Lifetime cancer incidence in individuals born as twins is similar to that of singletons (Chen et al., Reference Chen, Cnattingius, Nyman Iliadou and Oberg2016; Hemminki & Chen, Reference Hemminki and Chen2005). However, the risk of breast, testicular and some other adult cancers, and of childhood leukaemia for any member of a twin pair is substantially increased if their co-twin is diagnosed with the same cancer (Couto et al., Reference Couto, Chen and Hemminki2005; Lichtenstein et al., Reference Lichtenstein, Holm, Verkasalo, Iliadou, Kaprio, Koskenvuo, Pukkala, Skytthe and Hemminki2000; Swerdlow et al., Reference Swerdlow, De Stavola, Swanwick and Maconochie1997). Further, twins experience significantly fewer childhood cancers (<15 years of age) compared to singletons though it is not clear why or which specific tumors contribute to this reduced risk (Murphy et al., Reference Murphy, Bunch, Chen and Hemminki2008; Murphy et al., Reference Murphy, Whiteman, Hey, Griffith, Gill, Goldacre, Vincent and Bunch2001; Puumala et al., Reference Puumala, Carozza, Chow, Fox, Horel, Johnson, McLaughlin, Mueller, Reynolds, Von Behren and Spector2009).
The cancer burden in childhood is very different from that in adulthood, though there is some evidence of increased overall adult cancer risk in the families of children diagnosed with any childhood cancer (Neale et al., Reference Neale, Stiller, Bunch, Milne, Mineau and Murphy2013). The tumors occurring at teenage/young adult (TYA) ages show an intermediate pattern, with a mix of those typical of childhood, (though some rise to a peak incidence in young adulthood) with an increasing proportion of those typical of adulthood such as melanomas and carcinomas (Murphy et al., Reference Murphy, Bithell, Stiller, Kendall and O’Neill2013). Because of this transitional tumor profile between childhood and adulthood, we first aimed to clarify the risk of childhood cancer in twins in an updated meta-analysis, and then used the Swedish Multigeneration Register to explore the older ages to which this reduced overall tumor risk persists beyond childhood. We examined the contributions made by specific tumors to the overall cancer risk reduction in the childhood/teenage/young adult (CTYA) age group.
Materials and Methods
Literature Search
We systematically searched Medline and Embase for studies about childhood cancer risk in twins published in English. We conducted a literature search in October 2016 and updated it in March 2020, with a strategy modified particularly to capture known publications potentially containing data allowing calculation of total cancer risk among the increasing numbers of twins conceived after use of assisted reproductive technology (ART). We conducted further modified searches in 2021 and again, finally in November 2023, exploding the Search terms to capture all studies that we knew to be identified as potentially relevant. The Search strategies are described in the Supplement (which is available on the CUP website). From the principal searches conducted to 2021, we retrieved abstracts of 528 publication, and after deduplication we had 367 records for inspection by two of the authors (MM, RR). In the final 2023 search, we identified no publications containing directly relevant data of which we were previously unaware. All relevant studies already known to us were captured by the searches, apart from one publication which did not directly provide a childhood cancer risk estimate among twins (Pinborg et al., Reference Pinborg, Loft and Nyboe Andersen2004) that we had previously identified.
Meta-Analysis
Building on earlier meta-analyses (Inskip et al., Reference Inskip, Harvey, Boice, Stone, Matanoski, Flannery and Fraumeni1991; Murphy, Reference Murphy, Ward and Whittle1995; Murphy et al., Reference Murphy, Whiteman, Hey, Griffith, Gill, Goldacre, Vincent and Bunch2001), we identified four more recent studies about childhood cancer risk in twins versus singletons or the general population of births that contributed relevant data (Murphy et al., Reference Murphy, Bunch, Chen and Hemminki2008; Neale et al., Reference Neale, Mineau, Whiteman, Brownbill and Murphy2005; Pinborg et al., Reference Pinborg, Loft and Nyboe Andersen2004; Puumala et al., Reference Puumala, Carozza, Chow, Fox, Horel, Johnson, McLaughlin, Mueller, Reynolds, Von Behren and Spector2009). Some of the early studies examined cancer mortality as a measure of childhood cancer risk. Our updated meta-analysis was of incidence only, but of the four more recent studies we identified, risks in two (Pinborg et al., Reference Pinborg, Loft and Nyboe Andersen2004; Puumala et al., Reference Puumala, Carozza, Chow, Fox, Horel, Johnson, McLaughlin, Mueller, Reynolds, Von Behren and Spector2009) could only be estimated by odds ratios (ORs) rather than rate/risk ratios (RRs). One study (Pinborg et al., Reference Pinborg, Loft and Nyboe Andersen2004) was entirely of births following use of ART, where no cancers were observed among the twins against an expected number of about six (p = .014). To include their data in our meta-analysis, we calculated a ‘Peto Odds Ratio’ rather than using a continuity correction method (Bradburn et al., Reference Bradburn, Deeks, Berlin and Russell2007; Higgins & Green, Reference Higgins and Green2011; Sweeting et al., Reference Sweeting, Sutton and Lambert2004). In the published data from the other (case-control) study, only ORs adjusted for several variables, including birth weight, were presented as the risk estimates (Puumala et al., Reference Puumala, Carozza, Chow, Fox, Horel, Johnson, McLaughlin, Mueller, Reynolds, Von Behren and Spector2009). We therefore obtained from the authors directly the unpublished, unadjusted (matching factors only) ORs. Of the studies identified with relevant data, only one was excluded from our meta-analysis, because of both its size and quality. The published results in this incident case-control study permit calculation of an approximate, all cancer OR (95% CI) for being a twin of 1.3 (0.4-4.5) based on only six exposed cases (Savitz & Ananth, Reference Savitz and Ananth1994). Its inclusion in our analysis makes no difference to our effect estimate. Our meta-analysis was implemented in STATA 13.
