Pomegranate (Punica granatum L.) has been used for centuries to confer health benefits in a number of inflammatory diseases(Reference Lansky and Newman1, Reference Basu and Penugonda2). Fruits are widely consumed either fresh, or as a beverage. Dietary supplements containing pomegranate extracts are becoming popular in the Western world for the treatment and prevention of arthritis and other inflammatory diseases(Reference Lansky and Newman1, Reference Basu and Penugonda2). The health benefits of juice or pomegranate extracts have been attributed to the polyphenol content and composition of this fruit(Reference Basu and Penugonda2). The main polyphenols proven to have antioxidant and anti-inflammatory bioactivities in pomegranate include the ellagitannins and anthocyanins, which are concentrated in the peel and piths of the fruit(Reference Basu and Penugonda2). Pure punicalagin, total pomegranate tannin extract, or pomegranate juice have been shown to inhibit, in a dose-dependent manner, TNF-α-induced cyclo-oxygenase 2 (COX-2) expression in HT-29 human colon cancer cells(Reference Adams, Seeram and Aggarwal3). In addition, a whole pomegranate methanol extract has been shown to inhibit TNF-α expression and release in microglial cells activated with lipopolysaccharides(Reference Jung, Kim and Ha4). A recent study suggested that a pomegranate extract could be particularly promising in the dietary prevention of intestinal inflammation since it was able to inhibit NF-κB activity and to decrease inflammatory cytokine (IL-8) and PGE2 in human intestinal Caco-2 cells stimulated by cytokines(Reference Romier-Crouzet, Van De and During5).
In this context, a question often raised is whether the concentration of a plant or fruit extract constituent compound that has been used in in vitro experiments would be realistic or achievable in vivo. In a majority of the cases, this has to be denied because of the poor bioavailability of most constituents of plant or fruit extracts(Reference Seeram, Henning and Zhang6, Reference Shukla, Gupta and Rasheed7). In addition, the formation of bioactive compounds from molecules present in the original extract might be issued from intestinal and hepatic metabolism or from a too-often neglected source, namely the intestinal bacterial metabolism(Reference Kemperman, Bolca and Roger8). The human intestine harbours a complex microbial ecosystem comprising a considerable metabolic versatility, using biological pathways that humans have not evolved(Reference Qin, Li and Raes9). This capacity of the gut microbiome, encoded by the collective genomes of the gut microbiota or gut metagenome, includes the metabolism of indigestible polyphenols derived from fruit and vegetables(Reference Kemperman, Bolca and Roger8, Reference Selma, Espin and Tomas-Barberan10). This last consideration is of particular interest knowing that the gut microbiota is increasingly considered as a symbiotic partner for the maintenance of health(Reference Qin, Li and Raes9). Several data suggest that the activity of the gut microbiota is a factor to be taken into account when assessing the risk factors related to obesity, and associated disorders, such as dyslipidaemia, inflammation, insulin resistance and diabetes(Reference Backhed and Crawford11–Reference Neyrinck, Possemiers and Verstraete19). Indeed, alterations in the composition of the gut microbiota – known as dysbiosis – have been proposed to contribute to the development of obesity, thereby supporting the potential interest of nutrients acting on the gut microbes to produce beneficial effects on host energy metabolism.
The purpose of the present study was to determine whether the oral administration of a polyphenol-rich extract of pomegranate peel to mice could modulate the gut microbiota composition and offset increases in body weight, cholesterol profile, glucose intolerance, lipid storage and inflammation occurring in mice fed a high-fat (HF) diet. We decided to perform this in vivo study on Balb/c mice since it was demonstrated that (1) a HF diet induces hepatic lipid accumulation at a higher extent in Balb/c mice than in C57BL6J mice(Reference Nishikawa, Sugimoto and Okada20) and (2) Balb/c mice exhibit a higher capacity to respond to inflammatory stimulus (higher increase in plasma and hepatic levels of cytokines/chemokines upon caecal ligation and puncture inducing septic peritonitis) than C57BL6J mice(Reference Watanabe, Numata and Ito21).
