The evolution of weed resistance to herbicides (Heap Reference Heap2017) and a scarcity of new herbicide mechanisms of action (Duke Reference Duke2012) have pressured the agricultural industry to develop alternatives to chemical weed control. Australia has led the innovation stream with the development of harvest weed seed control (HWSC) systems. HWSC targets weed seeds that would typically be distributed by combine harvesters in the chaff residue and aims to destroy those seeds to prevent introduction into the seedbank (Walsh et al. Reference Walsh, Newman and Powles2013). There are a number of methods for HWSC use, including narrow windrow burning, chaff carts, direct-bale systems (Walsh et al. Reference Walsh, Newman and Powles2013), chaff tramlining (or chaff deck), and windrow rotting (or chaff lining) (Australian Herbicide Resistance Initiative 2014, 2015). An additional HWSC technology that has received substantial attention is the Harrington Seed Destructor™ (HSD), a tow-behind machine that processes chaff through a cage mill to devitalize weed seeds (Walsh et al. Reference Walsh, Harrington and Powles2012).
The benefits of using the HSD over other HWSC technologies are the retention of all residues across the field for nutrient cycling and moisture conservation and no additional labor required after harvest in each field (Walsh et al. Reference Walsh, Harrington and Powles2012). Additionally, there is physical processing of the weed seed rather than reliance on composting, burning, or residue removal as for some other HWSC methods. However, following commercialization, adoption of the HSD has been slow due to cost and a lack of desire by producers to tow a large machine (Australian Herbicide Resistance Initiative 2016). In March 2016 the commercialization of the Integrated Harrington Seed Destructor™ (iHSD) was announced, a system of two mills incorporated in the back of the harvester and powered by the harvester, providing the same method of weed control without the towing requirement (de Bruin Engineering 2017). The iHSD is based on the same cage mill as the original HSD; however, rather than two spinning cages making up the mill, the iHSD has only one cage spinning twice as fast. The cost of the iHSD is also less than that of the original unit (A$160,000 vs. A$200,000), making it a more viable system for producer use and providing equivalent efficacy (Australian Herbicide Resistance Initiative 2016). It was determined that adoption of the HSD was most economical when herbicide-resistant weeds (particularly to nonselective herbicides) were present, when crop yields were high and the annual cropping area was a minimum of 3,000 ha (Jacobs and Kingwell Reference Jacobs and Kingwell2016). However, this evaluation was done with the original HSD; the lower cost of the iHSD will make it more economical in other situations. As this analysis was based on Australian cropping systems and weed species through use of the Ryegrass Integrated Management simulation model (Lacoste and Powles Reference Lacoste and Powles2014; Pannell et al. Reference Pannell, Stewart, Bennett, Monjardino, Schmidt and Powles2004), the economics of adoption will likely differ in different countries and in different agroecoregions.
In Australia, the HSD has been >90% effective on rigid ryegrass (Lolium rigidum Gaudin), wild radish (Raphanus raphanistrum L.), wild oat (Avena fatua L.), and Bromus spp. (Walsh et al. Reference Walsh, Harrington and Powles2012). Control of the larger seeds (wild oat and brome grass: 99%) was greater than for wild radish (93%) due to its protective, hard silique (Walsh et al. Reference Walsh, Harrington and Powles2012). Other factors have been suggested to affect weed seed control with impact implements like the HSD, such as the impact speed (RPM), number of impacts, seed size, weed species, seed strength, seed natural defenses, moisture content, and chaff type, among others (Berry et al. Reference Berry, Fielke and Saunders2015; Walsh et al. Reference Walsh, Harrington and Powles2012). Understanding how crop and weed seed factors affect HSD efficacy will increase understanding of potential suitability of the HSD in new agroecoregions.
The objective of this study was to determine the effect of some crop and weed seed parameters on HSD efficacy to determine its potential with new weeds in new agroecoregions. Weed seed viability was used to measure HSD efficacy. The parameters included weed species, weed seed size, seed number (density) to simulate variable weed infestations or suboptimal harvester settings, chaff load to simulate different yielding crops, and chaff type for comparing efficacy between crop types.