Swedish Multigeneration Register and Cancer Incidence Linkage: Cohort Analysis
Statistics Sweden maintains the Multigeneration Register, in which individuals born in Sweden in 1932 and later are registered with their birth parents and organized as families (Hemminki et al., Reference Hemminki, Li, Plna, Granstrom and Vaittinen2001). Information on the database is available at the Nature Genetics website as ‘Supplementary information’ (Hemminki & Granstrom, Reference Hemminki and Granstrom2002). The data on families and cancers have complete coverage, barring some groups of deceased offspring born in the 1930s who died before 1991. Although this small group of offspring with missing links to parents has a negligible effect on risk estimates (Hemminki & Li, Reference Hemminki and Li2003), the present report was limited to offspring whose parents were known, to eliminate possibility of bias. The data were linked using the individually unique national registration number to the Swedish Cancer Registry for the years 1958 to 2002. Cancer registration has been considered to be close to 100% for a long time (Hemminki & Chen, Reference Hemminki and Chen2005). Twins were defined as children born to the same mother at the same time. Triplets and quadruplets were excluded from the analysis. Use of the data as described here was previously approved by the Ethical Board of the Karolinska Institute.
The Swedish Cancer Registry classifies site of cancer using a 4-digit diagnostic code according to the 7th revision of the International Classification of Diseases (ICD-7; World Health Organization, 1955). This is an imperfect classification system for CTYA cancers. We are therefore mainly constrained to report site-specific cancer risks, rather than categorizing tumors by tissue type. Cancer risks for twins were estimated using standardized incidence ratios (SIRs). The SIR is the ratio of the observed (O) to the expected (E) number of cases. No correction for multiple testing of the SIR measuring different tumor risks was applied. Reference cancer incidence rates for calculating expected numbers were those applying to the much larger number of singletons, using standardization by 5-year age, sex, 10-year period, and area (county) standardized rates. 95% CIs were calculated assuming a Poisson distribution (dos Santos Silva, Reference dos Santos Silva1999; Estève et al., Reference Estève, Benhamou and Raymond1994). Follow-up was started for each individual at birth or at immigration after January 1, 1958, whichever was the later. Follow-up was terminated on diagnosis of first cancer, death, emigration or the closing date of the study, December 31, 2002. This cohort study was analyzed using SAS 9.2.
Results
Table 1 references the earlier meta-analyses (Inskip et al., Reference Inskip, Harvey, Boice, Stone, Matanoski, Flannery and Fraumeni1991; Murphy, Reference Murphy, Ward and Whittle1995; Murphy et al., Reference Murphy, Whiteman, Hey, Griffith, Gill, Goldacre, Vincent and Bunch2001) and provides some details about each of the 10 studies we identified from 11 publications, from which we were able to extract analysable results about childhood cancer risk in twins versus singletons or the general population of births (Hewitt et al., Reference Hewitt, Lashof and Stewart1966; Inskip et al., Reference Inskip, Harvey, Boice, Stone, Matanoski, Flannery and Fraumeni1991; Jackson et al., Reference Jackson, Norris and Klauber1969; Murphy, Reference Murphy, Ward and Whittle1995; Murphy et al., Reference Murphy, Bunch, Chen and Hemminki2008; Murphy et al., Reference Murphy, Whiteman, Hey, Griffith, Gill, Goldacre, Vincent and Bunch2001; Neale et al., Reference Neale, Mineau, Whiteman, Brownbill and Murphy2005; Norris & Jackson, Reference Norris and Jackson1970; Pinborg et al., Reference Pinborg, Loft and Nyboe Andersen2004; Puumala et al., Reference Puumala, Carozza, Chow, Fox, Horel, Johnson, McLaughlin, Mueller, Reynolds, Von Behren and Spector2009; Rodvall et al., Reference Rodvall, Hrubec, Pershagen, Ahlbom, Bjurman and Boice1992; Windham et al., Reference Windham, Bjerkedal and Langmark1985). The summary estimate of relative cancer mortality risk in the older publications that examined this outcome (Hewitt et al., Reference Hewitt, Lashof and Stewart1966; Jackson et al., Reference Jackson, Norris and Klauber1969; Norris & Jackson, Reference Norris and Jackson1970; Rodvall et al., Reference Rodvall, Hrubec, Pershagen, Ahlbom, Bjurman and Boice1992) is 0.85 (95% CI [0.74, 0.95]) (Murphy et al., Reference Murphy, Whiteman, Hey, Griffith, Gill, Goldacre, Vincent and Bunch2001).