Experimental methods
Animals and diet
A total of eighteen male Balb/c mice (9 weeks old at the beginning of the experiment, Charles River Laboratories) were housed in groups of three per cage in a controlled environment (12 h daylight cycle, lights off at 18.00 hours) with free access to food and water. After 1 week of acclimatisation, the mice were divided into three groups (six per group): a control (CT) group, fed a control diet (AO4, SAFE), a group fed a HF diet and a group fed the same HF diet and receiving pomegranate peel extract (HF-PPE) at a dose of 0·2 % in tap water (resulting in average consumption of 6 mg/d per mouse). The full composition of both the HF diet (D12492, Research Diets) and the A04 standard diet is given in Table S1, available online. Extract (OXYLENT GR®, Stiernon S.A.) used in the study was derived from pomegranate peel in which polyphenol content reached 30 % (Folin–Ciocalteu method, equivalent gallic acid) and the concentrations of punicalagin and ellagic acid were 8 and 5 % (ultra-performance liquid chromatography method with diode array detection), respectively. Food intake was recorded taking into account spillage twice a week during 4 weeks. After 4 weeks and a 6 h period of fasting, mice were anaesthetised (ketamine/xylazine intraperitoneal, 100 and 10 mg/kg, respectively) and blood samples were harvested for further analysis. Liver, adipose tissues, caecal content and intestinal tissues (proximal colon and caecum) were carefully dissected and immersed in liquid N2 before storage at − 80°C. The animal experiments were approved by the local ethics committee and housing conditions were as specified by the Belgian Law of 6 April 2010 on the protection of laboratory animals (agreement no. LA 1230314).
Oral glucose tolerance test
After 3 weeks of treatment, an oral glucose tolerance test (OGTT) was performed on 6 h-fasted mice. Glucose was administered orally (3 g/kg body weight, 66 % glucose solution) and blood glucose levels were determined using a glucose meter (Roche Diagnostics) on 3·5 μl of blood collected from the tip of the tail vein both before ( − 30 min and 0 min) and after glucose administration (15, 30, 60, 90 and 120 min).
Microbial analysis of the caecal contents
At the end of the experiment, the total caecum content was collected and weighed before storage at − 80°C. Quantitative PCR for total bacteria, Bifidobacterium spp., Lactobacillus spp., Bacteroides–Prevotella spp. and Roseburia spp. were performed as reported by Neyrinck et al. (Reference Neyrinck, Possemiers and Verstraete19) using Mesa Fast qPCR™ (Eurogentec). Real-time PCR were performed with the StepOnePlus™ real-time PCR system and software (Applied Biosystems). The cycle threshold of each sample was then compared with a standard curve (performed in triplicate) made by diluting genomic DNA (5-fold serial dilution of Bifidobacterium animalis for Bifidobacterium spp., Bacteroides fragilis for Bacteroides–Prevotella spp., Lactobacillus acidophilus for Lactobacillus spp. and total bacteria) (BCCM/LMG, Ghent, Belgium). Cell counts before DNA extraction were determined by culture.
Blood parameters
Plasma insulin concentrations were determined using an ultrasensitive ELISA kit (Alpco™ immunoassay, Alere Healthcare). The insulin resistance index was calculated by multiplying the AUC for glucose, and the AUC for insulin, calculated from − 30 min until 15 min after glucose challenge(Reference Abdul-Ghani, Matsuda and Balas22, Reference Neyrinck, Bindels and De23). Concentrations of IL-1α, IL-1β, IL-6, monocyte chemoattractant protein 1 (MCP-1), TNFα, IL-10 and IL-13 were determined in 15 μl of plasma using a multiplex immunoassay kit (Bio-Plex Cytokine Assay, Bio-Rad) and measured using Luminex® technology (Bioplex ®, Bio-Rad).
Plasma TAG, cholesterol and NEFA concentrations were measured using kits coupling enzymatic reaction and spectrophotometric detection of reaction end-products (Diasys Diagnostic and Systems). HDL-cholesterol concentration was measured enzymatically after VLDL, chylomicron and LDL-cholesterol antibody precipitation (Diasys Diagnostic and Systems). LDL was estimated by the Friedewald formula(Reference Friedewald, Levy and Fredrickson24).
Lipid analysis in the liver
TAG and cholesterol were measured in the liver tissue after extraction with chloroform–methanol, as described by Neyrinck et al. (Reference Neyrinck, Possemiers and Verstraete19).
