Introduction
Hydrilla [Hydrilla verticillata (L. f.) Royle] is a submersed, invasive plant that has been described as the “perfect aquatic weed” due to numerous physiological adaptations that make it highly aggressive and competitive (Langeland Reference Langeland1996). Both monoecious and dioecious H. verticillata biotypes are present in the United States, and they have spread significantly from their initial introduction sites (Cook and Lüönd Reference Cook and Lüönd1982). Dioecious H. verticillata (DHV) was first introduced in Florida as an aquarium plant in the 1950s and is commonly found in the southern United States, while monoecious H. verticillata (MHV) was first reported near Washington, DC, and Raleigh, NC, in 1980. Although MHV is still most commonly found from North Carolina northward, it has spread to northern and southern Alabama and northern Georgia (Gettys et al. Reference Gettys, Haller and Petty2020; Gettys and Leon Reference Gettys and Leon2021).
Before 1986, the serine/threonine protein phosphatase inhibitor endothall (HRAC Group 31; Bajsa et al. Reference Bajsa, Pan, Dayan, Owens and Duke2012) was frequently used for H. verticillata control at concentrations of several milligrams per liter (ppm) in the water column. Following its registration for aquatic use in 1986, fluridone (HRAC Group 12) became the primary tool for H. verticillata management because of its high efficacy, selectivity, and ability to control tuber production (Nawrocki et al. Reference Nawrocki, Richardson and Hoyle2016). Fluridone is applied to the water column in micrograms per liter (ppb), and it was used intensively for decades (Dayan and Netherland Reference Dayan and Netherland2005; Nawrocki et al. Reference Nawrocki, Richardson and Hoyle2016). Fluridone’s extensive use without any herbicide rotation or mixture, eventually selected for fluridone-resistant DHV in Florida in the late 1990s (Arias et al. Reference Arias, Netherland, Scheffler, Puri and Dayan2005; Dayan and Netherland Reference Dayan and Netherland2005; Michel et al. Reference Michel, Arias, Scheffler, Duke, Netherland and Dayan2004; Netherland and Jones Reference Netherland and Jones2015; Puri et al. Reference Puri, MacDonald, Altpeter and Haller2007). Only the female form of DHV is found in the United States, and its spread is limited to vegetative reproduction (Michel et al. Reference Michel, Arias, Scheffler, Duke, Netherland and Dayan2004). Fluridone resistance in DHV has been the result of a somatic mutation, and this is the first case of evolved herbicide resistance in a plant that does not rely on sexual reproduction (Ortiz et al. Reference Ortiz, Nissen, Thum, Heilman and Dayan2020).
The development of fluridone-resistant DHV reverted management strategies in Florida back to including endothall as the standard for the last two decades (Sperry et al. Reference Sperry, Leary, Jones and Ferrell2021). The overreliance on a single, effective mechanism of action (MOA) without rotations or mixtures, has led to suspected endothall resistance in some H. verticillata populations in central Florida as well (Giannotti et al. Reference Giannotti, Egan, Netherland, Williams and Knecht2014).
The loss of fluridone for DHV management in Florida and the potential for new market opportunities were a driving force behind identifying and registering several new herbicide MOAs for aquatic plant management (Getsinger et al. Reference Getsinger, Netherland, Grue and Koschnick2008; Ortiz et al. Reference Ortiz, Nissen, Thum, Heilman and Dayan2020). In 2018, florpyrauxifen-benzyl (HRAC Group 4; Epp et al. Reference Epp, Alexander, Balko, Buysse, Brewster, Bryan, Daeuble, Fields, Gast, Green, Irvine, Lo, Lowe, Renga and Richburg2016) was registered for aquatic use. Florpyrauxifen-benzyl is an auxin-mimic herbicide highly active in H. verticillata, even though this plant is a monocot species (Beets et al. Reference Beets, Heilman and Netherland2019). Auxin-mimic herbicides are generally active on dicots, with minimal impact on monocots. Florpyrauxifen-benzyl is an excellent tool for H. verticillata control, as it is highly effective in both H. verticillata biotypes and is classified as a reduced-risk pesticide by the U.S. Environmental Protection Agency (Epp et al. Reference Epp, Alexander, Balko, Buysse, Brewster, Bryan, Daeuble, Fields, Gast, Green, Irvine, Lo, Lowe, Renga and Richburg2016).