Table 1. Cancer occurrence in twins versus single births or the general population of children (modified from Inskip et al., Reference Inskip, Harvey, Boice, Stone, Matanoski, Flannery and Fraumeni1991; Murphy, Reference Murphy, Ward and Whittle1995; Murphy et al., Reference Murphy, Whiteman, Hey, Griffith, Gill, Goldacre, Vincent and Bunch2001)
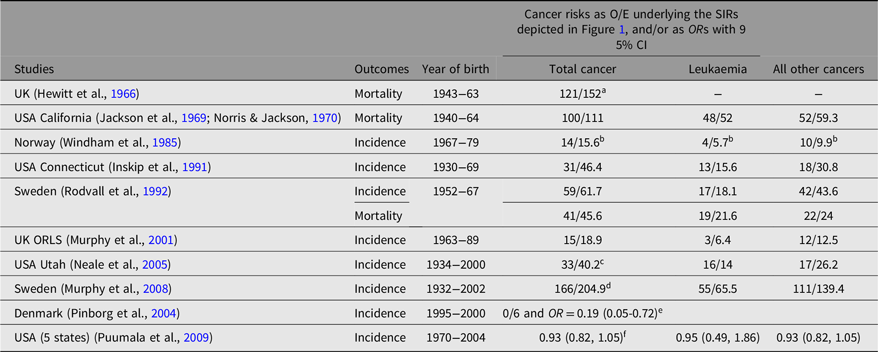
Note: O, observed; E, expected; SIRs, standardized incidence ratios; ORL, Oxford Record Linkage Study.
a Expected number of cancers adjusted upward to account for twins’ greater frequency of exposure to prenatal X-rays in Hewitt et al. (Reference Hewitt, Lashof and Stewart1966);
b Expected number of cancers estimated from relative risk and observed number of cancers for Windham et al. (Reference Windham, Bjerkedal and Langmark1985);
c Expected numbers of cancers estimated from adjusted relative risk and observed number of cancers for Neale et al. (Reference Neale, Mineau, Whiteman, Brownbill and Murphy2005; leukemia = hematopoietic);
d Expected number of cancers estimated from adjusted SIR and observed number of cancers for Murphy et al. (Reference Murphy, Whiteman, Hey, Griffith, Gill, Goldacre, Vincent and Bunch2008);
e Expected number of cancers estimated for twins from the cancer rate for singletons assuming same average follow-up time from birth in this pure IVF/ICSI cohort. OR calculated by ‘Peto Method for rare events’;
f Population-based record-linkage case-control study. Unpublished, unadjusted (matching factors only) ORs with twinning as exposure in babies weighing < or = 4000 g at birth obtained from the authors.
Figure 1 displays the results of each of the seven non-overlapping studies of childhood cancer incidence shown in Table 1. One study (Rodvall et al., Reference Rodvall, Hrubec, Pershagen, Ahlbom, Bjurman and Boice1992) presented results about both Swedish cancer mortality and cancer incidence. Their incidence data were excluded because they were entirely contained within another Swedish study we did include (Murphy et al., Reference Murphy, Bunch, Chen and Hemminki2008). For one study (Pinborg et al., Reference Pinborg, Loft and Nyboe Andersen2004), we calculated a ‘Peto Odds Ratio’ OR 0.19 (95% CI [0.05, 0.72]). The continuity corrected estimate based on the same data was OR 0.08 (95% CI [0.004, 1.37]). There is little heterogeneity between the seven studies and the summary risk estimate from a random effects analysis was OR 0.83 (95% CI [0.72, 0.94]). Excluding the extreme OR we calculated for the Danish ART study (Pinborg et al., Reference Pinborg, Loft and Nyboe Andersen2004) made little difference to the random effects risk estimate OR 0.86 (95% CI [0.79, 0.94]).The risk estimate from a fixed effects analysis of the seven studies was also very similar OR 0.86 (95% CI [0.78, 0.93).
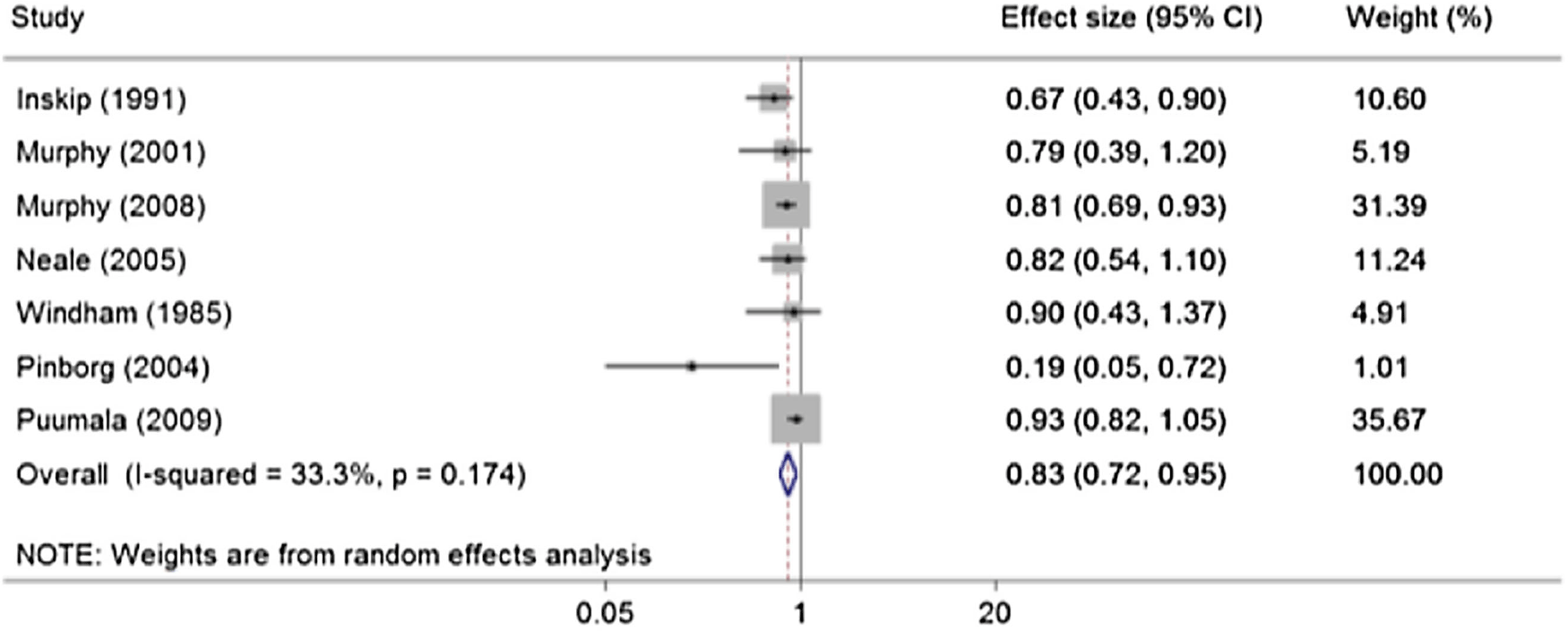
Figure 1. Meta-analysis of seven studies of childhood cancer incidence in twins.