Expression of selected genes in tissues
Total RNA was isolated using the TriPure isolation reagent kit (Roche Diagnostics Belgium). Complementary DNA was prepared by reverse transcription of 1 μg total RNA using the Kit Reverse transcription System (Promega). Real-time PCR were performed with the StepOnePlus™ real-time PCR system and software (Applied Biosystems) using SYBR Green for detection according to the manufacturer's instructions. RPL19 RNA was chosen as the housekeeping gene. Primer sequences for the targeted mouse genes are available on request (audrey.neyrinck@uclouvain.be). All samples were run in duplicate in a single ninety-six-well reaction plate and the data were analysed according to the 2− ΔC T method. The identity and purity of the amplified product were checked through analysis of the melting curve carried out at the end of amplification.
Statistical analysis
Results are presented as means with their standard errors. Statistical analysis was performed by the Mann–Whitney test (GraphPad Software). P< 0·05 was considered as statistically significant. A two-way analysis on repeated measures was performed for the evolution of body weight and the evolution of glycaemia upon OGTT.
Results
Oral administration of pomegranate peel extract promotes the growth of Bifidobacterium spp. in the caecal content of mice upon high-fat feeding
HF feeding decreased the weight of caecal content as compared to the control condition (Fig. 1(a)). Quantitative analysis performed in caecal content showed that HF diet decreased the number of total bacteria, the Gram-positive lactobacilli and Roseburia spp. as well as the Gram-negative bacteria such as Bacteroides–Prevotella spp. without effect on bifidobacteria content (Fig. 1). Surprisingly, the PPE treatment significantly increased the weight of caecal content and the caecal pool of Bifidobacterium spp. without modifying significantly other bacteria as compared to HF-fed mice. Of note, the increase of Bacteroides–Prevotella spp. due to PPE supplementation v. the HF-fed group was nearly of significance (P= 0·07).

Fig. 1 (a) Weight of caecal content, (b) caecal content of total bacteria, (c) caecal content of Bifidobacterium spp., (d) caecal content of Lactobacillus spp., (e) caecal content of Bacteroides–Prevotella spp. and (f) caecal content of Roseburia spp. Mice were fed a control (CT) diet, a high-fat (HF) diet or a HF diet with pomegranate peel extract (HF-PPE) in tap water for 4 weeks. * Values were significantly different from those of CT (P< 0·05). † Values were significantly different from those of HF (P< 0·05). ‡ Values were nearly significantly different from those of HF (P= 0·07).
The oral administration of pomegranate peel extract does not modify the high-fat-induced body weight gain, adiposity and glucose intolerance
A drop in body weight occurred within the first 3 d of treatment in both groups receiving the HF diet, signalling an adaptation to the dietary changes (Fig. 2(a)). The body weight evolution did not reveal any significance about time, treatment and interaction (time × treatment) after a repeated-measures analysis (two-way ANOVA). However, the HF diet significantly increased the body weight gain of mice when considering the difference between day 3 and day 30 (end of the experiment) (Fig. 2(b)). This effect was accompanied by fat accumulation as shown by the weight of the adipose tissues (Fig. 2(c)–(e)). Although body weight gain and adiposity were decreased upon PPE treatment, statistical analysis did not reveal any significant effect of PPE on those parameters. HF-fed mice exhibited glucose intolerance as shown by the glycaemia evolution upon OGTT, with a significant P value for time, treatment and interaction (time × treatment) (two-way ANOVA, P< 0·05) (Fig. 3(a) and (b)). The insulin response after the oral load of glucose was significant for the CT group only: insulinaemia was higher 15 min after the glucose load as compared to the insulinaemia measured before glucose administration (Fig. 3(c)). This was not the case when analysing values observed in the HF or HF-PPE groups (lack of post-OGTT change in insulin in those groups). Moreover, the insulin resistance index increased upon HF feeding (HF and HF-PPE v. CT mice) (Fig. 3(d)). The glycaemia evolution, the insulin concentrations and the insulin resistance index upon OGTT were not modified after PPE administration, suggesting that PPE did not affect insulin sensitivity.