Herbicide rotations and mixtures are widely recommended to mitigate the evolution of herbicide resistance (Beckie and Reboud Reference Beckie and Reboud2009). Terrestrial field research and computer simulation models suggest that combining herbicide MOAs as mixtures are the most effective measure at delaying resistance evolution (Beckie and Reboud Reference Beckie and Reboud2009; Busi et al. Reference Busi, Powles, Beckie and Renton2020; Evans et al. Reference Evans, Williams, Hager, Mirsky, Tranel and Davis2018). Herbicide mixtures ensure that weeds, terrestrial or aquatic, are treated with two different MOAs, and weeds potentially resistant to one herbicide MOA are still controlled by another herbicide acting at a different site of action (Busi et al. Reference Busi, Powles, Beckie and Renton2020).
To date, only 15 herbicides and 9 different MOAs are registered for aquatic use (Ortiz et al. Reference Ortiz, Nissen, Thum, Heilman and Dayan2020). Consequently, when resistance develops in aquatic weeds, the options for alternative chemical controls are very limited. To reduce the opportunity for aquatic weeds to evolve resistance, there has been more interest in applying herbicide mixtures; however, there is very little information about how herbicide behavior might change when products are applied as mixtures. There are examples of both herbicide antagonism and synergism in terrestrial weed management, and the same could occur in aquatic systems (Kyser et al. Reference Kyser, Madsen, Miskella and O’Brien2021; Wersal and Madsen Reference Wersal and Madsen2010, Reference Wersal and Madsen2012). To better understand how herbicide behavior might change when used in combination, we investigated the behaviors of endothall and florpyrauxifen-benzyl applied alone and in combination. The objectives of this research were to determine herbicide absorption and translocation patterns when these herbicides were applied alone and in combination in MHV and DHV over a 192-h time course.
Materials and Methods
Plant Material
MHV and DHV tubers were collected from Shearon Harris Lake, NC (35.61°N, 78.95°W), and Orange Lake, FL (29.46°N, 82.17°W), respectively, in spring 2016 and cultured under greenhouse conditions for the last 5 yr. To produce uniform plant material for this research, 10-cm apical sections were cut from the previously propagated plants, and the distal ends were planted in 16 cm by 12 cm by 6 cm (1,152 cm3) plastic pots filled with soil collected from Colorado State University’s organic research farm. Each pot was fertilized with 2 g of slow-release fertilizer (Osmocote Smart Release® Plant Food 15-9-12, Scotts Company, 14111 Scottslawn Road, Marysville, OH 43040), and six apical meristem shoots were planted in each pot for a total of 16 pots. Soil was covered by a 1-cm layer of washed sand. Plants were grown in dechlorinated tap water in 1.2 m by 1 m by 0.9 m (1,041 L) plastic tanks under greenhouse conditions until they produced roots. The photoperiod was 14:10-h light:dark, supplemental lighting was provided with 400-W sodium-halide light bulbs, and the greenhouse temperature was set at 24 C during the day and 18 C at night.
Approximately 3 wk after being transplanted, propagated apical shoots reached 13 to 15 cm in length. Shoots were removed from their original pots, and plants with well-developed roots were selected for absorption and translocation experiments. Roots were washed with tap water to remove soil residue and replanted into plastic test tubes (15-ml Conical Centrifuge Tubes, Thermo Fisher Scientific, 81 Wyman Street, Waltham, MA 02451). Test tubes were filled with unwashed silica sand. After plants were transferred into test tubes, a low melting point eicosane wax (Eicosane 99%, ACROS Organics, 81 Wyman Street, Waltham, MA 02451) was used to seal the top of each tube to isolate the root system from the water column (Frank and Hodgson Reference Frank and Hodgson1964; Ortiz et al. Reference Ortiz, Nissen and Gray2019). Plants were moved to 4-L plastic tanks (22.7-cm tall by 17-cm diameter) filled with dechlorinated tap water and were allowed to acclimate for 24 h in the laboratory prior to herbicide treatment.