Although not shown in Figure 1, an additional estimate of risk in a cohort of twins conceived with ART has become available. By comparison with national childhood cancer rates, an SIR (95% CI) of 0.95 (0.68, 1.30) was published for this cohort of ART births studied across Great Britain (Williams et al., Reference Williams, Bunch, Stiller, Murphy, Botting, Wallace, Davies and Sutcliffe2013). Its inclusion in the meta-analysis makes little difference to the result, with the random effects estimate becoming 0.84 (0.75, 0.95).
Table 2 shows the all-cancer SIR (95% CI) for all twins, all like-sex pairs (male-male [MM] or female-female [FF]) and all unlike-sex pairs (male-female [MF]), together with the numbers of affected twins on which the SIRs are based, for the nested age groups to attained age 34 years. SIR was low among all twins until about 30 years attained age, with both like- and unlike-sex pairs contributing to the reduction. Age-band specific risks for all twins from age 15−19 to age 30–34 are not included in the table but were estimated by subtracting the O and the E between the nested age groups shown, which for all twins showed a steady increase in all-cancer SIRs in the four age-groups (0.85; 0.94; 1.04; 1.09) across the age range, but none were significantly raised or lowered.
Table 2. Total cancer risk in Swedish twins by age group
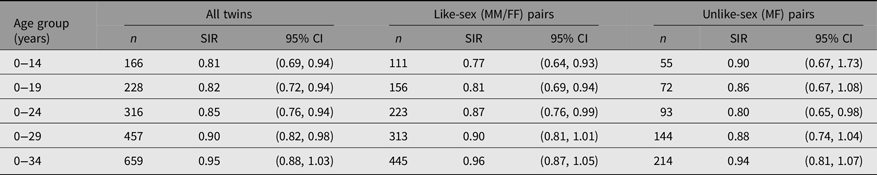
The aggregate reduced risk to age 30 years is based on nearly 3 times the number of observed cancers compared to the childhood risk at age 0–14 years. Concordance increased from four pairs in childhood (affected by hematopoietic tumors only) to seven pairs by age 30 years (five haematopoietic, one central nervous system, one breast) together with a further pair of testicular cancer cases where one concordant twin pair member was diagnosed above age 30 years.
Table 3 indicates for the age group 0–29 years (beyond which attained age the all-twin SIR rapidly approaches 1 and becomes non-significant) greater detail of the contribution made to the reduced overall cancer incidence by the range of specific sites/types provided by the Swedish Cancer Registry, and by sex combination of twin pairs. A variety of sites/types contribute, notably with a marginally significant risk reduction for leukemias in all twins (significantly so in all like-sex twins, particularly MM) and a significant reduction for renal tumors (five Wilms, one other renal) in all twins, particularly among all like-sex twins. A reduced risk for connective tissue tumors, in all twins and again particularly in all like-sex twins, is also observed.
Table 3. Cancer occurrence in Swedish twins aged 0−29 years
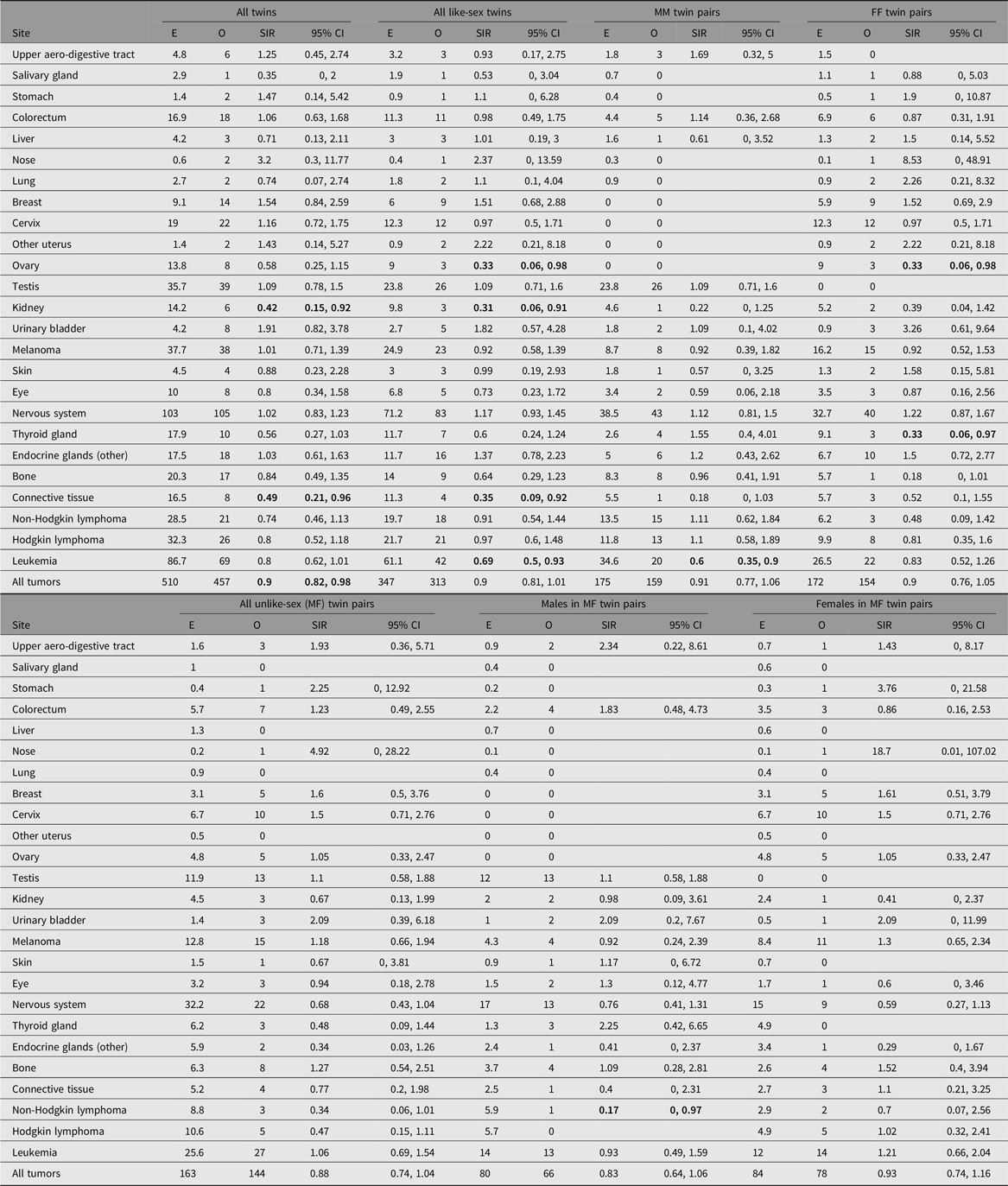
Note: O, observed; E, expected, SIR, standardized incidence ratio; MM, male-male; FF, female-female; MF, male-female. Results are not shown for nine sites (esophagus, small intestine, anus, pancreas, endometrium, other female genitals, prostate, other male genitals, and myeloma) because the observed number of cases was zero in each case. SIRs whose 95% CI does not include 1 are highlighted by bold font.
Discussion