Fig. 2 (a) Body weight evolution, (b) body weight gain (from day 3 to day 30) and (c) weight of visceral, (d) subcutaneous and (e) epididymal adipose tissues in mice fed a control (CT, ![]() ) diet, a high-fat (HF,
) diet, a high-fat (HF, ![]() ) diet or a HF diet with pomegranate peel extract (HF-PPE,
) diet or a HF diet with pomegranate peel extract (HF-PPE, ![]() ) in tap water for 4 weeks. * Values were significantly different from those of CT (P< 0·05). OGTT, oral glucose tolerance test.
) in tap water for 4 weeks. * Values were significantly different from those of CT (P< 0·05). OGTT, oral glucose tolerance test.

Fig. 3 Oral glucose tolerance test performed in mice fed a control (CT; (a)![]() , (b–d) □) diet, a high-fat (HF; (a)
, (b–d) □) diet, a high-fat (HF; (a)![]() , (b–d) ■) diet or a HF diet with pomegranate peel extract (HF-PPE; (a)
, (b–d) ■) diet or a HF diet with pomegranate peel extract (HF-PPE; (a)![]() , (b–d)
, (b–d) ![]() ) in tap water for 3 weeks. (a) Plasma glucose levels after the oral glucose load, (b) area under the curve (AUC) of the glucose excursion, (c) plasma insulin levels 30 min before and 15 min after the oral glucose load, (d) insulin resistance index. * Values were significantly different from those of CT (P< 0·05). † Values were significantly different from those of CT ( − 30 min) (P< 0·05).
) in tap water for 3 weeks. (a) Plasma glucose levels after the oral glucose load, (b) area under the curve (AUC) of the glucose excursion, (c) plasma insulin levels 30 min before and 15 min after the oral glucose load, (d) insulin resistance index. * Values were significantly different from those of CT (P< 0·05). † Values were significantly different from those of CT ( − 30 min) (P< 0·05).
Oral administration of pomegranate peel extract reduces the high-fat-induced hypercholesterolaemia
HF feeding negatively affected cholesterol content in the serum (increase in total cholesterol, LDL-cholesterol and HDL-cholesterol), but decreased triacylglycerolaemia as compared to the CT group (Table 1). Interestingly, the administration of PPE was able to counteract the HF-induced hypercholesterolaemia, in particular total cholesterol and LDL-cholesterol without provoking any change in HDL-cholesterol. The lipid content in the liver was not different between the groups (Table 1).
Table 1 Lipid contents in the serum and the liver‡ (Mean values with their standard errors)
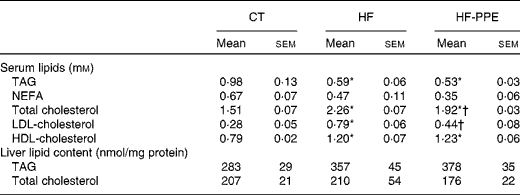
CT, control; HF, high-fat; PPE, pomegranate peel extract.
* Values were significantly different from those of CT (P< 0·05).
† Values were significantly different from those of HF (P< 0·05).
‡ Mice were fed a CT diet, a HF diet or a HF diet with PPE in tap water for 4 weeks.
Oral administration of pomegranate peel extract decreases high-fat-induced expression of inflammatory markers in the colon and the visceral adipose tissue
In contrast to what happens in the liver, a higher expression of inflammatory markers was observed both in the colon and the visceral adipose tissue in mice fed a HF diet v. control mice with a significant P value for IL-1β (Fig. 4). PPE administration significantly down-regulated COX-2 induction in colonic and adipose tissues but not in the liver. Moreover, PPE decreased the mRNA levels of IL-6 and IL-1β, with a significant P value for IL-6 mRNA in the colon and IL-1β mRNA in the visceral adipose tissue. Several inflammatory markers measured in the serum, including of IL-6 and IL-1β, were neither significantly affected by the HF diet nor by PPE administration (Data S1, available online).

Fig. 4 mRNA levels of inflammatory markers in (a) the colon, (b) the visceral adipose tissue and (c) the liver of mice fed a control (CT) diet, a high-fat (HF) diet or a HF diet with pomegranate peel extract (HF-PPE) in tap water for 4 weeks. Values are expressed relative to CT group (set at 1). * Values were significantly different from those of CT (P< 0·05). † Values were significantly different from those of HF (P< 0·05).