Herbicide Exposure
Twelve 4-L glass beakers (25-cm tall by 15-cm diameter) were filled with 3.5 L of dechlorinated tap water (pH 7.1). Six beakers were treated with [14C]endothall (56.6 mCi mmol−1 specific activity, Moravek Biochemicals, 577 Mercury Lane, Brea, CA 92821) combined with formulated dipotassium salt of endothall (Cascade®, United Phosphorus, 630 Freedom Business Center, Suite 402, King of Prussia, PA 19406) to achieve a final concentration of 2 mg L−1 in the water column. Three of these beakers were treated with endothall only, and the other three beakers were treated with endothall as described plus 3.8 μg L−1 of formulated, nonradiolabeled florpyrauxifen-benzyl (ProcellaCOR™ EC, SePRO, 11550 North Meridian Street, Suite 600, Carmel, IN 46032). The other six beakers were treated with [14C]florpyrauxifen-benzyl (57.1 mCi mmol−1 specific activity, Moravek Biochemicals) at 3.8 μg L−1. Three of these beakers were treated with [14C]florpyrauxifen-benzyl only, and the other three beakers were treated with [14C]florpyrauxifen-benzyl plus 2 mg L−1 of formulated, nonradiolabeled dipotassium salt of endothall (Cascade®).
Each treatment was replicated three times for a total of 12 treatment beakers. Each [14C]endothall-treated tank contained 33.8 ± 1.0 kBq L−1, while each [14C]florpyrauxifen-benzyl–treated tank contained 12.4 ± 0.5 kBq L−1. The radioactivity in each treatment tank was confirmed using liquid scintillation spectroscopy (LSS) (Packard 2500R, PerkinElmer, 940 Winter Street, Waltham, MA 02451).
Each beaker contained 6 MHV, 6 DHV plants, and one empty tube waxed with a toothpick simulating a plant stem as a control to test the wax barrier efficacy. All plants were held by a round test tube rack (No-Wire Round Rack, Bel-Art Scienceware, 661 Route 23 South, Wayne, NJ 07470). During the experiment, plants were maintained in the laboratory at 22 C, with a 14:10-h light:dark period, supplemented with LED grow lights. Beakers were stirred once a day, and treatment water volume was maintained by adding more water to the tanks daily. Plants were harvested at 6, 12, 24, 48, 96, and 192 h after treatment (HAT). Three replicates of each biotype were randomly harvested from a different tank at each time point, rinsed four times in clean tap water, and divided into shoots and roots. After separation, plant parts were dried at 60 C for at least 48 h, dry biomass was recorded for each plant part, and plant tissues were combusted in a biological oxidizer (OX500, R.J. Harvey Instrument, 11 Jane Street, Tappan, NY 10983) for 2 min, and absorbed 14C was collected by a 14C-trapping cocktail (OX161, R.J. Harvey Instrument). The efficiency of the oxidizer was tested before oxidizing plant parts, and it was always greater than 96%. After oxidation, radioactivity was quantified by LSS. The study was repeated twice.