Our updated meta-analysis of childhood cancer in twins confirms both their aggregate cancer mortality and incidence risk reduction of about 15% compared to singletons. It extends to young adult ages more detailed analyses of the Swedish Multigeneration Register/Cancer Registry data, using which we have previously demonstrated a reduced childhood cancer risk that was included in our updated meta-analysis (Murphy et al., Reference Murphy, Bunch, Chen and Hemminki2008). The lifetime risk of cancer in twins has been found to be otherwise unremarkable compared to singletons in the same dataset (Chen et al., Reference Chen, Cnattingius, Nyman Iliadou and Oberg2016; Hemminki & Chen, Reference Hemminki and Chen2005). Our results demonstrate a reduced aggregate cancer risk to ∼30 years of age, contributed by several specific cancer sites. Some of these tumor types contributed to the significant reduction in aggregate childhood cancer risk seen previously. The numbers observed in childhood sometimes constitute the majority of those observed at age 0–29; for example, renal tumors. Nevertheless, the reduced risk is extended in the 0–29 age grouping to tumor types (e.g., connective tissue tumors) similar to those for which significant protection was not observed when considering childhood risks alone, albeit based on very small numbers in childhood and much more substantial numbers by age 30 years.
How far the reduced childhood cancer risk observed in twins extends into the TYA age group has not previously been examined. There are 3 times as many observed cancers on which to base risk estimates to age 30 years, compared to childhood occurrence only, which can lead to narrower confidence intervals around risk estimates. Some tumors occur frequently in both childhood and young adulthood and some do not, with increasing numbers of carcinomas represented in the older age groups. Testicular cancer risk in Swedish twins was (non-significantly) raised in childhood (Murphy et al., Reference Murphy, Bunch, Chen and Hemminki2008), and is barely so here though two overlapping previous Swedish studies of testicular cancer (Braun et al., Reference Braun, Ahlbom, Floderus, Brinton and Hoover1995; Hemminki & Chen, Reference Hemminki and Chen2006) found it to be raised, one significantly so. These studies included cases to older ages than TYA. A meta-analysis also suggests risk of testicular cancer in twins is truly raised across the entire age range in which males are potentially affected (Neale et al., Reference Neale, Carriere, Murphy and Baade2008). This might incriminate the hormonal influences found in a twin pregnancy. Risks of different types of testicular cancer have been related to both extremes of birth weight (high and low) and individual members of twin pairs are generally of lower average birthweight than singletons (Michos et al., Reference Michos, Xue and Michels2007), so it is uncertain how intrauterine growth relates to risk of testicular tumors.
Early explanations to account for the childhood cancer deficit in twins, despite a greater frequency of exposure to X-rays in utero, included the low birth weight distribution of twins and/or selective early mortality of twin fetuses or infants who would otherwise have developed a clinical cancer (Inskip et al.; Reference Inskip, Harvey, Boice, Stone, Matanoski, Flannery and Fraumeni1991; Wakeford & Bithell, Reference Wakeford and Bithell2021). The prenatal environment of twins and singletons does clearly differ. The proportion of twin pregnancies arising from subfertility treatment in the parents is much greater than for singletons (Monden et al., Reference Monden, Pison and Smits2021). Twin pregnancies are also more likely to be complicated by conditions such as pre-eclampsia, gestational diabetes, and hyperemesis. But none of these factors are yet convincingly related to childhood cancer risk, though they may all exert effects on in utero growth patterns.
