Introduction
Gallium (Ga) and germanium (Ge) are listed among the most critical elements for industry in the European Union (European Commission, 2020), as well as in other technologically advanced countries (Fortier et al., Reference Fortier, Nassar, Lederer, Brainard, Gambogi and McCullough2018; Hofstra et al., Reference Hofstra, Lisitsin, Corriveau, Paradis, Peter, Lauzière, Lawley, Gadd, Pilote, Honsberger, Bastrakov, Champion, Czarnota, Doublier, Huston, Raymond, VanDerWielen, Emsbo, Granitto and Kreiner2021). Gallium is mostly used to manufacture integrated circuits and optoelectrical devices such as laser diodes, light-emitting diodes (LEDs), photodetectors and solar cells (Moskalyk, Reference Moskalyk2003; Butcher and Brown, Reference Butcher, Brown and Gunn2014; USGS, 2021a). Germanium also has uses in electronics and solar applications such as fibre/infrared optics or in chemical catalysis (Moskalyk, Reference Moskalyk2004; Melcher and Buchholz, Reference Melcher, Buchholz and Gunn2014; USGS, 2021b). Gallium is mostly extracted as a by-product of bauxite processing and, to a much lesser extent, from Zn-processing residues and coal (Butcher and Brown, Reference Butcher, Brown and Gunn2014; Frenzel et al., Reference Frenzel, Hirsch and Gutzmer2016; USGS, 2021a). In contrast, Ge is mostly associated with Zn or Pb–Zn–Cu sulfide ores and coals (Frenzel et al., Reference Frenzel, Ketris and Gutzmer2014; USGS, 2021a,b), and many old deposits and prospects of Zn ores have recently been (re-)examined because of the potential to recover Ge (Saini-Eidukat et al., Reference Saini-Eidukat, Melcher and Lodziak2009; Mondillo et al., Reference Mondillo, Herrington, Boyce, Wilkinson, Santoro and Rumsey2018a,Reference Mondillo, Arfè, Herrington, Boni, Wilkinson and Mormoneb). In addition, so-called ‘metalliferous coals’ highly enriched in Ge (grades in the order of hundreds of ppm) located especially in China and Russia are also considered as potential sources of Ge (Melcher and Buchholz, Reference Melcher, Buchholz and Gunn2014 and references therein).
During the pyrometallurgical processing of sulfide ores, Ga and Ge mostly end up in the slag phase due to a strong affinity with Al (Ga) and Si (Ge) (Piatak and Ettler, Reference Piatak, Ettler, Piatak and Ettler2021). Recent laboratory experiments simulating Cu pyrometallurgy confirmed that Ga was partitioned predominantly into the slag phase, but Ge, present in significantly lower concentrations, was almost entirely vaporised (Avarmaa et al., Reference Avarmaa, Klemettinen, O'Brien, Taskinen and Jokilaakso2019). However, under reducing Pb smelting conditions, Ge was incorporated into silicate slag, with minor volatilisation and only a limited amount dissolved into the Pb metal (Yan and Swinbourne, Reference Yan and Swinbourne2003). Old slag deposits have also been reported as a potential source of Ga and Ge. As an example, the 15 Mt slag heap in Lubumbashi, Democratic Republic of Congo produced by processing Kipushi Ge-rich Zn–Cu ores and stratiform Cu–Co ores contains Ge concentrations ranging from 100 to 250 ppm, and is potentially the largest known occurrence of Ge (Melcher and Buchholz, Reference Melcher and Buchholz2012).
Other interesting sites occur in Otavi Mountain Land in northern Namibia, where some Ge-rich sulfide deposits (e.g. Tsumeb and Khusib Springs) have been discovered with ore grades up to 0.83% Ge found predominantly in sulfides (Melcher, Reference Melcher2003; Melcher et al., Reference Melcher, Oberthur and Rammlmair2006; Höll et al., Reference Höll, Kling and Schroll2007; Kamona and Günzel, Reference Kamona and Günzel2007). These Ga- and Ge-bearing polymetallic sulfide ores mined in the area were historically processed in the Tsumeb smelter, and old slag deposits are now reported as a potential source of Ge (and Ga) (Höll et al., Reference Höll, Kling and Schroll2007; Frenzel et al., Reference Frenzel, Ketris and Gutzmer2014).