Statistical Analysis
Before data from repeated experiments were combined for statistical analyses, Levene’s test for homogeneity of variance (α = 0.05 level of significance) was performed using R (v. 4.0.0, R Project) (Vassios et al. Reference Vassios, Nissen and Brunk2011). Means and standard errors for each experiment were back-calculated from dry weight, considering 90% of water content, determined based on 10 H. verticillata plants. Data collected from these experiments were analyzed using RStudio (v. 1.4.1717) and MS Excel and plotted with Prism 9 (GraphPad Software, 2365 Northside Drive, Suite 560, San Diego, CA 92108). Absorption and translocation over time were analyzed using a nonlinear regression analysis to fit a hyperbolic function (Kniss et al. Reference Kniss, Vassios, Nissen and Ritz2011), where y is the predicted absorption at time x, and a and b are constants.
The plant concentration factor (PCF) was calculated to determine bioaccumulation, a metric often used in aquatic plant research to compare absorption across different herbicide concentrations and in different species. The equation used to calculate PCF was adapted from de Carvalho et al. (Reference de Carvalho, Bromilow and Greenwood2007) and can be defined as:
The nonlinear regression equations resulting from these analyses were used to calculate two other values: predicted absorption at 192 HAT (A192) based on the radiolabeled/nonradiolabeled herbicide ratio in the water column and the predicted time required for 90% of that absorption (t 90). The A192 value was used to compare the theoretical maximum absorption among different plant parts, plant species, and herbicides. The t 90 was used to compare the absorption rate or how quickly the plant reached maximum absorption.
Results and Discussion
Plant roots were effectively isolated from the radiolabeled treatment solutions through the eicosane wax barrier. Only 0.041 ± 0.012 Bq ml−1 (n = 6) and 0.022 ± 0.007 Bq ml−1 (n = 6) of radioactivity were found in the non-plant toothpick test tubes 192 HAT for [14C]endothall and [14C]florpyrauxifen-benzyl treatments, respectively. There was no detected radioactivity in 5 of the 12 combined test tubes. Based on these data, this insignificant amount of radioactivity did not impact the results of the study.
Endothall absorption did not reach a maximum asymptote in either biotype when applied alone or in the presence of florpyrauxifen-benzyl (Figure 1). Although the asymptotic rise to maximum function is the most biologically relevant function to describe herbicide absorption (Kniss et al. Reference Kniss, Vassios, Nissen and Ritz2011), previous research also demonstrated that endothall at 3 mg L−1 did not reach maximum asymptote for DHV and MHV (Ortiz et al. Reference Ortiz, Nissen and Gray2019).

Figure 1. [14C]endothall and [14C]florpyrauxifen-benzyl bioaccumulation in Hydrilla verticillata biotypes (DHV, dioecious; MHV, monoecious) over a 192-h time period expressed as plant concentration factor (PCF). Filled circle, herbicide bioaccumulation in MHV alone; open circle, bioaccumulation in MHV in combination; filled square, herbicide bioaccumulation in DHV alone; open square, bioaccumulation in DHV in combination. Data presented are means and error bars are the standard error of the mean (n = 6).
The PCF192 of [14C]endothall applied alone was 20.2 ± 1.3 and 25.8 ± 0.7 in DHV and MHV, respectively. When applied in combination with florpyrauxifen-benzyl, [14C]endothall PCF192 in DHV and MHV was 19.0 ± 1.5 and 30.2 ± 0.9, respectively (Figure 1; Table 1), which was not statistically different than [14C]endothall alone. The accumulation of this herbicide at 3 mg L−1 was 11.0 ± 0.9 and 6.6 ± 0.7 for DHV and MHV, respectively (Ortiz et al. Reference Ortiz, Nissen and Gray2019). The reason for greater herbicide accumulation in this study may be due to the difference in herbicide rate, as a lower concentration allows the plant to be more physiologically active and maintain a stronger concentration gradient. A similar endothall accumulation response was observed in curly-leaved pondweed (Potamogeton crispus L.) with PCF192 of 4.8 ± 0.1 when exposed to 0.75 mg L−1 endothall and a lower PFC192 of 3.9 ± 0.1 when exposed to a higher endothall concentration of 3 mg L−1 (Ortiz et al. Reference Ortiz, Nissen and Gray2022).