Like-sex (particularly MM) twin pairs are generally underrepresented among childhood cancer cases, which might result from selective elimination in utero, infancy or childhood of (one or both) cancer prone twins (Inskip et al., Reference Inskip, Harvey, Boice, Stone, Matanoski, Flannery and Fraumeni1991). A recent study has provided some indirect support for this hypothesis (Bruckner et al., Reference Bruckner, Catalano, Das and Lu2021). The starting point for considering selective loss in pregnancy is the primary (conception) sex ratio. Some have reported the number of males and females is usually equal at this time, but others disagree, and it is uncertain whether intrauterine loss is always greater for one than the other throughout the trimesters of pregnancy (James & Grech, Reference James and Grech2020; Orzack et al., Reference Orzack, Stubblefield, Akmaev, Colls, Munné, Scholl, Steinsaltz and Zuckerman2015). However, the secondary (birth) sex ratio almost always favors males in both singletons and twins. Although many twin pairs ‘vanish’ in utero, in natural as well as ART conceptions (at least in MM and FF pairs), the sex ratio at birth is lower than for singletons but still favors males (Chen et al., Reference Chen, Du, Zhao, Lv, Wang, Chen, Zhang, Hu, Jin, Shen, Hu, Xiong, Chen and Ling2017; James & Grech, Reference James and Grech2020; Orzack et al., Reference Orzack, Stubblefield, Akmaev, Colls, Munné, Scholl, Steinsaltz and Zuckerman2015). Although stillbirth rates have been falling for a long time and contribute little numerically to the overall sex ratio at birth, there remains a male excess at stillbirth registration, which has been even higher in the past than now (Shaw, Reference Shaw1989). Thus, considering birth registration data for England and Wales, among nearly 180,000 twin pairs born over the period 1993–2011, 4450 twin pairs included at least one stillbirth. Their average livebirth sex ratio was about 1.01 and the stillbirth ratio about 1.1. In about 3% of MM pairs there was at least one stillbirth, in FF about 2.7%, but among unlike-sex pairs stillbirth occurrence was about 1% for both males and females. Over the same 19-year period, among nearly 12.5 million livebirths there were nearly 75,000 deaths before age 18 years. Low birth weight, being male and a member of a multiple birth were among the factors separately influencing death rates (Watkins et al., Reference Watkins, Kotecha and Kotecha2016). So the potential for selective loss of males throughout the period from birth is clear. It has been suggested that one way to begin to unpick the influence of selective elimination of cancer-prone individuals as a contributor to the reduced cancer risk in twins versus singletons, is to conduct a competing-risks analysis. We believe such a study would need to involve a large historical cohort followed to adulthood, encompassing all births and vital events of interest probably through nationwide record-linkage with causes of stillbirth/death recorded, but also information on a number of potential confounding factors such as subfertility (treatment) and other baby/parental characteristics. Such an analysis is beyond the scope of the present study.
Selective elimination as a potential contributing explanation may also be compatible with the other main hypothesis to explain childhood cancer risk reduction, namely the in utero growth pattern of twins. Males on average weigh more than females at all gestational ages, whether born as singletons or (probably) as members of a like- or unlike-sex twin pair (McKeown & Record, Reference McKeown and Record1952). Although this is generally considered to be due to the effects of androgens on in utero growth, in twins this is complicated by effects of sex-combination of the pair, zygosity, and chorionicity, which may all affect growth velocity and birth weight (discordance) of the pair (Derom et al., Reference Derom, Loos, Thiery, Vlietinck, Fryns and Fryns2005; González-Quintero et al., Reference González-Quintero, Luke, O’Sullivan, Misiunas, Anderson, Nugent, Witter, Mauldin, Newman, D’alton, Grainger, Saade, Hankins and Macones2003; Jelenkovic et al., Reference Jelenkovic, Sund, Yokoyama, Hur, Ullemar, Almqvist, Magnusson, Willemsen, Bartels, Beijsterveldt, Bogl, Pietiläinen, Vuoksimaa, Ji, Ning, Pang, Nelson, Whitfield, Rebato and Silventoinen2018; Luke et al., Reference Luke, Hediger, Min, Brown, Misiunas, Gonzalez-Quintero, Nugent, Witter, Newman, Hankins, Grainger and Macones2005). All twins normally have restricted in utero growth (velocity) compared to singletons from about 30 weeks gestation, and consequently a lower average birthweight per baby of 1000 grams compared to singletons (McKeown & Record., Reference McKeown and Record1952). The in utero growth of twins, and any restriction thereof, may be conditioned by a different balance of factors from those operating in singleton pregnancies (Hall, Reference Hall2003), but the genetic (if not epigenetic) control of in utero growth in twins and singletons is similar (Beck et al., Reference Beck, Pool, van de Weijer, Chen, Krapohl, Gordon, Nygaard, Debrabant, Palviainen, van der Zee, Baselmans, Finnicum, Yi, Lundström, van Beijsterveldt, Christiansen, Heikkilä, Kittelsrud, Loukola and Hottenga2021; van Dongen et al., Reference van Dongen, Gordon, McRae, Odintsova, Mbarek, Breeze, Sugden, Lundgren, Castillo-Fernandez, Hannon, Moffitt, Hagenbeek, van Beijsterveldt, Jan Hottenga, Tsai, Min, Hemani, Ehli, Paul and Boomsma2021).