Materials and Methods
To investigate specific factors and their effects on HSD weed seed destruction, seed and chaff samples were processed while the HSD was stationary (Figure 1); stationary processing through the HSD minimized variability between samples but also required collection of threshed chaff, as the seed destructor is designed to process chaff and not whole-plant samples. To facilitate stationary threshing, chaff was collected in the fall of 2015 during harvest of unsampled plot areas at Lacombe, AB, and with the assistance of a local producer who used a chaff cart in the harvest of his field pea (Pisum sativum L.) crop. Chaff was collected from areas with minimal weed presence. Chaff samples were stored in canvas totes to allow for air-drying until use.

Figure 1 Stationary threshing setup of the Harrington Seed Destructor. Arrows indicate (A) the intake (B) the Harrington Seed Destructor, and (C) the collection cyclone. (Photo credit: Josh Kirsch, PAMI.)
Volunteer canola was chosen as the primary weed species for testing the HSD. Its rapid germination, minimal dormancy, and high viability made it an ideal species for these studies. Additionally, volunteer canola is an increasingly prominent weed and is often introduced from harvest losses of canola crops (Cavalieri et al. Reference Cavalieri, Harker, Hall, Willenborg, Haile, Shirtliffe and Gulden2016). Untreated F2 canola (‘CF 46H75’ in most cases) was used to simulate volunteer canola seed. Seeds were counted using an Agriculex ESC-1 seed counter (Agriculex, Guelph, ON, Canada). For most studies (see details below), 10,000 seeds were used for each sample. Additional species tested included kochia [Kochia scoparia (L.) Schrad.], green foxtail [Setaria viridis (L.) Beauv.], false cleavers (Galium spurium L.), and wild oat. These species were chosen for their range of seed size and for being common problem species in western Canada. Preliminary viability testing was conducted on multiple seed lots of all species through germination testing in 100 by 15 mm petri dishes with blue germination blotting paper (Anchor Paper, St Paul, MN). Each dish received 7 ml of water and was germinated in the dark at room temperature (~22 C) for 2 wk. For each species, 50 seeds were germinated to determine viability prior to HSD processing and select highest germinability seed lots (unpublished data).
Processing of samples with the HSD occurred at the Prairie Agriculture Machinery Institute (PAMI) in Humboldt, Saskatchewan. For all experiments, four replications of each sample were used, and each experiment was conducted twice. Each sample consisted of 20 L of chaff, measured by filling 20-L pails with chaff and intermixing the seed samples. Using an approximate 3:1 ratio of grain to chaff production (M Walsh, personal communication) and an average barley (Hordeum vulgare L.) yield of 4,500 kg ha−1, assuming 20 L of chaff weighs 1 kg (M Walsh, personal communication), the 20 L of barley chaff used in most samples would come from approximately 6.7 m2 of land. The 10,000 canola seeds dispersed would result in a volunteer canola seed density of 1,500 seeds m−2, which is slightly lower than typical harvest losses of this species (2,500 to 6,100 seeds m−2) (Cavalieri et al. Reference Cavalieri, Harker, Hall, Willenborg, Haile, Shirtliffe and Gulden2016). A lower average barley yield would change the area and seed distribution ranges. To ensure relatively homogenous samples, chaff and seed were mixed just prior to processing to ensure distribution of seeds throughout the sample and prevent settling and separation. Samples were introduced into the HSD intake (Figure 1) once the machine had reached full RPM (i.e., 1,450). Each sample took approximately 30 s to input into the seed destructor, and the machine was allowed to run for an additional 30 s after input to ensure the entire sample was processed and expelled. To account for decreased airflow due to the HSD being separated from the harvester, compressed air was used at the intake and just prior to the sample entering the cage mill. This ensured that the entire sample entered the cage mill, resulting in improved accuracy and minimal contamination between samples. After cage-mill processing, samples were expelled into a large cyclone attached to the machine, which allowed the air and extremely fine dust to escape out the top without loss of the sample. Samples were collected, labeled, and returned to Lacombe for processing.