Table 1. Predicted plant concentration factor at 192 h after treatment (HAT) (PCF192), herbicide absorption (μg g−1) at 192 HAT (A192), and the time in hours required to reach 90% of A192 (t 90). a

a Values represent the mean, and error terms represent the standard error of the mean (n = 6).
b Hydrilla verticillata biotypes: DHV, dioecious; MHV, monoecious.
The PCF192 for [14C]florpyrauxifen-benzyl alone was 299.4 ± 21.3 and 433.5 ± 25.4 in DHV and MHV, respectively (Figure 1). Previous research reported that florpyrauxifen-benzyl PCF192 at 10 µg L−1 was 90 ± 20 and 10 ± 2 for DHV and MHV, respectively (Haug et al. Reference Haug, Ahmed, Gannon and Richardson2021). Greater herbicide accumulation in the current study is likely due to differences in the ratio of radiolabeled and nonradiolabeled herbicide, herbicide rate, and different herbicide formulations (Vassios et al. Reference Vassios, Nissen and Brunk2011). In the presence of 2 mg L−1 endothall, [14C]florpyrauxifen-benzyl accumulation in DHV and MHV was 219 ± 12.8 and 364.0 ± 17.0, respectively (Table 1) and was not statistically different than [14C]florpyrauxifen-benzyl alone.
Florpyrauxifen-benzyl high log Kow (5.5) is very similar to 2,4-D butoxyethyl ester’s (BEE) (log Kow 5.3), another auxin-mimic herbicide used for aquatic plant management. The 2,4-D BEE PCF192 in Eurasian watermilfoil (Myriophyllum spicatum L.) and hybrid watermilfoil (Myriophyllum spicatum × Myriophyllum sibiricum Kom.) was 35.11 ± 1.33 and 52.11 ± 1.09, respectively, substantially lower than florpyrauxifen-benzyl in DHV and MHV. Both florpyrauxifen-benzyl and 2,4-D BEE are pro-herbicides that are bioactivated into their acid forms once they are absorbed by the plant (Nandula et al. Reference Nandula, Riechers, Ferhatoglu, Barrett, Duke, Dayan, Goldberg-Cavalleri, Tétard-Jones, Wortley, Onkokesung, Brazier-Hicks, Edwards, Gaines, Iwakami, Jugulam and Ma2019). Depending on water chemistry and temperature, both herbicides can be hydrolyzed to the free acid in the water column and absorbed by plants. The acid form of florpyrauxifen-benzyl is significantly more lipophilic than 2,4-D acid, which could explain its higher accumulation.
Endothall absorption at 192 HAT (A192) in DHV and MHV shoots was similar, 307.9 ± 20.2 µg g−1 and 382.3 ± 13.6 µg g−1, respectively (Table 1); in combination with florpyrauxifen-benzyl, it was only impacted in MHV, with a 40% increase in herbicide absorption (DHV = 260.9 ± 15.1 µg g−1; MHV = 535.6 ± 25.6 µg g−1). Although endothall absorption increased by 40% in the presence of florpyrauxifen-benzyl, the time required to reach 90% of total herbicide absorbed (t 90) only increased by 18% (Table 1), meaning that endothall absorption by MHV was slower when in combination with florpyrauxifen-benzyl. In contrast, while florpyrauxifen-benzyl A192 was not impacted in the presence of endothall, its absorption was significantly lower than endothall’s, which was not unexpected, considering the rates at which each herbicide is applied (endothall = 2 mg L−1 and florpyrauxifen-benzyl = 3.8 µl−1). Florpyrauxifen-benzyl absorption was very similar between the two biotypes, 3.1 ± 0.4 µg g−1 and 4.9 ± 0.3 µg g−1 in DHV and MHV, respectively (Table 1). A previous study using another herbicide formulation indicated similar florpyrauxifen-benzyl absorption in MHV, but four times greater absorption in DHV (Haug et al. Reference Haug, Ahmed, Gannon and Richardson2021). The reason for the discrepancy between the two studies is unknown but may be attributable to the use of different herbicide formulations.