Our previous investigations of the Tsumeb metallurgical slags of various ages and produced from different technologies, reported typical concentrations of Ga and Ge in the order of tens ppm (up to 48 ppm Ga, 123 ppm Ge; Ettler et al., Reference Ettler, Johan, Kříbek, Šebek and Mihaljevič2009a; Jarošíková et al., Reference Jarošíková, Ettler, Mihaljevič, Kříbek and Mapani2017). Another screening study has shown recently that some old Cu slags from Tsumeb can contain ~100 ppm Ga (Lohmeier et al., Reference Lohmeier, Lottermoser, Schirmer and Gallhofer2021). Recovery of Ga and Ge from waste materials and intermediate products is mostly carried out via hydrometallurgical processing (Fayram and Anderson, Reference Fayram and Anderson2008; Liu et al., Reference Liu, Liu, Li, Liu, Li and Li2016; Drzazga et al., Reference Drzazga, Prajsnar, Chmielarz, Benke, Leszczyńska-Sejda, Ciszewski, Bilewska and Krawiec2018) or chloride fuming processes (Dey et al., Reference Dey, Hutton-Ashkenny, Grogan, Bowell, Guilders, Chueng, Williams, Evans and Goldsack2010). However, the design of the metallurgical technology should be based on a detailed knowledge of solid-phase partitioning of target elements within the material. Chirkst et al. (Reference Chirkst, Chistyakov and Cheremisina2008) proposed a complex chloride leaching and a sorption-based hydrometallurgical method for Zn and Ge recovery from Ga- and Ge-rich slags from Tsumeb. The challenge with the proposed technology was the poor understanding of contaminant elements within the slag and incorporation of these into the products that compromised the quality of the metal oxides produced. In addition, the sensitive nature of the proposed D-403 anionic ion exchange resin made the project uneconomic as a metal process at the time of evaluation. To improve the opportunity to recover these metals from the slag it is essential that the material is characterised in terms of mineralogy and distribution of Ga and Ge in the individual phases. This work aims to characterise the speciation and mineralogy of Ga and Ge within the Tsumeb lead blast-furnace slag materials through a combination of mineralogical and chemical methods such as powder X-ray diffraction (PXRD), scanning electron microscopy and electron microprobe with field emission gun electron source (FEG-SEM-EPMA), and laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS).
Experimental methods
Sample collection and processing
Slags were collected (with the assistance of smelter staff) in the Tsumeb smelter area during a field campaign in 2012 (GPS coordinates: –19.2255, 17.7226) from the historic reduced Pb blast-furnace slag, produced up to 1996. The smelter was officially commissioned in 1963 by Tsumeb Corporation Limited (TCL). At the time of working, the smelter consisted of a Pb section (including a refinery), a Cu section, and plants that produced Cd, Na antimonite and As2O3. Production commenced in early 1964, with 3500 tonnes of Cu and 6000 tonnes of Pb being produced per month. The slag originated from the second stage of the reverberatory furnaces that produced Pb from Cu-depleted feed material. This part of the smelter is currently being dismantled (Dundee Precious Metals, personal communication, 2021).
The dumps were composed mainly of granulated slags, but massive slag fragments were also encountered (Fig. 1). Samples denoted as T17 corresponded to ‘Ge slags’ (aged ~30 years at the time of sampling), and samples denoted as T18 corresponded to ‘Zn slags’ (aged ~20 years at the time of sampling) (H. Nolte and M. Trust, personal communication, 2012). Five samples were collected: three massive slag specimens grabbed on the dump surfaces and two representative granulated slags collected as composite samples from several randomly selected sites of the dump. Höll et al. (Reference Höll, Kling and Schroll2007) estimated that the two dumps of Ga- and Ge-rich slags account for 2.9 Mt of material grading 9% Zn, 2.05% Pb, 183 ppm Ge, 200 ppm Ga, and could yield ~800 t Ge, thus representing a major Ge source. Today we know that only 2.2 Mt of these slags remain at the dumps, because some of it was used to cover the nearby tailing pile to reduce the air dispersion of tailing-derived dust particles.