Due to extreme mold growth when entire samples (all chaff and fine particles) were germinated, a cleaning process was used to eliminate as much of the fine dust and chaff as possible. Each sample was initially passed through hand sieves (4.77 mm round hole) to remove larger residue components. Samples were then passed through an Almaco Air Blast Seed Cleaner (Seedburo Equipment, Des Plaines, IL) with very low wind to remove fine residues without losing seeds. Finally, samples were passed through a Clipper air and sieve cleaner (A.T. Ferrel, Bluffton, IN) twice to refine the sample to whole and partial seeds as much as possible (sieves selected were appropriate for seeds in the sample). These samples were then germinated in 16.6 by 24.1 by 4.4 cm germination boxes with blue blotting paper (Seedburo Equipment, Des Plaines, IL) on the bottom and white filter paper on the top to ensure moisture levels were maintained. Distilled water amounts were adjusted based on seed size; for canola seed samples, 36 ml was used. For other species 36 ml was the starting point, and moisture was increased in 6-ml increments as required due to water uptake by the seeds. Preliminary germinations with test samples indicated that all viable seeds were in the cleaned fractions and not in the chaff that had been screened out. On two samples there were exceptions where one viable seed was found outside the cleaned fraction; all samples were visually checked for potentially viable seeds during cleaning as a result. Samples were germinated for 2 wk in the dark at room temperature (~22 C), at which point any ungerminated seeds were subjected to a press test to determine viability (Sawma and Mohler Reference Sawma and Mohler2002). The total number of viable seeds in the processed sample was equivalent to the number of germinated seeds and the number of seeds evaluated as viable during the press test.
Five factors that may affect weed seed devitalization by the HSD were investigated. The first factor was weed seed species. Species were selected across a gradient of 1,000-seed weights (TSW) to account for variations in the types of weed seeds that are problematic in western Canada (Table 1). We used kochia, green foxtail, false cleavers, volunteer canola, and wild oat (Table 1). Seed lots had been collected over a number of years and stored for use in weed management trials in which population establishment was required. Weed seed size was the second factor investigated and used F2 canola seed (‘73-75 RR’) (Dekalb, Monsanto, St. Louis, MO) that had been passed through multiple hand sieves to separate the seed into size categories. Sieves included 6-mm round holes (R), 5.5-mm R, 5-mm R, and 4.5-mm R. This resulted in five seed sizes—the seeds that remained in each of the sieves plus those seeds that passed through the 4.5-mm R sieve (Table 1). The TSW was calculated for seed from each of these sieve sizes and used for data analysis (Table 1). Using sized canola seed minimizes differences in HSD efficacy due to different seed shape, external protrusions, etc.; the targeted difference between treatments in this experiment was seed size. Seed number was another factor investigated. Sample seed numbers ranged from 10 to 1 million in logarithmic steps (Table 1) dispersed through the same 20-L volume of chaff. Chaff load was also investigated. Samples of 10,000 canola seeds were intermixed with chaff amounts ranging from no chaff to 160 L (eight 20-L pails of chaff) (Table 1). These samples were processed within the same target time frame of 30 s, resulting in a range of chaff volume processed within a unit time. Chaff type was the final factor investigated. Samples of 10,000 canola seeds were intermixed with 20L of barley, canola, or pea chaff, chosen for their differences in plant structure and the resulting variation in chaff composition (Table 1).
Table 1 Treatments used in each of the five experiments to determine effects of weed species, seed size, seed number, chaff type, and chaff load on Harrington Seed Destructor efficacy.
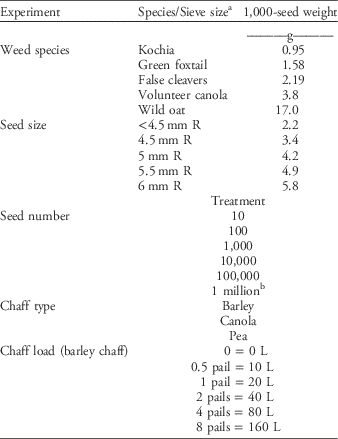
a R, round holes.
b Based on a single experiment.
Experimental design varied by experiment. The chaff type was run as a randomized complete block design. All other experiments were nonrandomized and organized to prevent contamination between samples. For example, in the chaff-load experiment, treatments started with zero chaff and increased to the highest amount with four replications of each treatment. This is similar to an herbicide dose–response study wherein increasing rates of an herbicide would be applied to eliminate risk of contaminating lower-rate treatments with higher-rate residues. For seed number there is lower risk of contamination when a sample of 1 million seeds follows one of 10 seeds compared with the reverse order. There is no reason to expect differences in processing over time, as the machine was run at a constant speed for each sample; therefore, minimized contamination was the goal, rather than randomization.
