Introduction
The processing tomato (Solanum esculentum L.) industry, a key component of California’s agricultural economy, is under serious threat from the reemergence of the parasitic weed branched broomrape [Phelipanche ramosa (L.) Pomel] (Fatino and Hanson Reference Fatino and Hanson2022; Osipitan et al. Reference Osipitan, Hanson, Goldwasser, Fatino and Mesgaran2021). California accounts for more than 95% of the ∼12.8 Mg of tomatoes produced in the United States in 2023, underscoring the importance of this crop (USDA-NASS 2024). At high infestation levels, broomrape (Philipanche aegyptiaca Pers.) parasitism can lead to yield losses of up to 80% (Eizenberg and Goldwasser Reference Eizenberg and Goldwasser2018). In California, P. ramosa is currently at relatively low levels; however, it is a California Department of Food and Agriculture (CDFA) “A” list quarantine pest, a regulatory status that can lead to crop destruction and field quarantine (CDFA 2020). Phelipanche ramosa is currently of greatest concern to the processing tomato industry in California’s northern growing regions, but the entire tomato industry and other potential host crop commodities are at risk (Martin et al. Reference Martin, Swett, Vinchesi-Vahl and Parreira2021). Because of its quarantine status and limited management options once established, preventing establishment in uninfested fields (exclusion) and reducing spread within infested fields is critical (Martin et al. Reference Martin, Swett, Vinchesi-Vahl and Parreira2021).
A single P. ramosa plant can yield tens or even hundreds of capsules in a single season, resulting in an annual dispersal of between 10,000 and 500,000 seeds (Baird and Riopel Reference Baird and Riopel1986; Ye et al. Reference Ye, Chen, McErlean, Zhang, Yu and Ma2016). The seeds are tiny, with various measurements reported in the literature, from 0.12 by 0.14 mm (Joel and Bar Reference Joel, Bar, Joel, Gressel and Musselman2013) to 0.35 by 0.25 mm (Gibot-Leclerc et al. Reference Gibot-Leclerc, Sallé, Reboud and Moreau2012). Although Phelipanche spp. seed can be transported by water and wind, as well as by animals and people entering fields (Eizenberg et al. Reference Eizenberg, Aly and Cohen2012; Ginman et al. Reference Ginman, Prider, Matthews, Virtue and Watling2015), farm machinery (e.g., tillage and harvest equipment) is likely one of the primary means of P. ramosa seed dispersal across the farmscape because of the amount of soil and plant debris that can be moved on this large, complex equipment (Hershenhorn et al. Reference Hershenhorn, Eizenberg, Dor, Kapulnik and Goldwasser2009; Osipitan et al. Reference Osipitan, Hanson, Goldwasser, Fatino and Mesgaran2021). Processing tomato production in California is highly mechanized, with operational consolidations associated with custom planting and harvest resulting in frequent shared machine use across farms (Baur and Iles Reference Baur and Iles2023). While some equipment is typically used within a small geographic region and represents primarily a field-to-field dispersal risk, other equipment, such as commercial harvesters, transplanters, and fruit transport trailers, is commonly used across multiple counties and represents a risk for long-distance dispersal of P. ramosa seeds to currently uninfested tomato-producing areas of California (Martin et al. Reference Martin, Swett, Vinchesi-Vahl and Parreira2021).
To reduce the risk of spreading P. ramosa seeds, farm equipment should be cleaned and sanitized before being deployed into the next field (Osipitan et al. Reference Osipitan, Hanson, Goldwasser, Fatino and Mesgaran2021). At the time this study was initiated, there were no specific guidelines for effective P. ramosa-targeted sanitation of farm equipment or any guidelines for other soilborne pests that could be similarly managed in this system. However, equipment and facility sanitation protocols used in the food processing, brewing, and dairy industries, where human health pathogens are of high concern (Sansebastiano et al. Reference Sansebastiano, Zoni, Bigliardi, McElhatton and Marshall2007), provide some basic parameters for sanitizing field equipment. Standard cleaning and sanitization procedures for food-contact surfaces typically follow a four- or five-step protocol (Sansebastiano et al. Reference Sansebastiano, Zoni, Bigliardi, McElhatton and Marshall2007). These processes commonly involve a combination of mechanical actions, such as scrubbing, spraying, and scraping, along with chemicals or technologies like heat or radiation as the final sanitation step (Cai et al. Reference Cai, Phinney, Heldman and Snyder2020). Chemical sanitizing agents approved for use on food-contact surfaces include halogens (e.g., chlorine- and iodine-based products), ozone, peroxides (e.g., peracetic acid), and quaternary ammonium compounds (QACs) (Berk Reference Berk2018; Sansebastiano et al. Reference Sansebastiano, Zoni, Bigliardi, McElhatton and Marshall2007).
QACs are surface-active chemicals widely used as sanitation solutions in the food processing industry and health care (Osimitz and Droege Reference Osimitz and Droege2021; Vereshchagin et al. Reference Vereshchagin, Frolov, Egorova, Seitkalieva and Ananikov2021). The chemical structure (a central nitrogen atom bonded to four organic groups and an anion) of QACs provides versatility for various uses, including medical, industrial, household, and agricultural sanitation (Fedorowicz and Sączewski Reference Fedorowicz and Sączewski2024). In addition to having strong antibacterial effects, QACs are effective corrosion inhibitors, a crucial factor for protecting the mild steel and electronics in modern farm equipment (Kudryavtsev et al. Reference Kudryavtsev, Panteleeva, Yurina, Voloshina, Lukashenko and Zobov2011; Migahed et al. Reference Migahed, Shaban, Fadda, Awad and Negm2015). Initial research on the efficacy of QACs suggests that they can effectively prevent the germination of Egyptian broomrape (Orobanche aegyptiaca Pers.; Philipanche aegyptiaca Pers.) (Hershenhorn et al. Reference Hershenhorn, Eizenberg, Dor, Kapulnik and Goldwasser2009) and P. ramosa seed (Hosseini et al. Reference Hosseini, Osipitan and Mesgaran2022) at relatively long-duration exposures. Based on this work, the industry has begun using QAC compounds for equipment sanitation to mitigate the spread of P. ramosa in California. Problematically, given the rapid movement of machines during a hot and dry time of the year, the duration of QAC exposure is typically only a few minutes, and in some cases (such as trailer washes), only a few seconds. Furthermore, the time committed to physically cleaning these large and complex machines often is quite limited due to personnel costs and intense operational schedules; as a result, soil and plant (vegetative and fruit) debris loads often can still be very high at the time of sanitizer applications. The effect of equipment-borne debris loads and debris types on QAC activity on P. ramosa seed is not well known. However, as a charged compound, QACs are known to be sensitive to inactivation by organic matter and soil (Mulder et al. Reference Mulder, Siemens, Sentek, Amelung, Smalla and Jechalke2018). These discrepancies raised concern that QAC-based treatments intended to reduce broomrape spread were being ineffectively utilized by the industry, representing both an economic loss to the producer and a threat to the state, as the movement of broomrape may not be effectively prevented.
At the time this study was initiated, broomrape had become established in only a single county in the northern Central Valley of California, in a relatively small percentage of the total processing tomato acreage in the state (Z Bagley, personal communication), presenting a critical window in which to prevent establishment in more fields in this region and reduce the risk of spread to other processing tomato production regions. The overall goal of this study was to optimize the efficacy of QAC-based sanitizers in preventing the germination of P. ramosa seeds under relatively short exposure durations in the presence or absence of soil and plant debris. This information is key to developing best management guidelines for field equipment cleaning practices to reduce the spread of this critical soilborne quarantine pest in the California processing tomato and other specialty crop industries.
Specific objectives of this study were to: (1) establish the efficacy of individual QAC compounds under short, industry-relevant, exposure durations, and identify the most effective compounds; (2) evaluate the efficacy of commercial sanitizers containing one or more effective QAC under short exposure durations; (3) evaluate how field soil, vegetative plant debris, and fruit debris influence commercial sanitizer efficacy, and whether inhibition could be overcome with the addition of a surfactant or higher QAC concentrations; and (4) place this in an in machina context by evaluating effects of soil-dominated and plant-dominated debris collected from field equipment on commercial sanitizer efficacy. This study, together with in situ field equipment hazard assessment and cleaning studies (Swett et al., unpublished data) are being used to develop best management practices for equipment sanitation that can effectively reduce the risk of P. ramosa dispersal on farm equipment.