Fig. 1. Field photographs of Ga- and Ge-bearing slags from Tsumeb, Namibia, taken in 2012. (a) View from the top of the dump; (b) massive fragment of slag on the surface of granulated slag dump.
For the microscopic observations, SEM, EPMA and LA-ICP-MS analyses, aliquot parts of the samples were embedded in an epoxy resin and prepared as polished (thin) sections. Other sample aliquots were pulverised in an agate planetary mill (Retsch PM 400, Germany) and used for bulk chemistry and phase-composition determinations.
Bulk chemistry
Determination of major-element composition of the slags was carried out via standard silicate analysis used for rock materials (combination of gravimetric, volumetric, and spectrometric analyses) as described in Ettler et al. (Reference Ettler, Johan, Kříbek, Šebek and Mihaljevič2009a). The contents of total sulphur (Stot) and total organic carbon (Corg) were determined using a combination of ELTRA CS 530 and ELTRA CS 500 TIC analysers (ELTRA, Germany). For the determination of trace elements in slags, we used two digestion procedures (modified from Strnad et al., Reference Strnad, Mihaljevič and Šebek2005) because when preparing samples for the Ge analysis, the usage of HClO4 or HCl during the digestion procedures may lead to Ge loss due to the formation of volatile GeCl4 (Biver and Filella, Reference Biver and Filella2018). The following procedure was used for all the elements studied except Ge: a mass of 0.2 g of sample was dissolved in a closed system (Teflon beakers, Savillex, USA) in a mixture of 10 ml of HF (49% v/v) and 0.5 ml HClO4 (70% v/v) on a hot plate (130°C). The mixture was evaporated to dryness, and this procedure was repeated with 5 ml of HF and 0.5 ml of HClO4. The residue was then evaporated to near dryness, dissolved in 2% HNO3 (v/v), and diluted to 100 ml before the analysis. A modified digestion procedure was used for the analysis of Ge and avoided the use of chlorate: a mass of 0.2 g of sample was dissolved in a closed system (Teflon beakers, Savillex, USA) in a mixture of 10 ml of HF (49% v/v) and 0.5 ml HNO3 (65% v/v) on a hot plate (130°C). The mixture was evaporated to dryness, and this procedure was repeated with 5 ml of HF and 0.5 ml of HNO3. The residue was then evaporated to near dryness, dissolved in 2% HNO3 (v/v) and diluted to 100 ml before the analysis. All chemicals were reagent-grade (Sigma-Aldrich, Germany), and deionised water obtained by a Millipore Academic (Millipore, USA) system was used for the dilutions.
The digests were analysed for a series of metals and metalloids using a combination of inductively coupled plasma optical emission spectrometry (ICP-OES, Agilent 5110, USA; As, Ba, Cu, Mo and Zn) and quadrupole-based inductively coupled plasma mass spectrometry (ICP-MS, ThermoScientific, iCAP-QTM, Germany; Ag, Bi, Cd, Co, Cr, Cu, Ga, Ge, In, Ni, Pb, Sb, Se, Sn and V). The 71Ga and 74Ge isotopes were used during the ICP-MS analysis, while the isotopes of other elements were selected as recommended by the manufacturer.
Quality control/quality assurance (QC/QA) of the bulk-digestion procedures and analyses was checked by parallel processing of several certified reference materials: NIST 1633 (Coal Fly Ash), NIST 2782 (Industrial Sludge), NIST 2780 (Hard Rock Mine Waste), BCR-2 (Basalt, British Columbia, USGS), AGV-2 (Andesite, Guano Valley, USGS). The results were found to be satisfactory (Supplementary Table S1).