Statistical Analysis
Percent viability was calculated using Equation 1 for all treatments. Percent viability was then converted to percent of control by Equation 2 and divided by 100 to result in proportional control.
Seed number in each percent control calculation was adjusted for the viability of the seed source at the time of final processing based on a germination box test with 100 seeds. PROC GLIMMIX in SAS v. 9.4 (SAS Institute 1995) with a beta error distribution was used to analyze proportional control data with trial repeat, treatment, and their interaction as fixed effects and replicate as a random effect. If trial repeat and its interactions were not significant on proportion of seeds controlled, trial repeats were combined and reanalyzed. From this analysis, LSmeans and standard errors were obtained and converted back to percent control for presentation. For the chaff type and weed species experiments, a pdiff statement with a Tukey adjustment was included in the GLIMMIX ANOVA for comparison of means (α=0.05). For seed size (canola) and chaff load, Proc Reg was used to perform linear (Equation 3) and quadratic (Equation 4) regressions, respectively. In the linear regression equation (Equation 3), Y is the proportion of seeds controlled, x is TSW, m is the slope of the line, and b is the intercept.
In the quadratic regression equation (Equation 4), Y is the proportion of seeds controlled, x is the chaff load volume, a and b are slope values, and c is the intercept.
For the seed number experiment, an exponential reciprocal model (Equation 5) was fit using DeltaGraph (Red Rock Software, Salt Lake City, UT).
where Y is the percentage of seeds controlled, x is the seed number, a is the asymptote, and b is the slope parameter.
For all experiments, with the exception of seed size (species), trial repeat was not a significant factor, and there was no significant interaction with treatment. Therefore, trials were combined for further analysis.
Results and Discussion
Weed Species
For weed species, trial repeat was a significant factor (P=0.019). However, the LSmeans for each species were not significantly different between trial repeats, so combined data are presented. Weed seed control by the HSD showed limited significant differences (kochia significantly different than all weeds except wild oat) and is unlikely to have high biological impact (Figure 2). There was not, as hypothesized, a linear increase in control with increased TSW but rather a significant quadratic regression (unpublished data). The quadratic regression was not consistent with the hypothesis that increased seed mass results in more energetic impacts and increased control. It is likely that other properties of the seeds (shape, external structures, seed coat strength, etc.) also affect the level of control by the HSD (Figure 2).

Figure 2 Percent control of various weed species by the Harrington Seed Destructor. The 1,000-seed weight of each species is listed in parentheses (g 1,000 seeds−1). Bars denote standard errors of the mean. Letters denote significant differences between control of species based on a Tukey adjusted comparison of means (α=0.05).
Control of the tested species ranged from 97.7% of cleavers to 99.8% control of kochia (Figure 2). Overall, there was a high level of control of all of the tested species across a wide range of TSWs. Control of some species (i.e., kochia) may be artificially high. Kochia had low seed lot viability (34%) by the time of processing and germination, and the adjustment of the seed number for viable seeds may have increased the control of kochia to an artificially high level (underestimation of the number of viable seeds in the sample). We do not believe that this adjustment makes the measurement inaccurate, because all species are still within a very narrow control-level range. Additionally, the star-shaped hull that typically covers kochia seed was removed prior to seed counting to allow for differentiation of seeds and chaff; while this hull is fragile (Friesen et al. Reference Friesen, Beckie, Warwick and Van Acker2009), it could offer additional protection to the seed that may result in lower control values than those observed in this experiment.
Control of cleavers (Figure 2) may be slightly less than other tested species due to external protrusions on the seed; the burr-like hooks on the outside of the seed may have protected the seed embryo from damage. Other very large seeds with burr-like protrusions and hard seed coats [i.e., common cocklebur (Xanthium strumarium L.)] have also been reported to have slightly lower but not significantly different control than other species tested in the United States with the integrated HSD (Schwartz et al. Reference Schwartz, Norsworthy and Walsh2017). This experiment highlights high levels of efficacy across a number of species with a wide range of seed sizes, structures, and shapes.