Materials and Methods
Seed and Chemicals
The P. ramosa seeds used in these experiments were collected from greenhouse-grown plants from seeds originally collected near (38.757°N, 121.768°W) Woodland, CA in 2019, or were collected directly from plants in a tomato field in the same area in August/September 2022. Seed capsules were collected at maturity, transported to a research site under appropriate state plant pest permit conditions, dried at room temperature, and then crushed and sifted to extract seeds. The seeds were stored in a dark environment at the Contained Research Facility at the University of California, Davis, at 4 C until use in experiments, allowing them to afterripen.
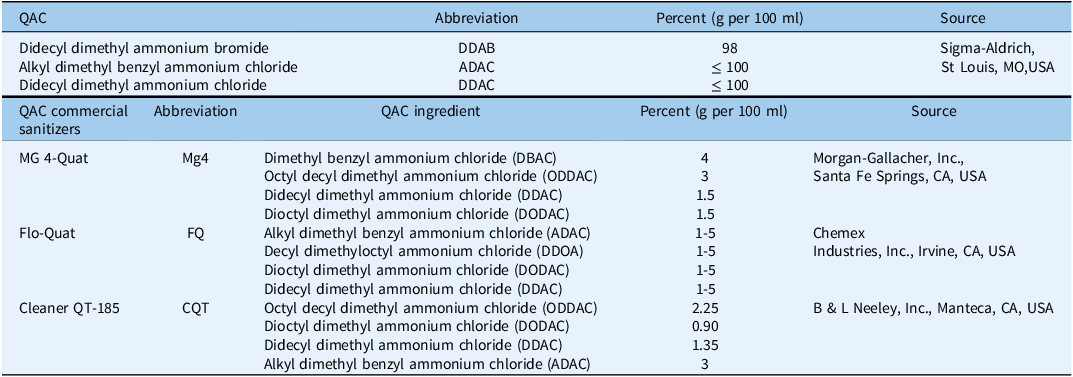
Six chemicals, including three pure QAC compounds and three commercial sanitizers containing these QACs, were used in this study. The QAC compounds were didecyl dimethyl ammonium bromide (DDAB) (CAS No. 2390-68-3), alkyl dimethyl benzyl ammonium chloride (ADAC) (CAS No. 27587-56-0), and didecyl dimethyl ammonium chloride (DDAC) (CAS No.7173-51-5) were obtained from Sigma-Aldrich (3050 Spruce Street, St Louis, MO 63103, USA). The three commercially available QAC sanitizers used in this research were MG4-Quat (Mg4), Flo-Quat (FQ), and Cleaner QT-185 (CQT) (Table 1). Each commercial sanitizer was a mixture of four QAC compounds, including one to three of the individual QACs tested in the initial single-compound experiment.
Table 1. Quaternary ammonium compound (QAC) and QAC commercial sanitizers and their chemical characteristics.
General Methods for All Experiments
Experimental units were approximately 30 to 40 P. ramosa seeds that were initially placed in 2-ml Eppendorf tubes. Sanitizer treatments were conducted by adding a 1-ml aliquot of the specified concentration of each sanitizer chemical to the test tube. Once the appropriate exposure time (which varied among experiments) had elapsed, all seeds were transferred to a cell strainer (pluriStrainer® Mini, 70 µm pluriSelect USA, Inc., El Cajon, CA, USA), rinsed thoroughly three times with distilled water, and placed onto a Whatman filter paper in a 60-mm-diameter petri dish. One ml of deionized water was added to all petri dishes, dishes were sealed using Parafilm® and kept in darkness for 10 d in a 25 C incubator as a preconditioning step. In all experiments, the control treatment was treated with 0.01% v/v Mg4 for 1 min to surface sterilize the seed and prevent fungal contamination in the petri dishes. After preconditioning, the petri dishes were unsealed and air-dried, and 0.7 ml of GR24 (10−5 M MiliQ water; PhytoAB, Cat: PHY0GR24) was added. The petri dishes were then sealed with Parafilm and placed in a dark 25 C incubator for 10 d for germination and radicle elongation.
Ten days after GR24 treatment, all petri dishes were unsealed and allowed to partially dry overnight to reduce sheen from free water before imaging. Images of the seeds and seedlings in each petri dish were captured using a camera (Sony, E3ISPM Series C-mount USB3.0 CMOS) and ToupView image capture software (ToupTek Photonics, Hangzhou, China). Germinated and nongerminated seeds were enumerated manually from the images, and the percent germination was used for data analysis (Figure 1). Seeds were considered germinated when a protruded radicle was visible.
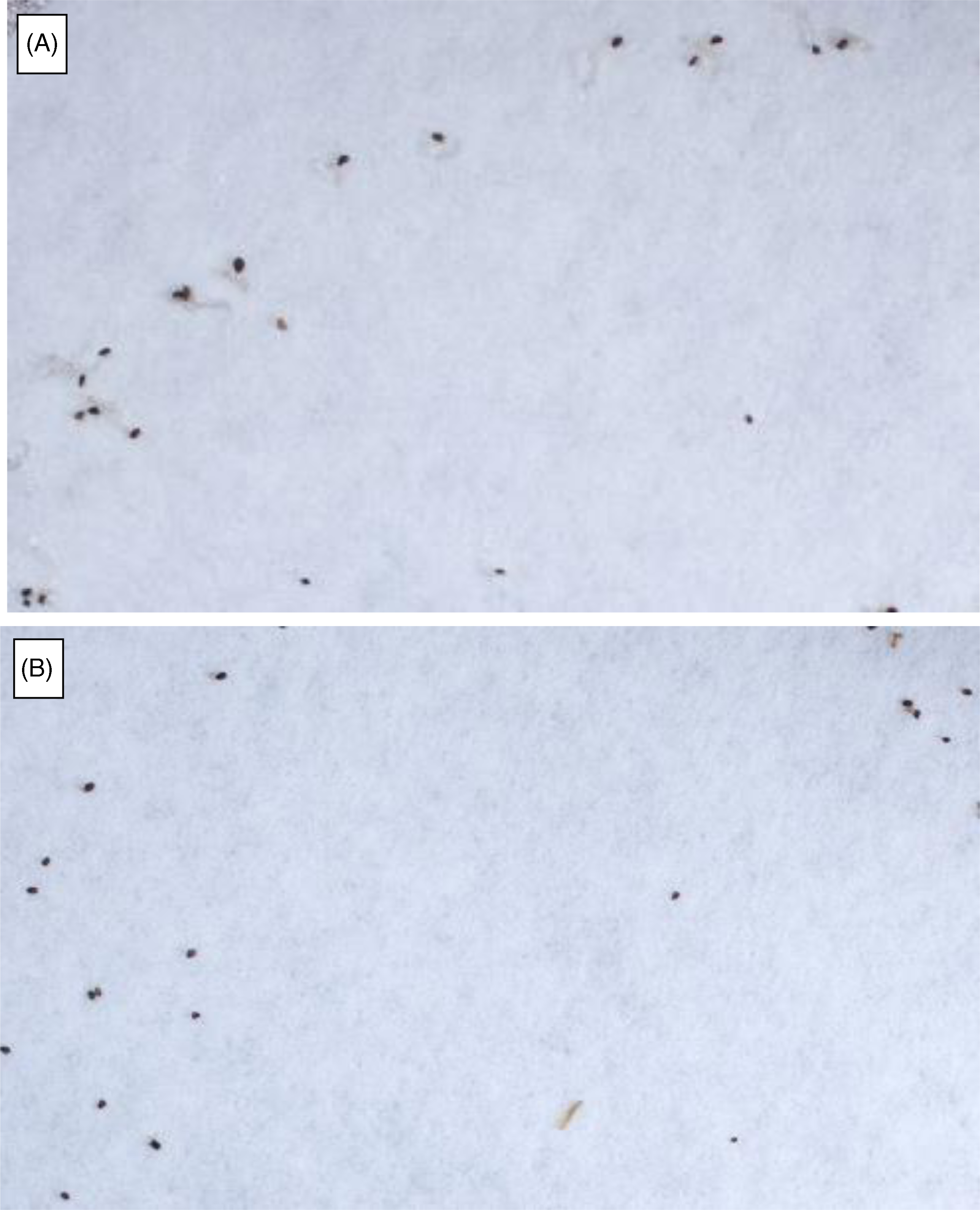
Figure 1. Phelipanche ramosa seed germination response to GR24 (10−5 M MiliQ water) application. (A) Germination observed in control seeds preconditioned with deionized water. (B) No germination observed in seeds treated with QAC (quaternary ammonium compound), where seeds were exposed to 1% (v/v) Mg4 (MG 4-Quat) for 1 min before preconditioning. GR24 (germination stimulator) was applied to both treatments 10 d after preconditioning to induce germination.