Many studies have demonstrated an association of intrauterine growth and higher birth weight with increased risks of many childhood cancers, and often have included twin birth weights in their analyses (O’Neill et al., Reference O’Neill, Murphy, Bunch, Puumala, Carozza, Chow, Mueller, McLaughlin, Reynolds, Vincent, Von Behren and Spector2015), though some studies have been restricted to singleton births (Paltiel et al., Reference Paltiel, Lemeshow, Phillips, Tikellis, Linet, Ponsonby, Magnus, Haberg, Olsen, Granstrom, Klebanoff, Golding, Herceg, Ghantous, Hirst, Borkhardt, Ward, Holst Soegaard and Dwyer2019; Paltiel et al., Reference Paltiel, Tikellis, Linet, Golding, Lemeshow, Phillips, Lamb, Stoltenberg, Haberg, Strom, Granstrom, Northstone, Klebanoff, Ponsonby, Milne, Pedersen, Kogevinas, Ha and Dwyer2015). One of the largest international studies of birth weight and childhood cancer risks included twins and demonstrated a 15% change in the aggregate cancer risk for each 1000 g change in birth weight, very comparable to the reduction in childhood cancer risk observed here in our meta-analysis of twins (O’Neill et al., Reference O’Neill, Murphy, Bunch, Puumala, Carozza, Chow, Mueller, McLaughlin, Reynolds, Vincent, Von Behren and Spector2015). One USA study (Puumala et al., Reference Puumala, Carozza, Chow, Fox, Horel, Johnson, McLaughlin, Mueller, Reynolds, Von Behren and Spector2009), which also examined higher multiples, did not find evidence that birth weight accounted for the reduced risk of aggregate childhood cancer seen in twins. Nevertheless, childhood leukemia, renal tumors, and soft tissue sarcomas were among the tumors most strongly related to birth weight in the large international study mentioned above (O’Neill et al., Reference O’Neill, Murphy, Bunch, Puumala, Carozza, Chow, Mueller, McLaughlin, Reynolds, Vincent, Von Behren and Spector2015). Although the tumor classification systems used in that study and used here are not identical, similar tumor types appear to be among those for which low birth weight is associated with reduced cancer risk in childhood, and for which a CTYA risk reduction is most evident here in twins, supporting the idea that intrauterine growth-related mechanisms underpin the risk reduction observed.
There has been a steadily increasing number of twins born worldwide following treatment for subfertility, particularly with use of ART since the 1980s (Monden et al., Reference Monden, Pison and Smits2021). Most of the new information we acquire from now on about CTYA cancer risks in twins and higher multiple births will derive from deliveries following ART. Medical conditions underlying the use of ART for subfertility, and the various forms of ART may affect the birthweight of the offspring differently, at least in singletons (Purkayastha et al., Reference Purkayastha, Roberts, Gardiner, Brison, Nelson, Lawlor, Luke and Sutcliffe2021), but subfertility treatments, including different types of ART, are not clearly associated in meta-analyses with increased CTYA cancer risk among singletons or multiple births (Zhang et al., Reference Zhang, Gao, Chen, Xu, Yang, Zeng, Sun, Zhang, Hu and Qin2020). In fact, the limited published evidence available suggests twins conceived using ART may experience the same kind of childhood cancer risk reduction compared to singletons as natural conceptions (Pinborg et al., Reference Pinborg, Loft and Nyboe Andersen2004). CTYA cancer risk was not importantly altered after maternal ART in the reports from three major studies, though with predominantly childhood cancer outcomes measured (Spector et al., Reference Spector, Brown, Wantman, Letterie, Toner, Doody, Ginsburg, Williams, Koch, Schymura and Luke2019; Sundh et al., Reference Sundh, Henningsen, Kallen, Bergh, Romundstad, Gissler, Pinborg, Skjaerven, Tiitinen, Vassard, Lannering and Wennerholm2014; Williams et al., Reference Williams, Bunch, Murphy, Stiller, Botting, Wallace, Davies and Sutcliffe2018; Williams et al., Reference Williams, Bunch, Stiller, Murphy, Botting, Wallace, Davies and Sutcliffe2013). These three studies (in four Nordic countries, the UK and the US) included 622 childhood cancer cases among nearly 280,000 singleton babies and 210,000 born as part of a multiple birth following IVF/ART, but did not report the twin/singleton risk contrast directly. Three other ART studies around the same time (Hargreave et al., Reference Hargreave, Jensen, Hansen, Dehlendorff, Winther, Schmiegelow and Kjær2019; Reigstad et al., Reference Reigstad, Larsen, Myklebust, Robsahm, Oldereid, Brinton and Storeng2016; Spaan et al., Reference Spaan, van den Belt-Dusebout, van den Heuvel-Eibrink, Hauptmann, Lambalk, Burger and van Leeuwen2019) reported similarly, though the Dutch study (Spaan et al., Reference Spaan, van den Belt-Dusebout, van den Heuvel-Eibrink, Hauptmann, Lambalk, Burger and van Leeuwen2019) included a simple, age-adjusted relative hazard (of 0.99) among children and teenagers combined, for all multiples versus singletons, in its Supplementary tables. An updated report from that group allows calculation of an unadjusted, all cancer risk ratio (95% CI) among all multiples versus singletons up to age 18 years of 0.85 (0.66, 1.08; Spaan et al., Reference Spaan, Pontesilli, van den Belt-Dusebout, Burger, van den Heuvel-Eibrink, Ravelli, Goddijn, Lambalk, Roseboom and van Leeuwen2023). Similarly, a recent study from the US (Luke et al., Reference Luke, Brown, Wantman, Schymura, Browne, Fisher, Forestieri, Rao, Nichols, Yazdy, Gershman, Sacha, Williams, Ethen, Canfield, Doody, Eisenberg, Baker, Williams and Lupo2022), related to the earlier cited study (Spector et al., Reference Spector, Brown, Wantman, Letterie, Toner, Doody, Ginsburg, Williams, Koch, Schymura