables 1 and 2 and led us to falsely accept the null hypothesis for test cohorts with non-significant coefficients in the tables (Chang et al., Reference Chang, Tiao and Chen1988). We detected no outliers for males and one (i.e., high value in February 2009) for females. Adjusting our test for the single outlier among females did not change the results shown in Table 2.
We offer two ways to understand the strength of our results. First, taking the antilog of the pared model coefficients in Table 1 suggests that the odds of a twin among male births fell 33% below expected levels in September 2011 and 31% below expected levels in October. Doing the same for females born in February 2012 implies that the odds rose 29% above the expected value.
We calculated another familiar metric for strength of association, change in explained variance (i.e., ‘R 2’). Autocorrelation in the dependent variable for males, as expressed by model 1 above, accounted for 8.5% of the series’ variance. The pared model shown in Table 1 accounted for 23.2% of the variance. For females, we calculated these values as 9.6% and 23.5%.
To connect our work with that on the secondary sex ratio in stressed populations, we also determined whether the secondary sex ratio among singletons fell in Norway after the Oslo Massacre. The Bruce Effect and the historical fitness rankings of Nordic gestations would suggest an effect of the massacre on the sex ratio of singletons, in addition to its effect on twins. Applying the three steps in our main test described above to the natural logarithm of the odds of a male among live singleton births yielded the results shown in Table 3. We found, consistent with the Bruce Effect and with timing reported in other literature (Karasek et al., Reference Karasek, Goodman, Gemmill, Falconi, Hartig, Magganas and Catalano2015), that the secondary sex ratio dropped significantly 5 months after the Oslo Massacre.
TABLE 3 Estimated Transfer Functions of the Natural Logs of the Male–Female secondary Sex Ratio Among Singleton Births in Norway From May 2007 Through March 2012
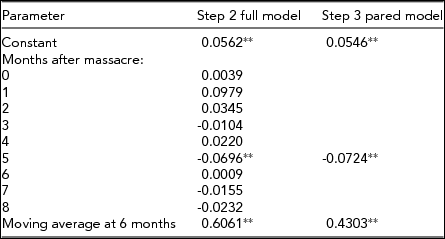
*p<.05; single-tailed test; **p<.01; single-tailed test.
Discussion
We inspected the likelihood of twins among male and female infants in Norwegian birth cohorts exposed and unexposed to the Oslo Massacre of July 2011. Consistent with the argument that the Bruce Effect appears in humans, we found fewer twins than expected among male infants born soon after the massacre (i.e., September and October), but more twins than expected among females born later (i.e., February 2012). The Bruce Effect via selection in utero may be vestigial in humans in that women currently realize small, if any, fitness benefit from avoiding male twins during stressful times, but the conserved response to environments that threaten offspring plausibly persists in the population.
We suggest that our findings may have implications for researchers who use twin sets to estimate the relative contribution of environment and genes to human behavior. The fact that the Bruce Effect may influence which twin sets survive gestation suggests that the environment may affect concordance in the response of twins to stimuli. Could the conversion, via spontaneous abortion, of a twin to singleton gestation during uncertain times select against twins likely to exhibit greater concordance in their response to stimuli? Would natural selection not conserve any mechanism that diversified offspring phenotypes in the face of uncertain threats to survival (Ellis et al., Reference Ellis, Boyce, Belsky, Bakermans-Kranenburg and van Ijzendoorn2011; Gordon et al., Reference Gordon, Joo, Powell, Ollikainen, Novakovic, Li and Alisch2012)? If so, would a sample of twin sets dominated by those in gestation during times of relatively great threat to infant survival not yield lower concordance in response to stimuli than a sample in gestation when threats to survival appeared lesser and more predictable?
Our article makes at least two contributions to the line of work reporting a drop in the secondary sex ratio following acute population stressors (Catalano, Reference Catalano2003; Catalano et al., Reference Catalano, Bruckner, Hartig and Ong2005; Torche & Kleinhaus, Reference Torche and Kleinhaus2012). First, by introducing the Bruce Effect into the literature concerned with selection in utero, we connect these heretofore separate but clearly complementary lines of inquiry. Second, the work concerned with the secondary sex ratio has focused primarily on the effects of selection in utero on male fetuses. We, however, find such an effect among female birth cohorts as well. Our findings suggest a Bruce Effect not only on male twins who would otherwise have been born in September and October 2011, but also on female twins who would have been born in February 2012. Earlier research reporting that loss of less fit female fetuses occurs early in gestation while that of less fit males occurs later (Boklage, Reference Boklage2005; Orzack et al., Reference Orzack, Stubblefield, Akmaev, Colls, Munné, Scholl and Zuckerman2015) predicts our findings.
As an observational test, ours cannot rule out that phenomena other than the Oslo Massacre induced our results. We note, however, that the logic of our analyses limits any such phenomena to those exhibiting no trends, cycles (including seasonality), or other forms of autocorrelation. Those phenomena, moreover, would have to first repress the birth of male, but then increase the birth of female, twins. We know of no mechanism other than selection in utero via the Bruce Effect that fits these constraints.
Our data do not allow us to determine the physical or emotional proximity of individual women to the massacre, so we cannot establish if our finding arose primarily from women who lost friends or family or if it represents a population-wide response. Several studies report that the psychological effects of mass trauma extend beyond the immediate survivors to the wider community and induce ‘communal bereavement’ (Catalano & Hartig, Reference Catalano and Hartig2001). For example, symptoms of post-traumatic stress disorder have been reported among individuals who witnessed the September 11 terrorist attacks only through media coverage (Galea et al., Reference Galea, Vlahov, Resnick, Ahern, Susser, Gold and Kilpatrick2003; Schlenger et al., Reference Schlenger, Caddell, Ebert, Jordan, Rourke, Wilson and Kulka2002) and among young adults with minimal direct exposure to missile attacks in the 2008–2009 military conflict in southern Israel (Neria & Sullivan, Reference Neria and Sullivan2011). Our findings suggest that the effects of mass trauma also shape the future population, by affecting which fetuses survive to birth.
Less dramatic circumstances than the Oslo Massacre or terrorist attacks may have a less detectable, but more persistent effect on natural selection in human populations. The level of anxiety and depression in Sweden, for example, predicts the secondary sex ratio (Catalano et al., Reference Catalano, Bruckner, Gould, Eskenazi and Anderson2005), and urbanization may influence resistance to infectious disease, via alterations in allele frequencies in the population (Barnes et al., Reference Barnes, Duda, Pybus and Thomas2011).
The contributions of our work to basic science include added support for the argument that natural selection may have conserved a Bruce Effect that protects or enhances reproductive fitness. The findings also add support for selection in utero and for the argument that that selection works against less fit fetuses.
We doubt that our theory or findings will, or should, affect what people do, or help others do, to cope with grief. The results suggest, however, that any interventions intended to avert spontaneous abortion among bereaved persons should broaden the target population to include pregnant women beyond those most obviously attached to the deceased.
Acknowledgments
This research was supported by the Robert Wood Johnson Health and Society Scholars Program.