QAC Chemical: QAC Compound Efficacy at Variable Doses and Exposure Durations
Three QAC compounds, DDAB, ADAC, and DDAC, were used for these experiments with the previously described general methods (Table 1). Two dose–response experiments with overlapping doses were conducted for each QAC. In the first experiment, seven concentrations of each QAC (including distilled water [0 dose] as the control) were evaluated across four exposure durations (Table 2). In the second experiment, seven concentrations of the QACs (0 as the control, excluding the three higher concentrations from the first experiment and including three additional lower concentrations) were tested with a 1-min exposure duration (Table 2). The three QACs were evaluated with four replicates per treatment in a factorial layout using a completely randomized design (CRD) experiment.
Table 2. Quaternary ammonium compound (QAC) sanitizer doses and exposure durations applied in different experiments. a
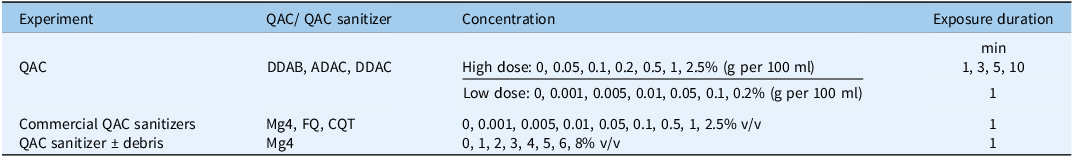
a Abbreviations: ADAC, alkyl dimethyl benzyl ammonium chloride; CQT, Cleaner QT-185; DDAB, didecyl dimethyl ammonium bromide; DDAC, didecyl dimethyl ammonium chloride; FQ, Flo-Quat Sanitizer; Mg4, MG 4-Quat.
Commercial QAC Sanitizers: Commercial Sanitizer Efficacy at Variable Doses under a Short Exposure Duration
Three QAC-containing commercial sanitizers, Mg4, FQ, and CQT, were applied to P. ramosa seeds at nine concentrations (including distilled water [0 dose] as the control) at a 1-min exposure duration (Table 2). The experiment was conducted twice with three replicates per treatment combination using a CRD experiment with a factorial layout.
QAC Sanitizer Experiments in the Presence of Debris
Seed Sanitation with Debris
To introduce debris into each experimental unit, fine particles of debris were mixed with seeds in the Eppendorf tubes at the seed sanitation step of the previously described general protocol (see Supplementary Figure 1 for an example of the debris). The fine debris particles were generated by drying and then grinding the source material and keeping the fraction that passed through a 63-micron sieve; these particles were smaller than P. ramosa seeds and could later be washed away through a 70-micron filter while retaining the treated P. ramosa seed. Five different types of debris were applied in these experiments:
-
1. Field soil: soil collected from a UC Davis research field from a site classified as loam with 44% sand, 36% silt, and 20% clay, with a pH of 7.4 before screening.
-
2. Plant: leaf and stem material from tomato seedlings grown in a greenhouse.
-
3. Fruit: locally purchased fresh, ripe tomato fruit.
-
4. Trailer soil: soil debris collected directly from tomato trailers at a processing facility, primarily from the frame and undercarriage of the equipment, estimated to be primarily soil with relatively little plant tissue.
-
5. Trailer plant: plant debris from surfaces of tomato trailers at a processing facility, primarily from vertical surfaces and surfaces that faced the harvest equipment, estimated to be primarily plant debris with relatively little soil.
Both field soil and trailer soil were oven-dried at 45 C for 2 d and screened with a 63-micron sieve. Plant, fruit, and trailer plant materials were oven-dried at 45 C for 4 d; ground using a high-speed, blade-type food grinder; and screened with a 63-micron sieve.
The three separate experiments on P. ramosa seeds and debris are explained here; each experiment was conducted twice and had three replicates:
-
1. The three commercial sanitizers (Mg4, FQ, and CQT) were tested at the recommended dosage of 1% v/v and 1-min exposure duration using a CRD experiment. Three debris types, including field soil, plant, and fruit, were added to the QAC solutions at three concentrations: 20, 40, and 80 mg ml−1 for plant and fruit powder and 100, 300, and 500 mg ml−1 for soil powder. Phelipanche ramosa seed germination without sanitizer (distilled water) and with sanitizers but without any debris was used as control.
-
2. A surfactant (Lansurf AEP63, Lankem (Lankem Ltd., Cheshire, UK)) at 0.5% v/v was added to the solution. Only Mg4 at 1% v/v of Mg4 and field soil at 100 mg ml−1 were used for this experiment. Exposure durations of 1, 5, and 10 min were used in this experiment, following a factorial layout in a CRD experiment. Phelipanche ramosa seed germination with and without Mg4 was used as the control.
-
3. Higher doses of Mg4, up to 8% v/v plus a no-QAC control treatment (Table 2), in the presence of all five debris types were applied to P. ramosa seeds for a 1-min exposure duration in a CRD experiment. In these experiments, debris loads were 100 mg ml−1 for field soil and trailer soil and 40 mg ml−1 for plant, trailer plant, and fruit material.
Data Analysis
Dose–response analyses for seed germination data were analyzed with the drc package (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015) in R software (R Core Team 2020) following the guideline provided by Keshtkar et al. (Reference Keshtkar, Kudsk and Mesgaran2021). Data from both experimental runs were pooled for all experiments (except the QAC chemical experiment), as there was no difference between them. A three-parameter log-logistic function (Equation 1) (Streibig Reference Streibig1993) best described the seed germination of P. ramosa in relation to the QAC concentrations of all dose–response experiments:
where Y represents the total percent seed germination of P. ramosa, e is the effective dose required to achieve a 50% response (ED50), u is the upper limit, and b is the relative slope at the inflection point (e).
To determine whether the model parameters differ between the QACs in each experiment, the error sizes were compared between a full model and various reduced models (with one or two parameters removed). An F-test was performed to compare the error between two types of models using the anova() function from the MASS package (Venables and Ripley Reference Venables, Ripley, Venables and Ripley2002) in R. For instance, to test the null hypothesis that the parameter e (ED50) does not vary among the ADAC, DDAB, and DDAC compounds, a reduced model was first fit, assuming a single e parameter across the dose–response curves of these three chemicals. A full model, incorporating individual e parameters for each compound, was then fit to the same data. The more complex full model is supported, and the three curves are considered to differ in parameter e if the model error is significantly smaller than that of the reduced model, as indicated by the F-test. Similar tests were conducted for all other model parameters to assess whether they differ across chemical compounds or exposure duration treatments (Hosseini et al. Reference Hosseini, Osipitan and Mesgaran2022; Keshtkar et al. Reference Keshtkar, Kudsk and Mesgaran2021). Non–dose response experiments were analyzed using ANOVA and LSD mean comparisons, with significance set at P < 0.05. A linear model (Equation 2) was used across three separate QAC experiments in the presence of varying amounts of debris.
Results and Discussion
QAC Chemical: QAC Compound Efficacy at Variable Doses and Exposure Durations
Overall, all three QACs effectively prevented the germination of P. ramosa seeds under at least one concentration by exposure duration treatment (Figure 2). Comparing various reduced models with the full three-parameter model showed that the upper limit, u, parameter could be fixed across curves of the three QACs without significantly (P = 0.11) reducing the goodness of fit (Tables 3 and 4). As represented by parameter u, total seed germination in the control treatments for the three experiments was 80% (Tables 3 and 4). The three individual QACs—ADAC, DDAB, and DDAC—effectively inhibited the germination of P. ramosa seeds. The dose for reducing germination by 50% (parameter e) varied significantly (P < 0.0001) among QACs and exposure durations, ranging from 0.001% (g per 100 ml) (SE = 0.003) at 10 min with DDAC to 0.35% (SE = 0.03) (g per 100 ml) at 1 min with ADAC (Table 3).
