Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Roberson, Erik D.
and
Sweatt, J. David
1995.
Regulation of adenylyl cyclase in LTP.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
485.
Kawamura, Satoru
1995.
Unsolved issues in S-modulin/recoverin study.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
479.
Bownds, M. Deric
and
Arshavsky, Vadim Y.
1995.
How many light adaptation mechanisms are there?.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
496.
Hurley, James B.
1995.
Recoverin, a calcium-binding protein in photoreceptors.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
497.
Sagoo, Mandeep S.
and
Lagnado, Leon
1995.
Modulation of the cGMP-gated channel by calcium.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
486.
Willardson, Barry M.
Yoshida, Tatsuro
and
Bitensky, Mark W.
1995.
Cyclic nucleotides as regulators of light-adaptation in photoreceptors.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
493.
Koch, Karl-Wilhelm
1995.
Crucial steps in photoreceptor adaptation: Regulation of phosphodiesterase and guanylate cyclase activities and Ca2+-buffering.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
480.
Hargrave, Paul A.
1995.
Future directions for rhodopsin structure and function studies.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
495.
Wensel, Theodore G.
and
Angleson, Joseph K.
1995.
More answers about cGMP-gated channels pose more questions.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
492.
Rasenick, Mark M.
1995.
Adenylyl cyclase, G proteins, and synaptic plasticity.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
484.
Hurwitz, Richard L.
Srivastava, Devesh
and
Hurwitz, Mary Y.
1995.
Channel structure and divalent cation regulation of phototransduction.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
478.
Bergen, A. A. B.
1995.
Genetic and clinical heterogeneity in tapetal retinal dystrophies.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
470.
Crouch, Rosalie K.
and
Corson, D. Wesley
1995.
The structure of rhodopsin and mechanisms of visual adaptation.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
472.
Molday, Robert S.
and
Hsu, Yi-Te
1995.
Further insight into the structural and regulatory properties of the cGMP-gated channel.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
500.
Oprian, Daniel D.
1995.
Structure of the cGMP-gated channel.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
482.
Sanada, Kamon
and
Fukada, Yoshitaka
1995.
Unique lipids and unique properties of retinal proteins.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
486.
Dratz, Edward A.
1995.
The key to rhodopsin function lies in the structure of its interface with transducin.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
473.
Yamazaki, Akio
1995.
Is the lifetime of light-stimulated cGMP phosphodiesterase regulated by recoverin through its regulation of rhodopsin phosphorylation?.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
494.
Heideman, Warren
1995.
Long term potentiation and CaM-sensitive adenylyl cyclase: Long-term prospects.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
477.
Gray-Keller, Mark P.
and
Detwiler, Peter B.
1995.
Does calmodulin play a functional role in phototransduction?.
Behavioral and Brain Sciences,
Vol. 18,
Issue. 3,
p.
475.
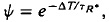 where ΔT is the decrease in saturation period, and where TR* is the slope of the line that relates T and ln If in a given state of adaptation. Dark- and light-adapted responses to flash intensities
where ΔT is the decrease in saturation period, and where TR* is the slope of the line that relates T and ln If in a given state of adaptation. Dark- and light-adapted responses to flash intensities 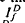 and
and 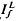 , respectively, exhibited similar absolute peak photocurrent and falling-phase kinetics when
, respectively, exhibited similar absolute peak photocurrent and falling-phase kinetics when 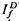 and
and 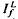 satisfied the relation,
satisfied the relation, 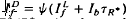 , where Ib is the background intensity. It is argued that ψ approximates the relative PDE*/R* gain of transduction, i.e. the relative peak level of activated cGMP phosphodiesterase (PDE*) produced by a given, small amount of photoactivated visual pigment (R*). Interpreted on this view, the results imply that light adaptation derives largely from a decrease in PDE*/R* gain, rather than from the stimulation of guanylate cyclase activity. The data are consistent with the possibility that modulation of the lifetime of PDE* underlies the background dependence of ψ.
, where Ib is the background intensity. It is argued that ψ approximates the relative PDE*/R* gain of transduction, i.e. the relative peak level of activated cGMP phosphodiesterase (PDE*) produced by a given, small amount of photoactivated visual pigment (R*). Interpreted on this view, the results imply that light adaptation derives largely from a decrease in PDE*/R* gain, rather than from the stimulation of guanylate cyclase activity. The data are consistent with the possibility that modulation of the lifetime of PDE* underlies the background dependence of ψ.