Endothall shoot-to-root translocation was 18.7 ± 1.4% and 16.2 ± 1.5% when applied alone in DHV and MHV (Figure 2), respectively, supporting previously published data that endothall can translocate to the roots of both H. verticillata biotypes (Ortiz et al. Reference Ortiz, Nissen and Gray2019). In combination with florpyrauxifen-benzyl, endothall translocation in DHV was not impacted (23.2 ± 2.2%), but it was greatly reduced in MHV (2.20 ± 0.09%) (Figure 2). This reduced translocation in MHV could be due to the fast-acting properties of florpyrauxifen-benzyl affecting the plant’s vascular tissue rapidly enough to reduce movement to belowground tissues. Florpyrauxifen-benzyl shoot-to-root translocation was 9.1 ± 1.1% and 1.3 ± 0.1% of total absorbed herbicide found in the roots of DHV and MHV, respectively (Figure 3). In combination with endothall, florpyrauxifen-benzyl translocation was reduced in both H. verticillata biotypes (DHV = 0.5 ± 0.1%; MHV = 0.2 ± 0.03%) (Figure 3). Although florpyrauxifen-benzyl is highly active in both H. verticillata biotypes, the dose that corresponds to 50% inhibition of growth (GR50) is lower in MHV than DHV (Netherland and Richardson Reference Netherland and Richardson2016; Richardson et al. Reference Richardson, Haug and Netherland2016). As both H. verticillata biotypes were exposed to the same dose of florpyrauxifen-benzyl (3.8 µg L−1), it may be that MHV vascular tissue is affected more rapidly than DHV, impacting herbicide translocation to the roots.
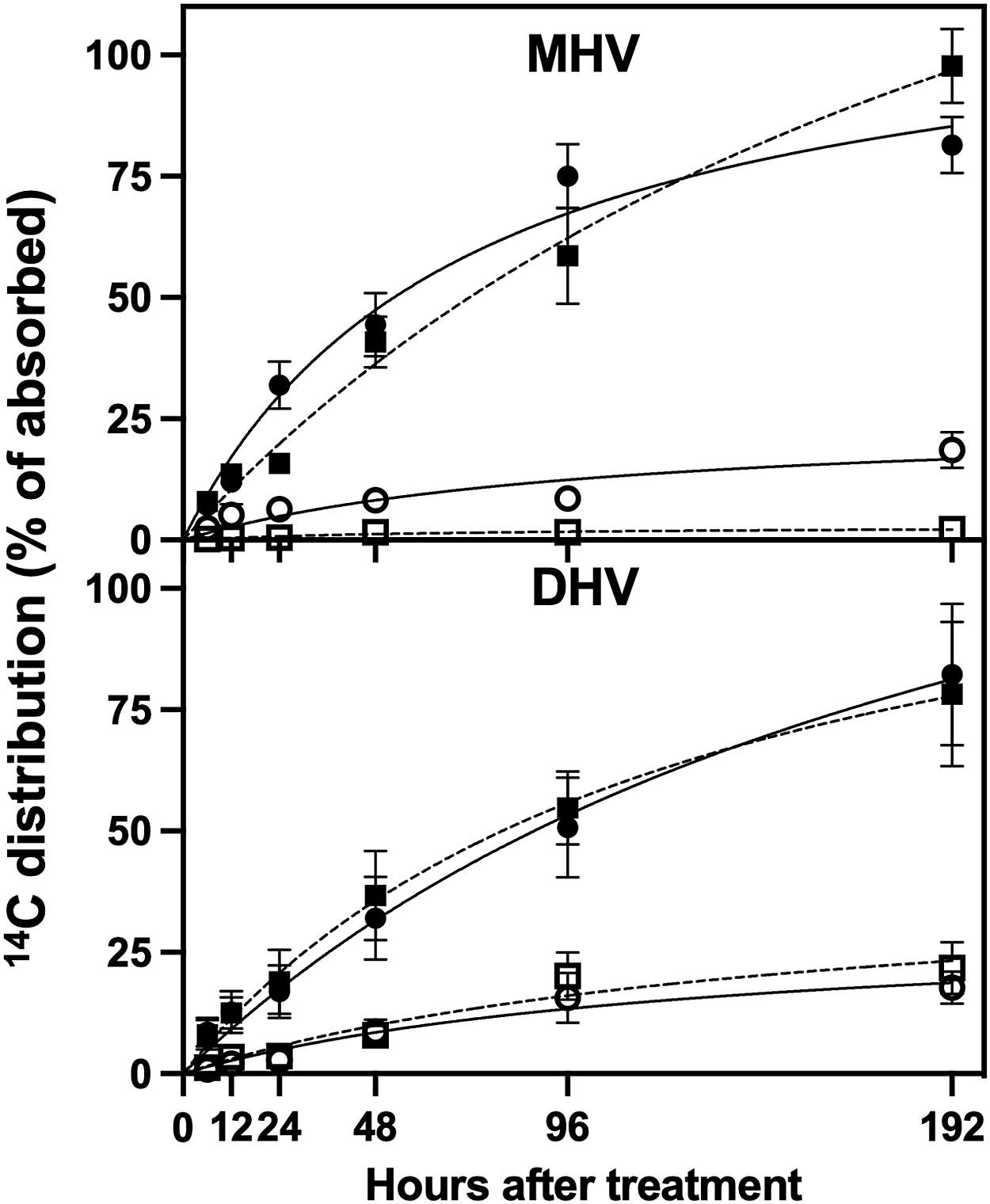
Figure 2. The 14C distribution in Hydrilla verticillata biotypes (DHV, dioecious; MHV, monoecious) over 192 h following exposure to [14C]endothall, expressed as percentage of total herbicide absorbed. Filled circle, percentage of [14C]endothall alone in shoots; open circle, percentage of [14C]endothall alone in roots; filled square, percentage of [14C]endothall in combination with florpyrauxifen-benzyl in shoots; open square, percentage of [14C]endothall in combination with florpyrauxifen-benzyl in roots. Data presented are means, and error bars are the standard error of the mean (n = 6).