Seed Size
Size of canola seed had a significant main effect on the level of control by the HSD (ANOVA P =0.0004). Control of canola increased linearly with TSW (Figure 3) (regression P<0.0001). The linear model explained 35% of variation in control of canola based on the adjusted R2; a limited range in control values likely contributes to this low R2 value. While the effect of seed size is statistically significant, the biological or practical effect of seed size is limited. Overall control values ranged from 98.4% to 98.8 % from the 2.2 g TSW to the 5.8 g TSW (Figure 3). This large range in canola seed size, which was visually apparent, would have a large effect on seeding rate if this was crop seed (almost a 3-fold difference in seeds per square meter). The limited effect on overall efficacy level is a positive result in terms of weed control. There are a number of small-seeded weeds globally including in western Canada. These results indicate that small-seeded weeds would still be controlled at high levels once introduced into the HSD. While this experiment provided the hypothesized linear relationship between seed weight and percent control, it is also consistent overall with the weed species experiment, in that control remains high across a variety of seed sizes. The linear equation in this case estimates a minimal control of 98% based on seed size alone; other factors could reduce this value (i.e., seed strength/silique strength of wild radish), but the overall implication of this experiment is that seed size will not likely be a limiting factor in weed control with the HSD.

Figure 3 The effects of canola seed size on percent control by the Harrington Seed Destructor. Bars denote standard errors of the mean. TSW, 1,000-seed weight.
Seed Number
Seed number had a significant effect on efficacy of the HSD (P<0.0001) and a significant exponential reciprocal regression (P<0.01) (Figure 4). The 1 million–seed treatment was only included in the first trial repeat due to the production of seed meal during the processing of those samples; other samples returned processed chaff with some processed seeds, while the 1 million–seed samples resulted in seed meal with small amounts of chaff. The oil in the seeds resulted in meal sticking to different parts of the HSD setup (i.e., the collection cyclone), increasing the risk of contamination between samples. As a result, that treatment was eliminated from the second repeat to ensure the ability to continue with other studies without risking sample contamination. Between 100 and 1 million seeds, control differed by just over a percent ranging from 97.3% to 98.5 % (Figure 4). The 10-seed treatment showed substantially less control than the other treatments (Figure 4); however, this was more likely an impact of sample size rather than poor control. With only 10 seeds, each surviving seed caused a loss of 10% control. As all other control is in the range of 98%, similar to the other studies, the lack of control in the 10-seed treatment is believed to be simply the effect of sample size. It is, however, possible that with fewer seeds there are less impacts per seed with the cage mill and other seeds or pieces of chaff, which decreased the control observed in that treatment. Overall, it appears that high seed inputs from high weed densities or an improperly set combine (e.g., high seed loss in canola) would be effectively controlled. In general, seed input density should not affect the ability of the HSD to control weed species. The treatments used would be approximately equivalent to 1.5 to 150,000 seeds m−2 (based on 20 L of chaff from 6.7m2). The 100,000-seed treatment is approximately equivalent to 15,000 seeds m−2. Extremes of the likely range for volunteer canola either through harvest losses or weed infestations were considered in this experiment and in general would be efficiently controlled by the HSD.

Figure 4 The effects of weed seed number on percent control of weed seeds by the Harrington Seed Destructor. Bars denote standard errors of the mean. Double asterisk (**) indicates significance at P=0.01
Chaff Load
The goal for each chaff-load sample was a 30-s input time. Across the 48 samples input, the input time ranged from 26 to 33 s, with the majority being input between 28 and 30 s. Considering samples were input manually, this was highly consistent between samples. A single pail (20L) of chaff is approximately equivalent to that produced on 6.7m2 of land, therefore 160 L would correspond to approximately 54m2 of land. While these areas are likely low compared to the area a typical harvester would cover in 30 s, it was the highest volume that physically could be fed into the HSD based on manual input and setup logistics. Chaff volume had a significant effect on HSD control (P<0.0001). HSD control of canola initially improved with increasing chaff load, until 80L of chaff (4 pails) (Figure 4). Between 4 and 8 pails (80 to 160 L), control declined again (Figure 5). Overall, control ranged between 97.9% with no chaff and 99% with 80L of chaff (Figure 5). The reason for the quadratic relationship (P<0.0001) is unclear. Increased chaff may initially increase the number of times seeds are impacted in the cage mill due to reflection and redirection, followed by protection of seeds by the chaff after a certain volume threshold. However, the dynamics of motion within the cage mill are not known and not easily observed. Regardless, the limited variability in the control of canola across a wide range of chaff volumes indicates limited effects of crop yield on HSD efficacy; processing of chaff from high- or low-yield crops should not have a large effect on the control of weed seeds that pass through the HSD. Results from this experiment also indicate that results of the other experiments in this study are likely applicable to weed control in both low- and high-yield crops.