Discussion
The health-promoting effect of plant constituents and extracts is subject to interesting developments, a phenomenon explaining that their consumption is on the rise in the Western world(Reference Lansky and Newman1, Reference Basu and Penugonda2, Reference Aggarwal and Shishodia25). The health benefits of pomegranate fruit and/or juice consumption have recently received considerable scientific focus. PPE used in this study is a source of polyphenol (30 %), with punicalagin concentration reaching 8 %. When added in the tap water of HF-fed mice during 4 weeks, PPE did not significantly modify body weight, adiposity and glucose tolerance. However, it decreased the HF-induced inflammatory tone not only in the gastrointestinal tract but also in the adipose tissue. It has been proposed that the anti-inflammatory effect of the pomegranate extract was dependent on both the inhibition of p38-mitogen-activated protein kinase (MAPK) pathway and inhibition of the activation of transcription factor NF-κB, as demonstrated in vivo in mouse skin exposed to 12-O-tetradecanoylphorbol-13-acetate, and in vitro in human chondrocytes(Reference Afaq, Saleem and Krueger26, Reference Ahmed, Wang and Hafeez27). The activation of p38-MAPK and NF-κB is intimately associated with the increased gene expression of TNF-α, IL-1β, IL-6, monocyte chemoattractant protein 1 (MCP-1), inducible NO synthase (iNOS) and COX-2 that are critical mediators of inflammation and the pathogenesis of inflammatory diseases(Reference Hayden and Ghosh28, Reference Schieven29). In particular, COX-2-mediated inflammation in visceral fat plays a pivotal role in the development of metabolic disorders associated with obesity induced by HF feeding(Reference Hsieh, Jin and Chiang30). In the present study, we have shown that PPE consumption down-regulated IL-1β, IL-6 and COX-2 in the colon and the visceral adipose tissue as compared to HF-fed mice without effect on the expression of those markers in the liver. These data demonstrate that bioavailable constituents of PPE or metabolites coming from the gut bacteria may reach host tissue such as visceral adipose tissue to exert their anti-inflammatory effects.
In fact, after drinking pomegranate juice containing punicalagins, ellagic acid was detected in plasma, suggesting acid hydrolysis of at least some of the ellagitannins releasing free ellagic acid, which is absorbed directly from the stomach or the proximal small intestine(Reference Seeram, Henning and Zhang6). Ellagitannin has also been detected in the plasma of rats after oral administration(Reference Cerda, Llorach and Ceron31). When the ellagitannins and/or ellagic acid reach the distal part of the small intestine and the colon, they are metabolised by the gut microbiota producing urolithins A and B, which are then absorbed along with ellagic acid(Reference Seeram, Henning and Zhang6, Reference Crozier, Del and Clifford32, Reference Larrosa, Garcia-Conesa and Espin33). Once the metabolites are absorbed, glucuronidation occurs in the intestinal cells and glucuronides are the main metabolites found in portal vein plasma(Reference Espin, Gonzalez-Barrio and Cerda34). The metabolites are further metabolised in the liver to produce diglucuronides, and/or sulphates, to produce a whole combination of metabolites secreted in the bile and in the urine. It seems that urolithins are absorbed preferentially, as their lipophilicity increases with plasma and that they undergo active enterohepatic circulation(Reference Crozier, Del and Clifford32, Reference Stoffel, Espinosa and Le Beau35).
Interestingly, it was shown that urolithins had anti-inflammatory action on colon fibroblasts activated with IL-1β(Reference Gonzalez-Sarrias, Larrosa and Tomas-Barberan36). Results obtained by Gonzalez-Sarrias et al. (Reference Gonzalez-Sarrias, Espin and Tomas-Barberan37) pointed out that kinase signalling pathways (MAPK) may be involved in the response of Caco-2 cells to urolithins. Therefore, it suggests that the gut microbiota could contribute to the anti-inflammatory effects of PPE through their capacity to metabolise polyphenols.