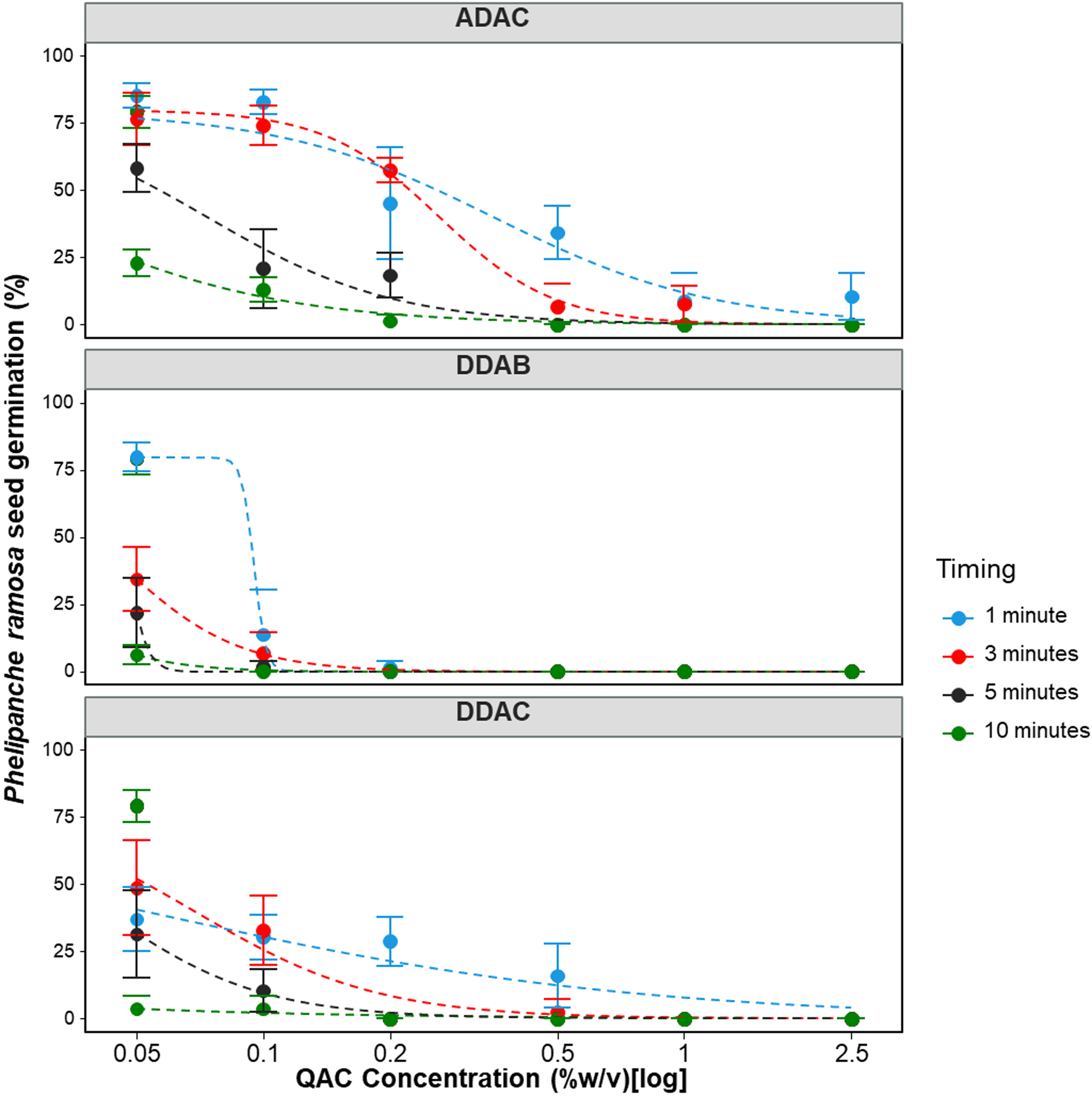
Figure 2.
Phelipanche ramosa seed germination in response to three different quaternary ammonium compounds (QACs) over various exposure durations from 1 to 10 min. A three-parameter logistic model (Equation 1) was fit to all data:
![]() $Y = {{u}\over{{\{ 1 + {\rm{exp}}[b(\log (x) - \log (e))]\} }}.{\rm{\;\;\;\;\;\;\;\;}}}$
Lines are fitted values, and solid circles indicate observed germination (n = 4). Error bars indicate 95% confidence intervals. Model parameter estimates are shown in Table 3. ADAC, alkyl dimethyl benzyl ammonium chloride; DDAB, didecyl dimethyl ammonium bromide DDAC; didecyl dimethyl ammonium chloride.
$Y = {{u}\over{{\{ 1 + {\rm{exp}}[b(\log (x) - \log (e))]\} }}.{\rm{\;\;\;\;\;\;\;\;}}}$
Lines are fitted values, and solid circles indicate observed germination (n = 4). Error bars indicate 95% confidence intervals. Model parameter estimates are shown in Table 3. ADAC, alkyl dimethyl benzyl ammonium chloride; DDAB, didecyl dimethyl ammonium bromide DDAC; didecyl dimethyl ammonium chloride.
Table 3. Estimated parameter values for the three-parameter log-logistic models used to describe Phelipanche ramosa seed germination in response to increasing doses of three quaternary ammonium compounds (QACs) at different exposure durations. a
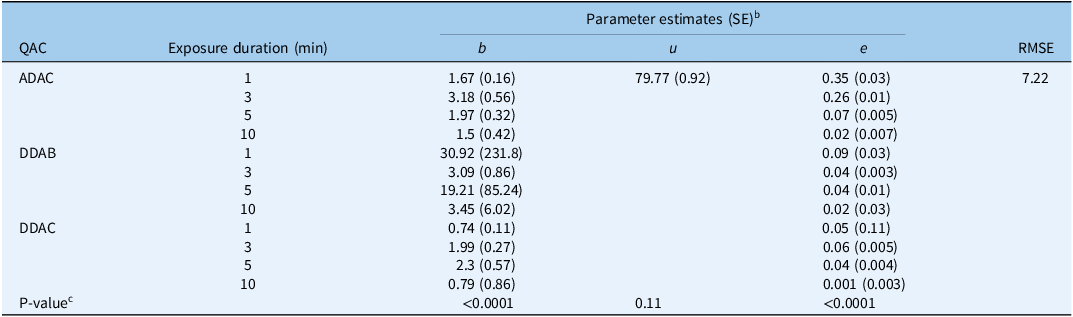
a Abbreviations: ADAC, alkyl dimethyl benzyl ammonium chloride; DDAB, didecyl dimethyl ammonium bromide; DDAC, didecyl dimethyl ammonium chloride; RMSE, root mean-square error.
b In the model, b represents the slope at the inflection point; u is the upper limit, i.e., maximum seed germination when the dose of the ammonium compound is zero; and e is the dose that produces a germination response half the u value (ED50).
c If there is a single value for the parameter, it means the parameter was fixed across ammonium compounds and exposure durations because of a nonsignificant P-value for the comparison of the full three-parameter model vs. the reduced models.
Table 4. Estimated parameter values for the three-parameter log-logistic models used to describe the Phelipanche ramosa seed germination responses to a lower dose range of different ammonium compounds over a 1-min exposure duration. a
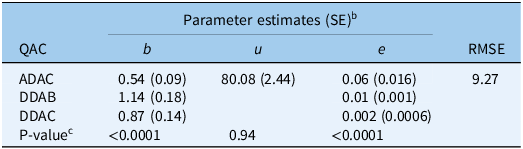
a Abbreviations: ADAC, alkyl dimethyl benzyl ammonium chloride; DDAB, didecyl dimethyl ammonium bromide; DDAC, didecyl dimethyl ammonium chloride; QAC, quaternary ammonium compound; RMSE, root mean-square error.
b In the model, b represents the slope at the inflection point; u is the upper limit, i.e., maximum seed germination when the dose of the ammonium compound is zero; and e is the dose that produces a germination response half the u value (ED50).
c If there is a single value for the parameter, the parameter was fixed across ammonium compounds and exposure durations because of a nonsignificant P-value for comparing full vs. reduced models.
The germination responses of P. ramosa seeds to DDAB and DDAC exhibited a sharp decline, with germination reduced by 50% even at the lowest dose of these two QACs following just 1 min of exposure (Figure 2). However, the decline rate in preventing germination with ADAC was comparatively slower (b = 0.54) than other compounds. The parameter e (ED50) values of DDAB, DDAC, and ADAC decreased with prolonged exposure durations (Table 3).
Repeating the experiment with lower doses and the shortest exposure duration (1 min) confirmed the findings of the initial experiments (Table 4; Figure 3). DDAB and DDAC exhibited a notable effect on reducing P. ramosa seed germination, with complete prevention achieved at the highest concentration tested (0.2% (g per 100 ml) for 1 min). ADAC, however, did not entirely prevent P. ramosa seed germination at 0.2% (g per 100 ml) and shorter exposure durations. The ED50 values in this experiment varied, ranging from 0.002 (SE = 0.0006) (g per 100 ml) for DDAB to 0.06 (SE = 0.016) (g per 100 ml) for ADAC.