Figure 5 The effect of chaff volume on percent control of weed seeds by the Harrington Seed Destructor. Bars denote standard errors of the mean.
Chaff Type
There was a significant effect of chaff type on HSD efficacy (Figure 6). Control of volunteer canola seeds in canola chaff was significantly less than control in barley or pea chaff (Figure 6). While this may be due to structural and component variation between the chaff types reducing control in the canola chaff, it is more likely due to an underlying presence of volunteer canola in the chaff in addition to the canola added for the treatment. Lower control may simply be due to the presence of additional canola seeds increasing the total number of seeds in the sample. Based on Equation 1, if the number of viable seeds used to calculate percent viability is lower than is actually present in the sample, percent viability will be overestimated and percent control underestimated. A postprocessing screening of the canola chaff determined that there was an inherent presence of canola seeds in the canola chaff that could have confounded the results for that chaff type. Regardless, while the control of canola in canola chaff was statistically decreased, there is less than 1% difference between chaff types (Figure 6). Demographically and biologically, the difference between 98% and 98.6% is unlikely to significantly impact overall weed population abundance. Additionally, the minimal differences among chaff types indicate that all the other experiments conducted on barley chaff in this study should be applicable to weed seeds in canola chaff and pea chaff as well. These chaff types are highly dissimilar in terms of their structure and components due to differences in original plant structures and drying rates. Similar levels of control would likely be attained with other chaff types that are biologically and structurally related to the chaff types investigated (i.e., wheat chaff should be similar to barley chaff).

Figure 6 The effect of chaff type on percent control of weed seeds by the Harrington Seed Destructor. Bars denote standard errors of the mean. The asterisk (*) indicates a significant difference between chaff types based on a Tukey adjusted comparison of means (α=0.05).
Conclusions
These studies investigated the potential effects of weed species, seed size, seed number, chaff load, and chaff type on HSD efficacy. Across the ranges of each of these factors, the HSD performed well, controlling around 98% of weed seeds in most cases. Ranges of control were small and consistent between samples. The 10-seed treatment from the seed number experiment showed the lowest control level (ca. 84%) of all the studies, although this was likely a result of very small sample size. The ranges of each of these factors indicate potentially high HSD efficacy in many cropping situations in western Canada and the Great Plains. These studies confirm that, as in Australia and the United States, the HSD will be highly effective on seeds it processes (Schwartz et al. Reference Schwartz, Norsworthy and Walsh2017; Walsh et al. Reference Walsh, Harrington and Powles2012). On-farm studies beginning in 2017 in Alberta will evaluate weed control efficacy of the HSD in spring wheat (Triticum aestivum L.), canola, and field pea, either swathed or direct harvested (direct combined). If efficacy is high, as suggested by the stationary evaluation results reported herein, then the limiting factor in weed control will be the degree of retention of seeds on target weed species produced at a collectible height at the time of swathing or direct harvest (Burton et al. Reference Burton, Beckie, Willenborg, Shirtliffe, Schoenau and Johnson2016, Reference Burton, Beckie, Willenborg, Shirtliffe, Schoenau and Johnson2017; Tidemann et al. Reference Tidemann, Hall, Harker, Beckie, Johnson and Stevenson2017).
Acknowledgments
We gratefully acknowledge the technical assistance of Jennifer Zuidhof, Larry Michielsen, Patty Reid, Elizabeth Sroka, and Josh Kirsch, as well as assistance provided by Tasha Valente and many students. We also gratefully acknowledge PAMI for use of its facilities and the Alberta Crop Industry Development Fund and Agriculture and Agri-Food Canada for funding. BDT also acknowledges the Alberta Wheat Commission, National Science and Engineering Research Council, and Izaak Walton Killam scholarships.