It is known that phenolic components of common foods readily contribute to gut bacteria modulation(Reference Selma, Espin and Tomas-Barberan10, Reference Parkar, Stevenson and Skinner38, Reference Tzounis, Vulevic and Kuhnle39). Bifidobacteria served as a model for the concept of prebiotics, which has been defined as ‘the selective stimulation of growth and/or activity(ies) of one or a limited number of microbial genus(era)/species in the gut microbiota that confer(s) health benefits to the host’(Reference Roberfroid, Gibson and Hoyles40). Interestingly, the number of bifidobacteria was inversely correlated with the development of fat mass, glucose intolerance, adipose tissue inflammation and lipopolysaccharide level(Reference Delzenne, Neyrinck and Backhed17, Reference Delzenne and Cani41, Reference Cani, Neyrinck and Fava42). Our data support that the gut microbiota may be an important actor in the health beneficial action of PPE. Indeed, we had demonstrated that PPE increased the caecal pool of bifidobacteria. Although, the potential prebiotic activity of pomegranate products has not been recognised yet, a recent study has shown that the pomegranate by-product, which is rich in oligomers composed of 2–10 repeating units of gallic acid, ellagic acid and glucose in different combinations, enhanced the growth of total bacteria, in particular Bifidobacterium spp., as well as the concentrations of SCFA using pH-controlled, stirred, batch culture fermentation systems reflective of the distal region of the human large intestine in the fermentation medium(Reference Bialonska, Ramnani and Kasimsetty43). It is worth noting that punicalagins did not affect the growth of bacteria in this in vitro system.
In addition to changes in the gut microbiota, the most important effect of PPE supplementation was to blunt HF-induced hypercholesterolaemia (total and LDL-cholesterol). Recently, we and other authors provided evidence that modulation of the gut microbiota–host metabolic interrelationship by dietary intervention has the potential to improve cholesterol homeostasis, which has relevance for cardiovascular health(Reference Martinez, Wallace and Zhang44, Reference Neyrinck, Possemiers and Druart45). Martinez et al. (Reference Martinez, Wallace and Zhang44) have highlighted through correlation analysis that the Bifidobacterium/Coriobacteriaceae equilibrium was important for plasma cholesterol levels in hamsters, with bifidobacteria being beneficial and coriobacteria being detrimental. In fact, the gut microbiota may affect host cholesterol metabolism through the modulation of the enterohepatic circulation of bile acids(Reference Ridlon, Kang and Hylemon46). We postulate that the modulation of the gut microbiota induced by PPE supplementation observed in the present study can be involved in its hypocholesterolaemic effects.
In conclusion, the present study has shown that oral administration of a PPE rich in polyphenols is able to modulate the gut microbiota in favour of bifidobacteria. This prebiotic effect was accompanied by a lower expression of key inflammatory expression in the colon and the visceral adipose tissue. The gut microbiota changes due to the PPE treatment were also accompanied by an improvement of atherogenic markers such as LDL-cholesterol in HF diet-induced obesity. Although mechanistic studies are needed in order to determine which bioactive constituent(s) or metabolite(s) coming from the gut bacteria were responsible for these effects, our results suggest that PPE can confer positive health impacts associated with gut microbiota modulation and may be a natural alternative in the prevention of obesity and CVD.
Acknowledgements
L. B. B. is a research fellow and P. D. C. is a research associate from the FRS-FNRS (Fonds de la Recherche Scientifique) in Belgium. N. M. D. and P. D. C. are recipients for grant from the FRS-FNRS. Financial support was provided by a grant from the Walloon Region (WalNut 20 Project, convention 5459). A. M. N. and N. M. D. conceived and designed the experiments, and wrote the paper. A. M. N. and V. F. V. H. analysed the data. A. M. N., V. F. V. H. and F. D. B. performed the in vivo experiments and biochemical analysis. V. F. V. H. and F. D. B. performed RNA extraction in tissues and measured mRNA levels by quantitative PCR. L. B. B. performed and interpreted gut microbiota analysis (quantitative PCR). P. D. C., N. M. D. and L. B. B. provided intellectual input on the paper and reviewed the paper. N. M. D. planned and supervised all experiments and the manuscript preparation. The authors declare that there are no conflicts of interest in relation to this study.
Supplementary table S1 and data are available online at http://www.journals.cambridge.org/bjn