Mineralogical investigations
Phase compositions of the slags were determined by powder X-ray diffraction analysis (PXRD) using a PANalytical X'Pert Pro diffractometer with Bragg-Brentano geometry (PANalytical, the Netherlands) and an X'Celerator detector (conditions: CuKα radiation, 40 kV, 30 mA, 2 theta range of 5–70°, a step size of 0.02° and counting time 150 s per step). The diffraction patterns obtained were analysed by the X'Pert High Score Plus 3.0 software coupled with the Crystallography Open Database (COD) (Gražulis et al., Reference Gražulis, Daškevič, Merkys, Chateigner, Lutterotti, Quirós, Serebryanaya, Moeck, Downs and Le Bail2012).
Polished (thin) sections of the specimens were first examined under a Leica DM LP polarising microscope (Leica, Germany). A JEOL JXA–8530F (JEOL, Japan) electron probe microanalyser (EPMA) equipped with a field emission gun source (FEG), energy dispersion spectrometer (EDS; JEOL JED–2300F), and five wave-dispersion spectrometers (WDS) was used for scanning electron microscopic (SEM) imaging, EDS analyses and quantitative analyses.
The lowest detection limits for our EPMA measurements of oxides and silicates (accelerating voltage of 15 kV and beam current of 20 nA) were 70 ppm for Ga and 90 ppm for Ge. For analyses of sulfides and metallic phases the EPMA was run at 20 kV and 30 nA, and the detection limits were slightly lower (Ga: 40 ppm and Ge: 60 ppm), however none of these elements were detected in any of these phases (see also results below). The standards for measurements were GaAs for Ga (Lα) and Rb-glass containing 54.09 ± 0.07 wt.% GeO2 for Ge (Lα) or germanium metal (Kα). Detailed analytical conditions and standards used for the instrument calibration are given in Supplementary Table S2.
Laser ablation ICP-MS
Laser ablation ICP-MS was carried out mainly on granulated slag samples (prepared as polished sections) because the textures in massive slags were too fine and complex (with crystals often <10 μm in size) such that it was almost impossible to find areas suitable for the analysis. The LA-ICP-MS was carried out on a NewWave Nd:YAG laser with an output wavelength of 213 nm coupled to a ThermoScientific iCAP-QTM ICP-MS. Ablated spots were 40–80 μm in diameter, fired with laser energy of 350 μJ at a repetition frequency of 10 Hz; total ablation time was 100 s. Aluminium (27Al) was employed as an internal standard, based on EPMA measurements of Al2O3 content in the studied phases. The data were processed externally in a MS Excel spreadsheet-based program, and 71Ga and 74Ge isotopes were used for the concentration determinations. BCR-2G certified reference material was used as an external calibrator of the LA-ICP-MS measurements. The measured values (Ga: 21 ± 1 ppm and Ge: 1.4 ± 0.1 ppm, N = 11) were in an excellent agreement with the certified (Ga: 23 ± 1 ppm) or reported values (Ge: 1.5 ± 0.1 ppm), as published in the GeoREM database as ‘preferred values’ (http://georem.mpch-mainz.gwdg.de). For detailed analytical protocol and correction strategy see Strnad et al. (Reference Strnad, Mihaljevič and Šebek2005, Reference Strnad, Ettler, Mihaljevič, Hladil and Chrastný2009).
Results
Bulk chemistry
The slags studied are typically Si–Ca–Fe rich materials with relatively high concentrations of trace elements (in ppm): Zn (84,700–110,000), Pb (14,500–24,800), Cu (2530–4080), Mo (2280–2570), As (1700–3650) and Ba (999–2640). The concentrations of Ga and Ge vary in the range of 135–156 ppm and 128–441 ppm, respectively (Table 1). Compared to our previous analyses of other Tsumeb slags (scatter plot in Fig. 2; Ettler et al., Reference Ettler, Johan, Kříbek, Šebek and Mihaljevič2009a; Jarošíková et al., Reference Jarošíková, Ettler, Mihaljevič, Kříbek and Mapani2017), the concentrations of Ga and Ge in the slags studied are substantially higher and correspond well to bulk chemistry data for these Ga- and Ge-rich slags reported elsewhere (Höll et al., Reference Höll, Kling and Schroll2007; Chirkst et al., Reference Chirkst, Chistyakov and Cheremisina2008).