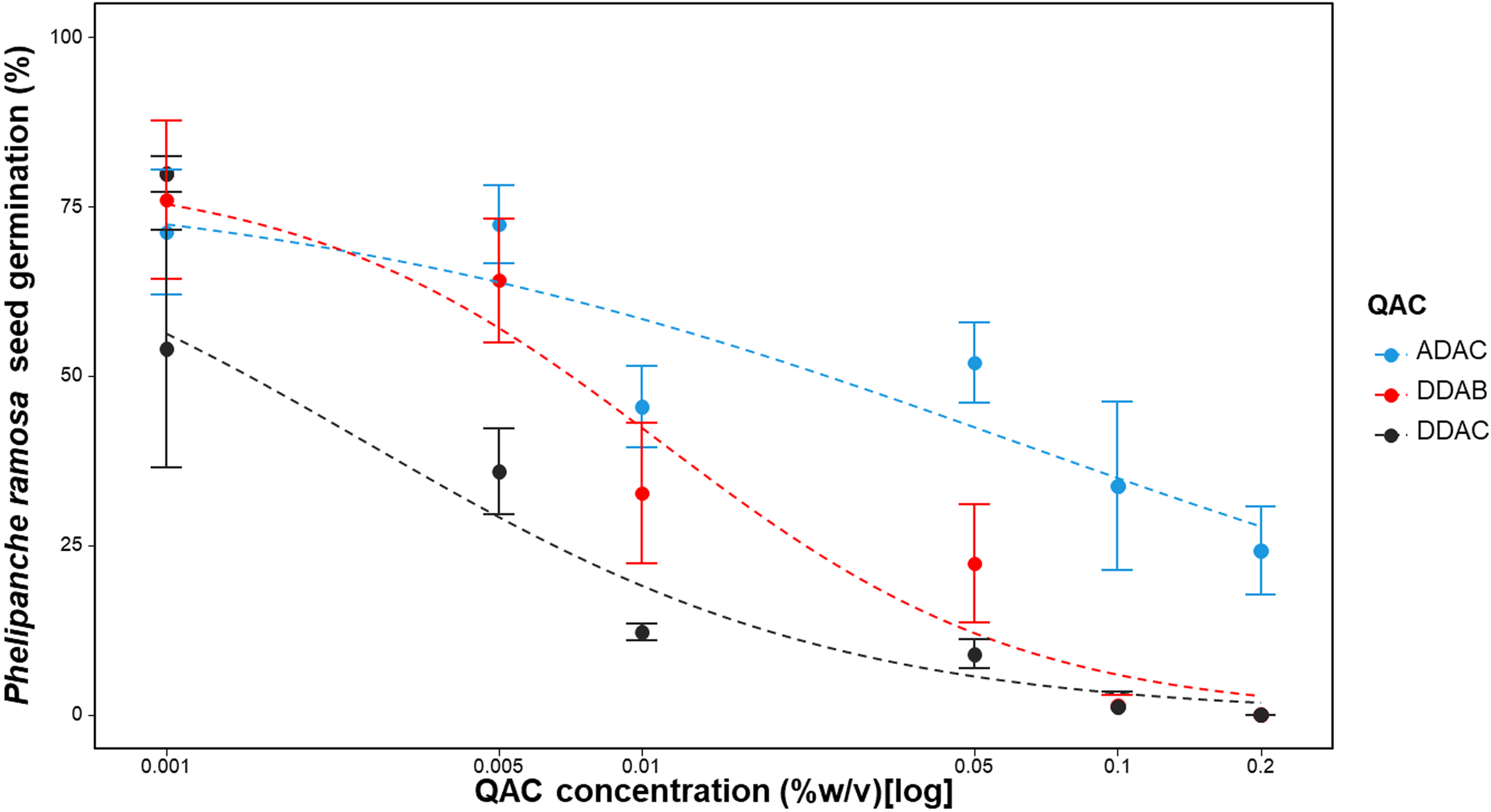
Figure 3.
Phelipanche ramosa seed germination in response to lower doses of three different quaternary ammonium compounds (QACs) at a 1-min exposure duration. A three-parameter logistic model (Equation 1) was fit to all data:
![]() $Y = {{u}\over{{\{ 1 + {\rm{exp}}[b(\log (x) - \log (e))]\} }}}$
. Lines are fitted values, and solid circles indicate observed germination (n = 4). Error bars indicate 95% confidence intervals. Model parameter estimates are shown in Table 4. ADAC, alkyl dimethyl benzyl ammonium chloride; DDAB, didecyl dimethyl ammonium bromide; DDAC, didecyl dimethyl ammonium chloride.
$Y = {{u}\over{{\{ 1 + {\rm{exp}}[b(\log (x) - \log (e))]\} }}}$
. Lines are fitted values, and solid circles indicate observed germination (n = 4). Error bars indicate 95% confidence intervals. Model parameter estimates are shown in Table 4. ADAC, alkyl dimethyl benzyl ammonium chloride; DDAB, didecyl dimethyl ammonium bromide; DDAC, didecyl dimethyl ammonium chloride.
The findings of the initial QAC experiments highlight the effectiveness of QACs in inhibiting P. ramosa seed germination and confirm the results of previous research on P. aegyptiaca and P. ramosa seeds (Hershenhorn et al. Reference Hershenhorn, Eizenberg, Dor, Kapulnik and Goldwasser2009; Hosseini et al. Reference Hosseini, Osipitan and Mesgaran2022). The three QAC chemical compounds exhibit slightly different efficacy on P. ramosa. DDAC showed the best efficacy, while ADAC was the least effective. Other researchers have noted that the efficacy of QAC disinfection on pathogens varies significantly, with later-generation QACs, from the fifth to the second generation, demonstrating greater effectiveness (Copes and Ojiambo Reference Copes and Ojiambo2023). In the current study, DDAB and DDAC, which are both fourth-generation QACs, were slightly more effective than ADAC, a second-generation QAC, in preventing P. ramosa seed germination under short exposure durations. Based on this work, three commercial sanitizers containing several QACs including ADAC and DDAC (Table 1) were evaluated in subsequent studies.
Commercial QAC Sanitizers: Commercial Sanitizer Efficacy at Variable Doses under a Short Exposure Duration
Commercial sanitizers (Mg4, CQT, and FQ), each containing a mix of four QACs as their active ingredients (Table 1), demonstrated effectiveness in preventing the germination of P. ramosa seeds (Figure 4). These sanitizers, like the individual QAC chemical compounds, reduced the germination of P. ramosa seeds within a short exposure time (1 min). Comparing various reduced models with the full three-parameter model showed that both the u (upper limit) and e (ED50) parameters could be fixed across curves of the three QAC sanitizers without significantly (P = 0.11) reducing the goodness of fit (Table 5). The parameter e did not vary among Mg4, FQ, and CQT, indicating that the three sanitizers were not significantly different in ED50. The decrease in P. ramosa seed germination was noticeable at concentrations as low as 0.05% v/v with all three commercial sanitizers. At the recommended concentration of 1% v/v, very little germination (approximately 5%) was observed with any of the QAC sanitizers (Figure 4).
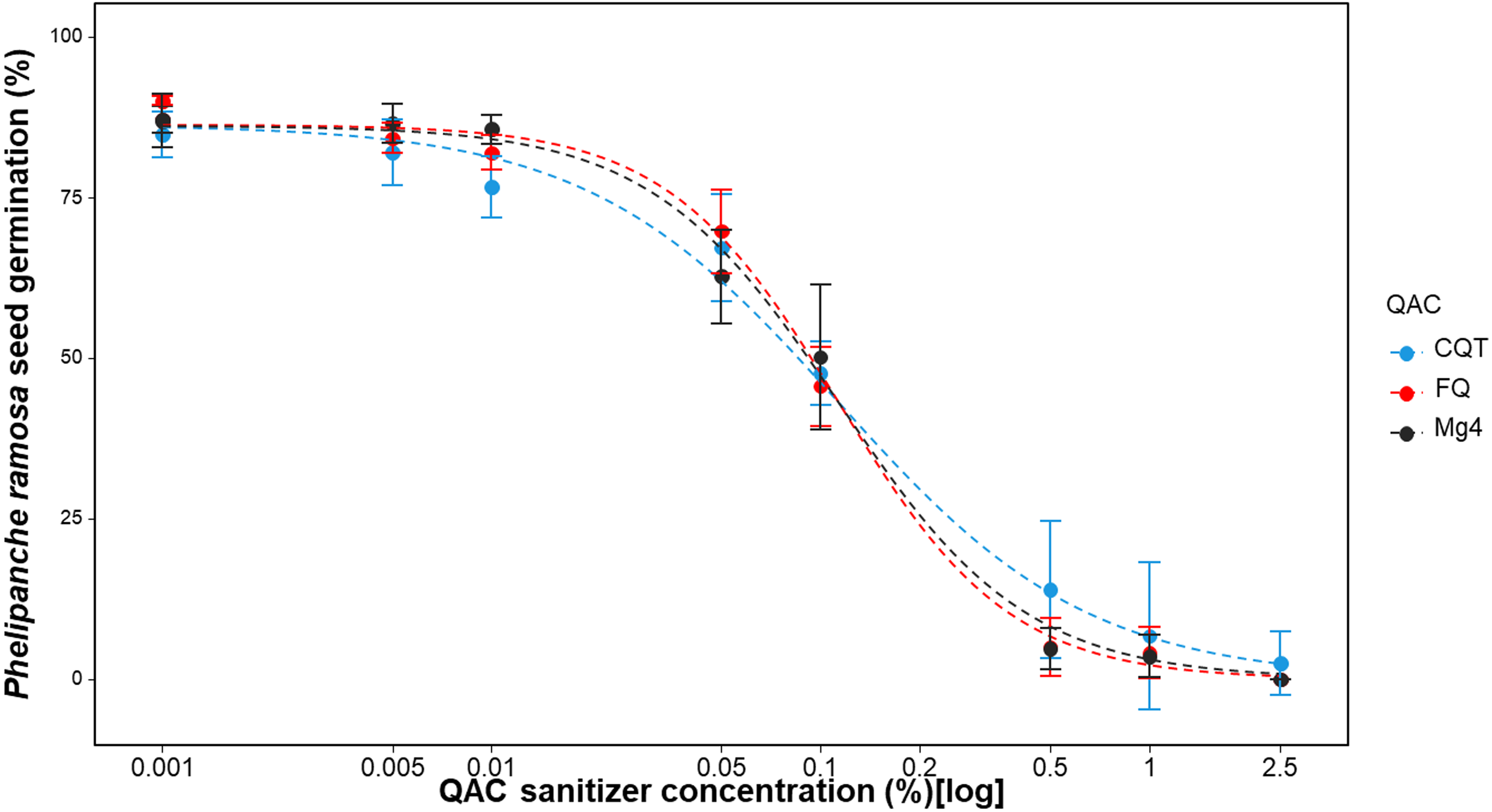
Figure 4.