and Luke2019), did report a heavily adjusted (not crude) childhood cancer risk for twins versus singletons (of 0.97), though the adjusted model did not include birth weight/gestation. Although a recent Taiwanese study (Weng et al., Reference Weng, Huang, Huang, Li and Chien2022) examined intrauterine growth as a confounder for the increased childhood cancer risks they observed following ART conception, the multiple/singleton risk contrast was not presented, nor was it in a further Nordic ART study of CTYA cancer (Sargisian et al., Reference Sargisian, Lannering, Petzold, Opdahl, Gissler, Pinborg, Henningsen, Tiitinen, Romundstad, Spangmose, Bergh and Wennerholm2022). Added to the large numbers contained in the earlier ART reports, these more recent studies combined could contribute data about CTYA cancer cases among about a further 100,000 multiple births, which might allow meaningful stratification of risk between twins and higher multiples separately and help to clarify causal pathways. Additional historical and contemporary data could also come from the NORCAN twin/cancer record-linkage studies (Skytthe et al., Reference Skytthe, Harris, Czene, Mucci, Adami, Christensen, Hjelmborg, Holm, Nilsen, Kaprio and Pukkala2019), whose results already corroborate studies using Swedish data alone about the lifetime cancer risks in twins (Chen et al., Reference Chen, Cnattingius, Nyman Iliadou and Oberg2016; Hemminki & Chen, Reference Hemminki and Chen2005), but which have not yet reported the twin/singleton risk contrast for CTYA cancers separately. In one of these (Skytthe et al., Reference Skytthe, Harris, Czene, Mucci, Adami, Christensen, Hjelmborg, Holm, Nilsen, Kaprio and Pukkala2019) significant risk reductions for a few cancer sites including kidney were noted, and in another study there was also a tendency towards the same reduction (Chen et al., Reference Chen, Cnattingius, Nyman Iliadou and Oberg2016). This corresponds with what we observed at much younger ages, though the nature of the kidney cancers incriminated may not be the same.
Our study has both strengths and some limitations. We believe our iterative literature searches captured every study with relevant published data and our updated meta-analysis includes information from all those studies. However, the mortality studies (from a time when childhood cancer mortality approximated incidence), although coherent, are very old, and there have been no new publications containing extractable twin childhood cancer incidence risks since 2013. Nevertheless, the summary mortality and incidence estimates agree very closely and the more numerous incidence data are not heterogeneous. Both random and fixed effects summary incidence estimates agree closely and sensitivity analyses suggest the results are robust. There are almost no recent published studies of the risk of childhood cancer in twins versus singletons conceived following subfertility treatment. Whether the findings from Denmark from a pure ART cohort (Pinborg et al., Reference Pinborg, Loft and Nyboe Andersen2004) will be sustained is uncertain, but is under investigation. Although no recent study has ever reported an increased risk of childhood cancer in twins versus singletons, we must consider our findings about the extension of risk reduction from childhood to young adulthood, the particular tumors that contribute to this aggregate risk reduction, and any possible influence of subfertility treatment on this risk as provisional.
In conclusion, we believe our analyses of both overall and specific cancer risks in twins contribute to understanding the important overlaps between childhood and TYA tumor risk factors. They point towards the importance of prenatal factors in the determination of risk for both age groups. Further work on both childhood and TYA cancers is needed to understand these risk factors.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/thg.2024.25.
Acknowledgments
Helen Elwell of BMA Library services (London) for assistance with literature searching (Supplement 1). David Smith at QIMR Berghofer (Australia) for suggesting the calculation of the ‘Peto Odds Ratio’. Kathryn Bunch for reading and commenting on an early draft of the manuscript. Much of this work was undertaken while Michael Murphy and Kathryn Bunch were members of the Childhood Cancer Research Group (CCRG) at the University of Oxford. The CCRG received support for its work from the Department of Health, the Scottish Ministers and Children with Cancer UK. We thank Logan Spector for providing unpublished data from the USA study (Puumala et al., Reference Puumala, Carozza, Chow, Fox, Horel, Johnson, McLaughlin, Mueller, Reynolds, Von Behren and Spector2009). Kari Hemminki’s work is supported by the European Union’s Horizon 2020 research and innovation programme, grant No 856620 and the SALVAGE project, reg.no: CZ.02.01.01/00/22_008/0004644. Jian-Rong He was supported by the Chinese Scholarship Council. Carrie Williams was supported by Cancer Research UK [C36038/A12535]; the National Institute for Health Research [405526 to Williams].
Data sharing
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Author contributions
Overall study design: MFGM, BC, KH. Swedish Multigeneration Register data assembly design and analysis: MFGM, BC and KH. Review, data extraction and meta-analysis of childhood cancer studies: MFGM, RR, JH, and CW. Initial drafts of paper were by MFGM, RR and JH. All authors contributed to the interpretation of the results, revision to and final approval of the manuscript. All authors take responsibility for the integrity of the data and the accuracy of its reporting in the analysis.
Competing interests
None.