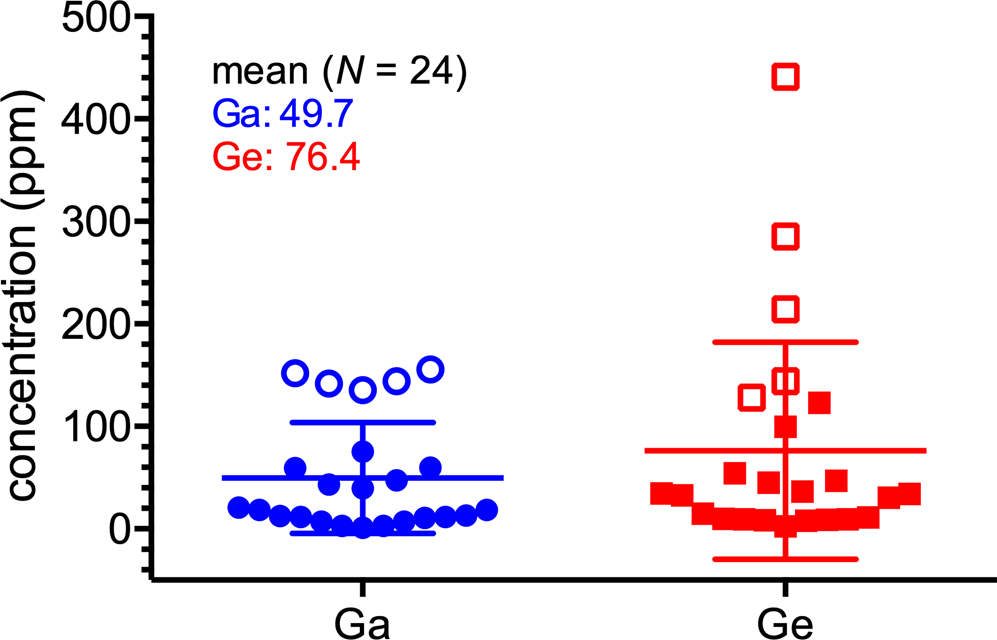
Fig. 2. Scatter plot showing the variability of Ga and Ge concentrations in the Tsumeb slags. Data taken from Ettler et al. (Reference Ettler, Johan, Kříbek, Šebek and Mihaljevič2009a), Jarošíková et al. (Reference Jarošíková, Ettler, Mihaljevič, Kříbek and Mapani2017), and unpublished results (Ga- and Ge-rich samples investigated in this study are indicated with empty symbols).
Table 1. Bulk chemical compositions of Ga- and Ge-bearing slags from Tsumeb, Namibia (mean ± standard deviation for trace elements, N = 2).*

* Stot – total sulphur; Corg – organic carbon; LOI – loss on ignition; Total = sum of oxides + Stot + Corg + LOI + metal(loid)s in elemental form.
Slag petrography
Representative slag textures and phase assemblages for massive and granulated slags are reported in Figs 3 and 4, respectively. Even though anthropogenic materials do not fulfil the criteria for being minerals, in this paper we use mineral names for their synthetic equivalents for the sake of clarity in defining solid phase compositions.