Phelipanche ramosa seed germination in response to doses of commercial sanitation agents for 1 min. A three-parameter logistic model (Equation 1) was fit to all data:
![]() $Y{\rm{\;}} = {{u}\over{{\{ 1 + {\rm{exp}}[b(\log (x) - \log (e))]\} }}}$
. Lines are fitted values, solid circles indicate observed germination (n = 6), and error bars indicate 95% confidence intervals. Model parameter estimates are shown in Table 5. QAC, quaternary ammonium compound; Sanitizers: CQT, Cleaner QT-185; FQ, Flo-Quat; Mg4, MG 4-Quat.
$Y{\rm{\;}} = {{u}\over{{\{ 1 + {\rm{exp}}[b(\log (x) - \log (e))]\} }}}$
. Lines are fitted values, solid circles indicate observed germination (n = 6), and error bars indicate 95% confidence intervals. Model parameter estimates are shown in Table 5. QAC, quaternary ammonium compound; Sanitizers: CQT, Cleaner QT-185; FQ, Flo-Quat; Mg4, MG 4-Quat.
Table 5. Estimated parameter values for the three-parameter log-logistic models used to describe the Phelipanche ramosa seed germination responses to 1-min exposure to commercial quaternary ammonium compound (QAC) sanitizers. a
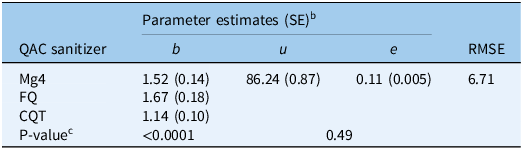
a Abbreviations: CQT, Cleaner QT-185; FQ, Flo-Quat Sanitizer; Mg4, MG 4-Quat; RMSE, root mean-square error.
b In the model, b represents the slope at the inflection point; u is the upper limit, i.e., maximum seed germination when the dose of the ammonium compound is zero; and e is the dose that produces a germination response half the u value (ED50).
c If there is a single value for the parameter, the parameter was fixed across ammonium compounds and exposure durations because of a nonsignificant P-value for comparing full vs. reduced models.
In line with these results, Bromosept 50, a QAC sanitizer containing a single QAC (DDAB), was used to prevent the germination of P. aegyptiaca (Hershenhorn et al. Reference Hershenhorn, Eizenberg, Dor, Kapulnik and Goldwasser2009). Hershenhorn et al. (Reference Hershenhorn, Eizenberg, Dor, Kapulnik and Goldwasser2009) reported that Bromosept 50 at 1% (g per 100 ml) concentration completely inhibited seed germination in petri dishes and on the pure seeds placed on a commercial tomato harvester. The same research demonstrated that Zoharquat 50 containing ADAC (a second-generation QAC) at a concentration of 1% (g per 100 ml) decreased P. aegyptiaca seed germination by 20% and that increasing the QAC concentration to 10% (g per 100 ml) resulted in complete inhibition of seed germination (Hershenhorn et al. Reference Hershenhorn, Eizenberg, Dor, Kapulnik and Goldwasser2009); however, it should be noted that those experiments were conducted in a debris-free environment.
QAC Sanitizer Experiments in the Presence of Debris
QACs significantly (P < 0.05) reduced P. ramosa seed germination in the absence of debris (Figure 5, left). The germination of P. ramosa seeds exceeded 80% in the absence of QAC, and all sanitizers reduced germination to less than 5% at the recommended dose in the absence of debris (Figure 5, left). However, when seeds were mixed with debris before exposure to QAC solutions, the sanitation efficacy was greatly reduced at all three debris levels (Figure 5, right). Soil at all doses inhibited all commercial sanitizers, resulting in seed germination comparable to the no-debris, no-sanitizer control at all soil concentrations. Plant debris also reduced QAC sanitizer efficacy, resulting in similar levels to untreated controls at high concentrations, but with some retained efficacy (45% germination) at lower debris levels (Figure 5, right). Fruit debris had the least effect on QAC sanitizer efficacy, with 25% germination at low and medium debris levels across products; however, germination levels were similar to those of untreated controls at the highest fruit debris level (Figure 5, right).
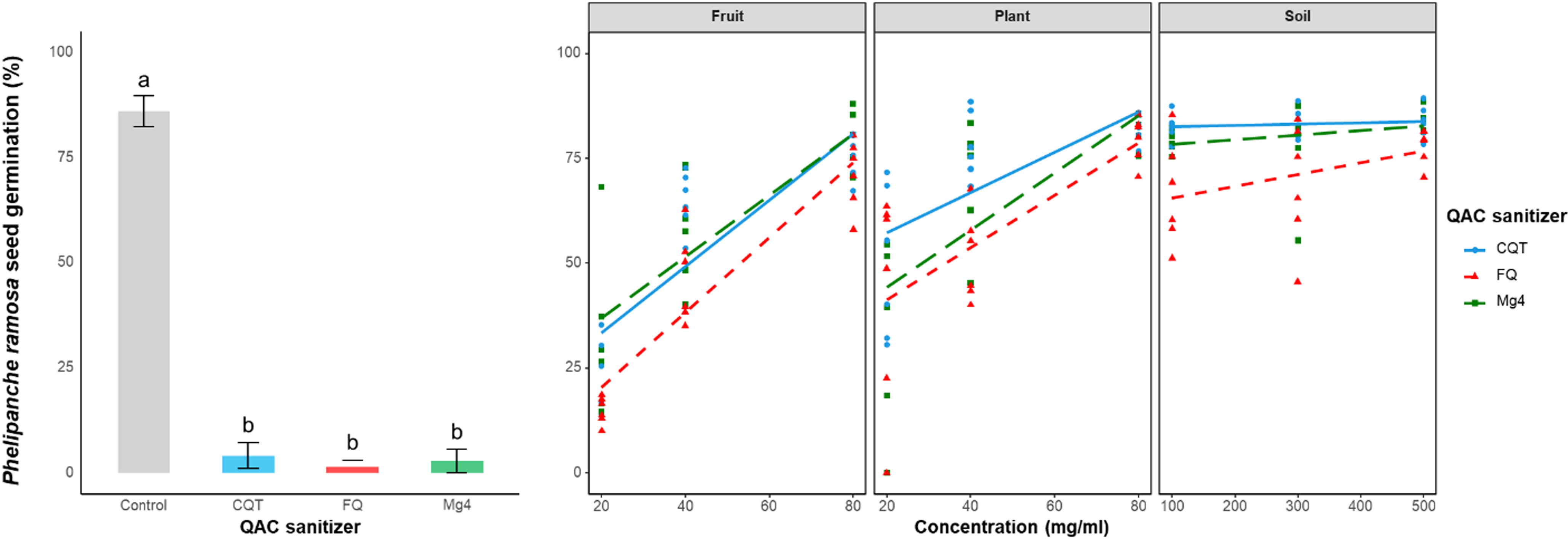
Figure 5. Phelipanche ramosa seed germination in the presence of three levels of soil, plant, and fruit debris (right). A linear model (Equation 2) was fit to all data. The control germination with no sanitizer and germination in the presence of each quaternary ammonium compound (QAC) sanitizer at 1% (v/v) (left). CQT, Cleaner QT-185; FQ: Flo-Quat; Mg4, MG 4-Quat. Data are means ± SE (n = 6), and treatments with various letters differ significantly according to one-way ANOVA. The debris levels were soil at 100, 300, and 500 mg ml−1 and plant and fruit powder at 20, 40, and 80 mg ml−1.