Figure 3. The 14C distribution in Hydrilla verticillata biotypes (DHV, dioecious; MHV, monoecious) over 192 h following exposure to [14C]florpyrauxifen-benzyl, expressed as percentage of total herbicide absorbed. Filled circle, percentage of [14C]florpyrauxifen-benzyl alone in shoots; open circle, percentage of [14C]florpyrauxifen-benzyl alone in roots; filled square, percentage of [14C]florpyrauxifen-benzyl in combination with endothall in shoots; open square, percentage of [14C]florpyrauxifen-benzyl in combination with endothall in roots. Data presented are means, and error bars are the standard error of the mean (n = 6).
In conclusion, although radioactive herbicide translocation to the roots was impaired when the herbicides were used in combination, this is the first study using radiolabeled herbicides to examine herbicide combination in aquatic plants; therefore, future research needs to be conducted to determine whether this reduced translocation negatively affects the overall effectiveness of this potential control strategy in the field. One major benefit of using endothall and florpyrauxifen-benzyl in combination is that both products require reduced exposure times relative to other systemic herbicides available for the aquatic system that are efficacious for H. verticillata. This study suggests that endothall and florpyrauxifen-benzyl have no negative impacts on each other’s absorption and accumulation in H. verticillata and can be an excellent tool to delay the development of herbicide-resistant aquatic weeds when used in combination.
Acknowledgments
We are grateful to the USDA National Institute of Food and Agriculture, Hatch project COL00785 (accession no. 1016591), UPL North America for providing partial funding to support this research project, and the many undergraduate students who helped maintain the aquatic plants stock tanks over the years. No conflicts of interest have been declared.