Consistent with our results, a study evaluating nine quaternary ammonium–based sanitizers on Fusarium oxysporum (Arango-Palacio et al. Reference Arango-Palacio, Pinzón-Núñez, Hoyos-Carvajal, Ospina-Galeano, Feria-Gómez, Izquierdo-García, Betancourt-Vásquez and Zapata-Henao2024) reported that QACs were 100% effective in the absence of soil. However, only one QAC (from the fifth generation) was effective against the pathogen in the presence of soil, emphasizing the influence of inorganic matter on QAC efficacy. The same research showed that fine-textured soil (clay) had a greater impact on QAC efficacy than sand. In the current study, our soil debris came from a soil with 20% clay and was finely sieved before use in the experiment—both factors may have enhanced the inhibitory capacity of this soil treatment. In other regions where soil has a lower clay content, soil debris may be less inhibitory. In addition to deactivation, soil and organic matter also absorb the QAC, which means that while QACs are still present in the soil or plant debris, they may be less effective because they are not freely available (Mulder et al. Reference Mulder, Siemens, Sentek, Amelung, Smalla and Jechalke2018).
The commercial QAC sanitizers did not differ from one another on P. ramosa seed germination in the presence of soil (Figure 5, right), although there was a trend in which FQ was less effective than the other two commercial products, especially at the lower soil levels. Mg4 was selected for further experiments, given its consistently high efficacy in the presence of all debris types and frequency of use in the industry in the P. ramosa-impacted region of California (Z Bagley, personal communication); in addition, this product contained DDAC, one of the two more highly effective QACs identified in the single-compound studies above.
Adding a surfactant to Mg4 and extending the exposure duration did not have a significant (P < 0.05) effect on efficacy (Figure 6). Because the surfactant and longer exposure times did not improve the inhibition of P. ramosa seed germination, it is inferred that the reduced activity is due to rapid deactivation or binding to the soil rather than to a seed-related exposure phenomenon (Figure 6). The QACs are cationic, and soil particles often carry negative charges; therefore, the interaction between QACs and soil particles is driven mainly by electrostatic interactions (Mulder et al. Reference Mulder, Siemens, Sentek, Amelung, Smalla and Jechalke2018).
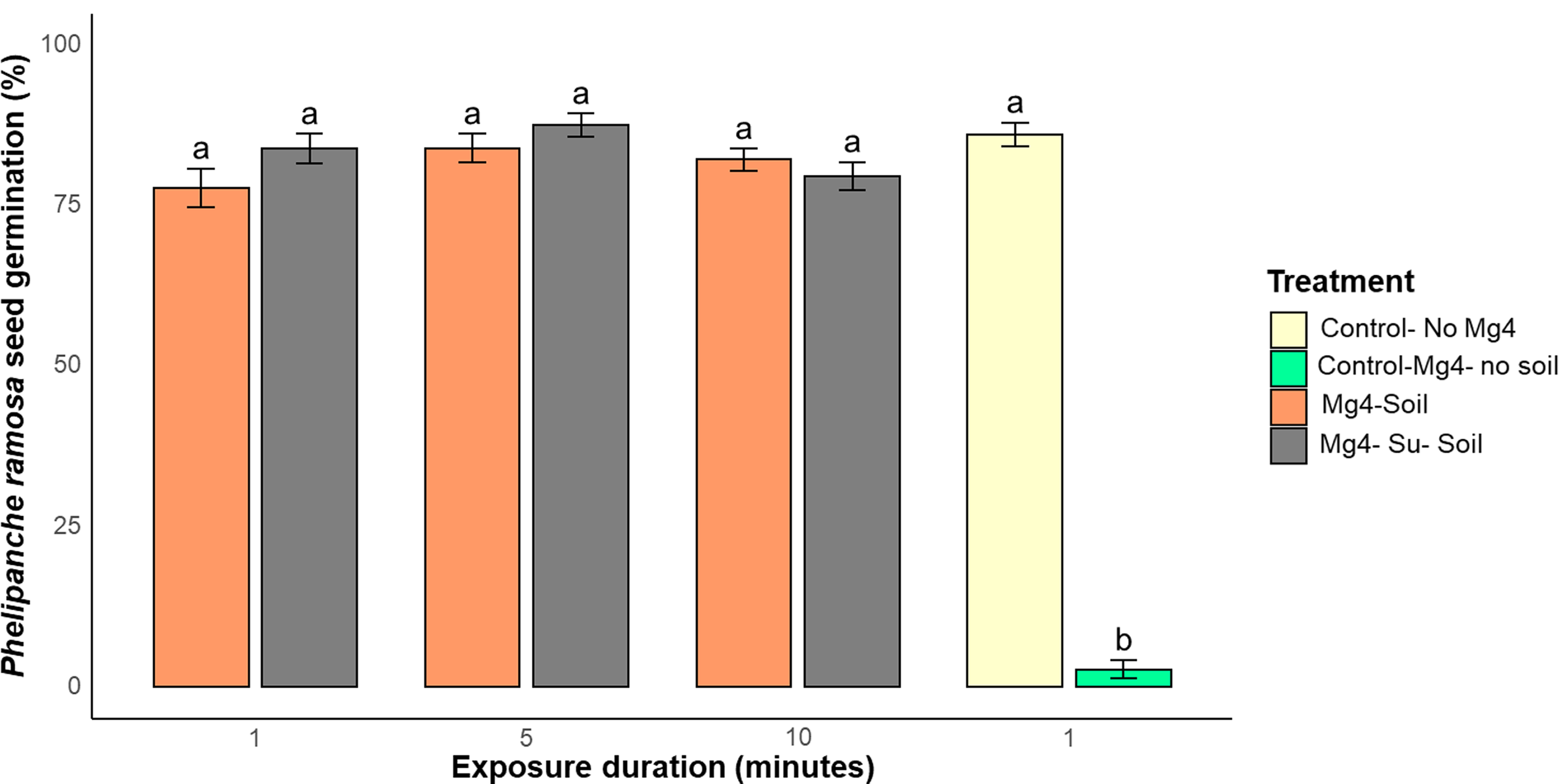
Figure 6. Phelipanche ramosa seed germination with MG 4-Quat (Mg4) sanitizer at 1% (v/v) concentration in the presence of 100 mg ml−1 soil powder for 1-, 5-, and 10-min exposure duration with or without surfactant. Su, Lansurf AEP63 surfactant. Data are means ± SE (n = 6), and treatments with the same letters did not differ according to two-way ANOVA.
To examine the effectiveness of Mg4 at higher concentrations in the presence of debris, the concentration of Mg4 was increased up to 8% v/v. Parameter estimates for the increasing Mg4 concentrations are shown in Table 6. Comparing various reduced models with the full model showed that both the upper limit, u, and slope, b, parameters can be fixed, and the reduced model was used for a comparison across the five debris categories without significantly (P = 0.11) reducing the goodness of fit (Table 6). The ED50 (parameter e) varied between plant and soil but remained relatively consistent between the two soil sources (field soil, trailer soil) and the trailer plant (>4% v/v). The QAC efficacy in the no-debris experiment suggested an ED50 of 0.11% (Table 5), while this experiment indicated that the presence of debris increased the ED50 to 3% to 5% v/v for the Mg4 product (Table 6), a 27- to 50-fold increase.
Table 6. MG 4-Quat (Mg4) dose (% v/v) resulting in 50% reduction in Phelipanche ramosa seed germination in the presence of soil and plant debris a .
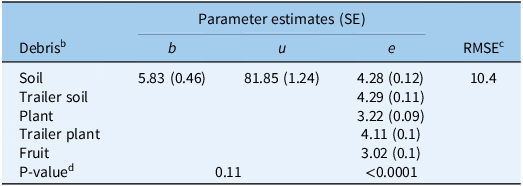
a Calculated using the three-parameter log-logistic models used to describe P. ramosa seed germination responses to increasing Mg4 dose. In the model, b represents the slope at the inflection point; u is the upper limit, i.e., maximum seed germination when the dose of the sanitizer (Mg4) is zero; and e is the dose that produces a germination response half the u value (ED50).
b Debris types: fruit, ripe tomato fruit; plant, from tomato seedlings; soil, field soil collected near Davis, CA, USA; trailer plant, plant debris collected from trailers; trailer soil, soil debris collected from trailers.
c RMSE, root mean-square error.
d If there is a single value for the parameter, it means the parameter is fixed across debris type because of the nonsignificant P-value for the comparison of full vs. reduced models.
The germination curve for soil, trailer soil, and trailer plant debris in the presence of a constant amount of debris showed a gradual decrease in P. ramosa seed germination as the Mg4 concentration increased to 3% v/v, with a steep decrease from 3% to 8% v/v (Figure 7). Higher concentrations of Mg4 (up to 8% v/v) did improve efficacy, particularly in the presence of lower amounts of soil and plant/fruit debris. This suggests that increasing QAC concentrations or higher volumes of sanitizer solution may partially overcome challenges with use on incompletely cleaned surfaces; however, more research is needed to validate and determine how this applies to the levels of debris encountered on field equipment, which can exceed 5 cm of thickly caked and compressed soil/plant debris.
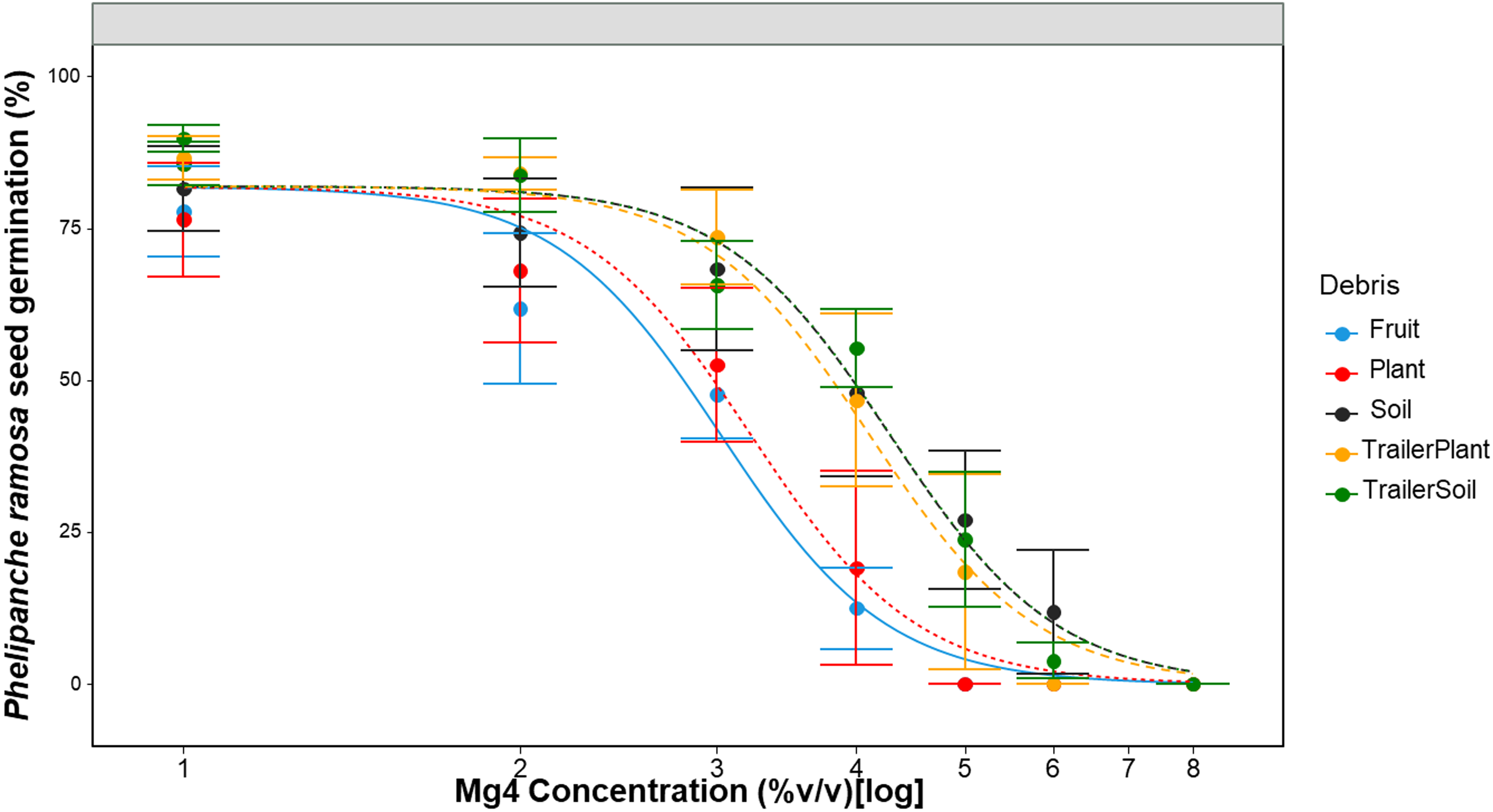
Figure 7. Phelipanche ramosa seed germination in response to increasing doses of Mg4 in the presence of various debris for 1 min. A three-parameter logistic model (Eq. 1) was fitted to all data. Lines are fitted values, solid circles indicate observed germination (n= 6), and error bars indicate 95% confidence intervals. Model parameter estimates are shown in Table 6. Abbreviations: Mg4: MG 4-Quat, fruit: tomato fruit powder at 40 mg ml−1 plant: tomato seedling powder at 40 mg ml−1, soil: soil powder collected from Davis fields at 100 mg ml−1, trailer plant: plant debris powder collected from trailers at 40 mg ml−1, trailer soil: soil debris powder collected from trailers at 100 mg ml−1.
The experimental approach used in this study followed methodologies previously established in similar seed germination inhibition research. Specifically, we adapted protocols from prior work on the effects of QACs on P. aegyptiaca (Hershenhorn et al. Reference Hershenhorn, Eizenberg, Dor, Kapulnik and Goldwasser2009) as well as standardized methods for testing chemical germination inhibitors in controlled laboratory conditions (López-Granados and García-Torres Reference López-Granados and García-Torres1996; Westwood and Foy Reference Westwood and Foy1999). It is important to note that while reductions in germination relative to the control treatment suggest that QAC treatments affect seed viability, this is not a direct measure of seed mortality. Assessing seed mortality would require additional assays, such as tetrazolium staining, although these techniques can be challenging on small seeds and can lack precision (Hezewijk et al. Reference Hezewijk, Beem, Verkleij and Pieterse1993). Developing methods to rapidly and accurately assess the viability of P. ramosa seed treated with QAC and other sanitizing treatments would is an area of potential future research.
This study indicates that QAC sanitizers can effectively prevent the germination of P. ramosa seeds but that soil and plant debris on farm equipment can reduce their efficacy. To maximize QAC treatment effectiveness, it is critical to remove soil from field equipment. While removing vegetative plant debris is moderately important, fruit debris is a lower priority. Given the limited time available for equipment cleaning, these findings can help tomato growers, shippers, and rest of the world prioritize efforts and reduce time spent on lower-priority debris. Although the impact of higher debris loads—such as those on harvesters and trailers—is uncertain, it is clear that sanitation efficiency improves when physical cleaning reduces debris to non-inhibitory levels before sanitizer application. Future research should focus on enhancing QAC efficacy under field conditions, particularly in the presence of varying debris types and amounts, to determine the cleanliness threshold needed for optimal performance.
Additionally, exploring alternative sanitation chemicals, sanitizer solution volumes, application methods, and sanitation technologies may provide further insights into practical strategies for managing the spread of P. ramosa. While broomrape, as a quarantine organism, represents the primary management target for the California tomato industry, studies to evaluate the efficacy of broomrape-targeted equipment cleaning protocols on other soilborne plant pathogens and insect pests in California processing tomato fields can provide an opportunity to develop protocols with broad-spectrum efficacy against the suite of important pests affecting this crop (Zimmerman et al., unpublished data). Insights from this reported and ongoing work are being used to rapidly improve sanitation methods and thus reduce the risk of spreading P. ramosa seeds to new fields and regions.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/wsc.2025.10028
Acknowledgments
The authors gratefully acknowledge the contributions and assistance of Mohsen B. Mesgaran, Kelley Paugh, Tong Zhen, Ni Tang, and Zach Bagley.
Funding statement
This research was supported by funding from the California Tomato Research Institute, the California League of Food Processors, and the California Department of Food and Agriculture Specialty Crop Block Grant Program (CDFA#22-0001-031-SF).
Competing interests
The authors declare no conflicts of interest.



