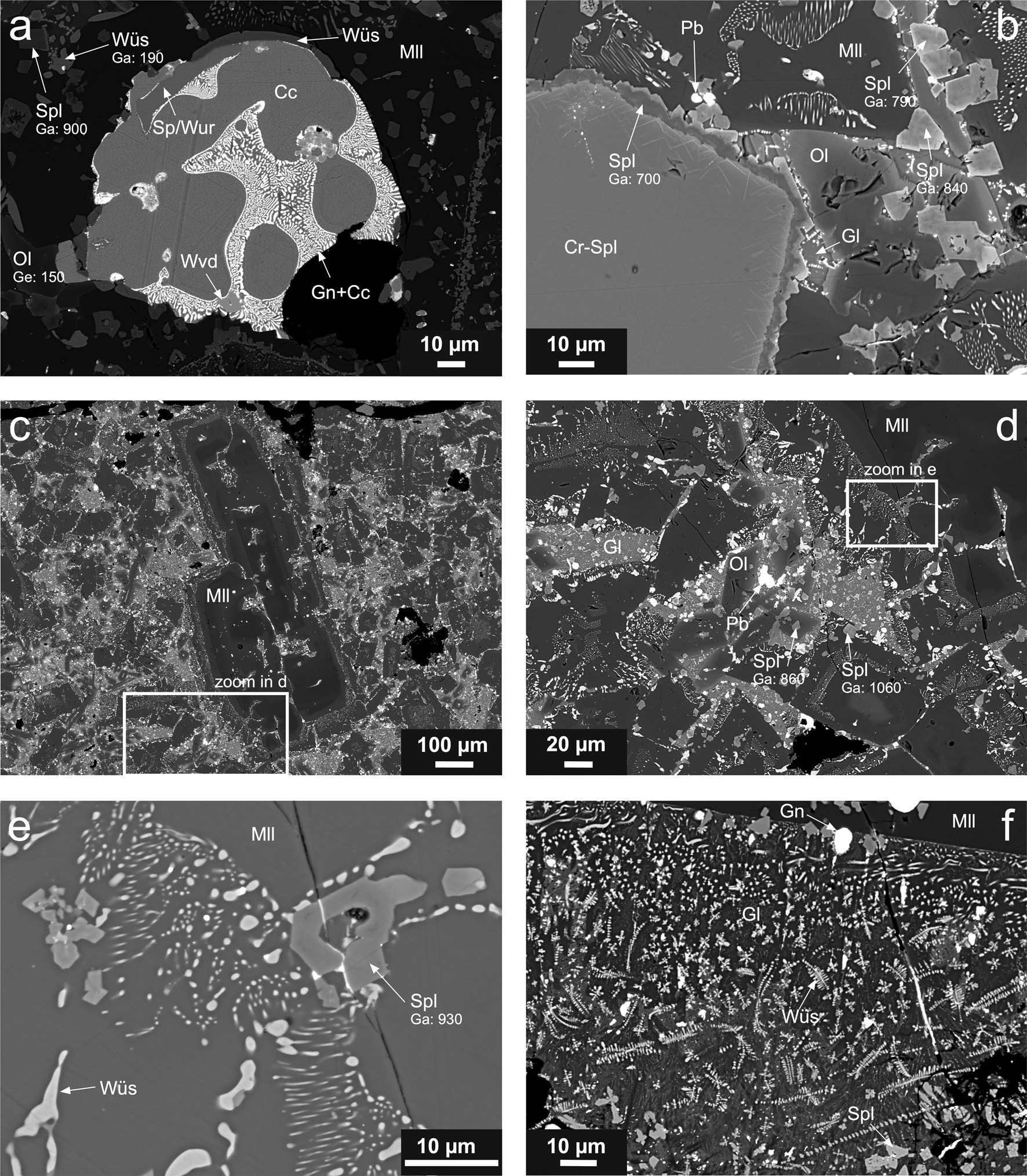
Fig. 3. Scanning electron micrographs of massive slag samples. (a) Sulfide droplet composed of chalcocite (Cc) and chalcocite–galena (Gn) myrmekite, with included crystals of sphalerite/wurtzite (Sp/Wur) and westerveldite (Wvd). The droplet is embedded in a silicate matrix composed of olivine (Ol) and melilite (Mll) with the wüstite (Wüs) and spinel (Spl) dendrites (sample T17-1A). (b) Large Cr-bearing spinel (Cr-Spl) crystal with a Ga-containing spinel rim and euhedral spinel crystals in a silicate matrix composed of melilite, olivine and glass containing Pb droplets (sample T17-1A). (c and d) Large melilite crystals associated with a silicate matrix composed of olivine and glass with euhedral spinel crystals and Pb inclusions (sample T17-1B). (e) Ga-bearing spinel crystals and wüstite exsolutions in melilite. (f) Euhedral spinel crystals, galena droplets and wüstite dendrites within a glass associated with a large melilite crystal (sample T18-1). Phase abbreviations according to Warr (Reference Warr2021). Gallium and germanium concentrations (in ppm) in individual phases are indicated.
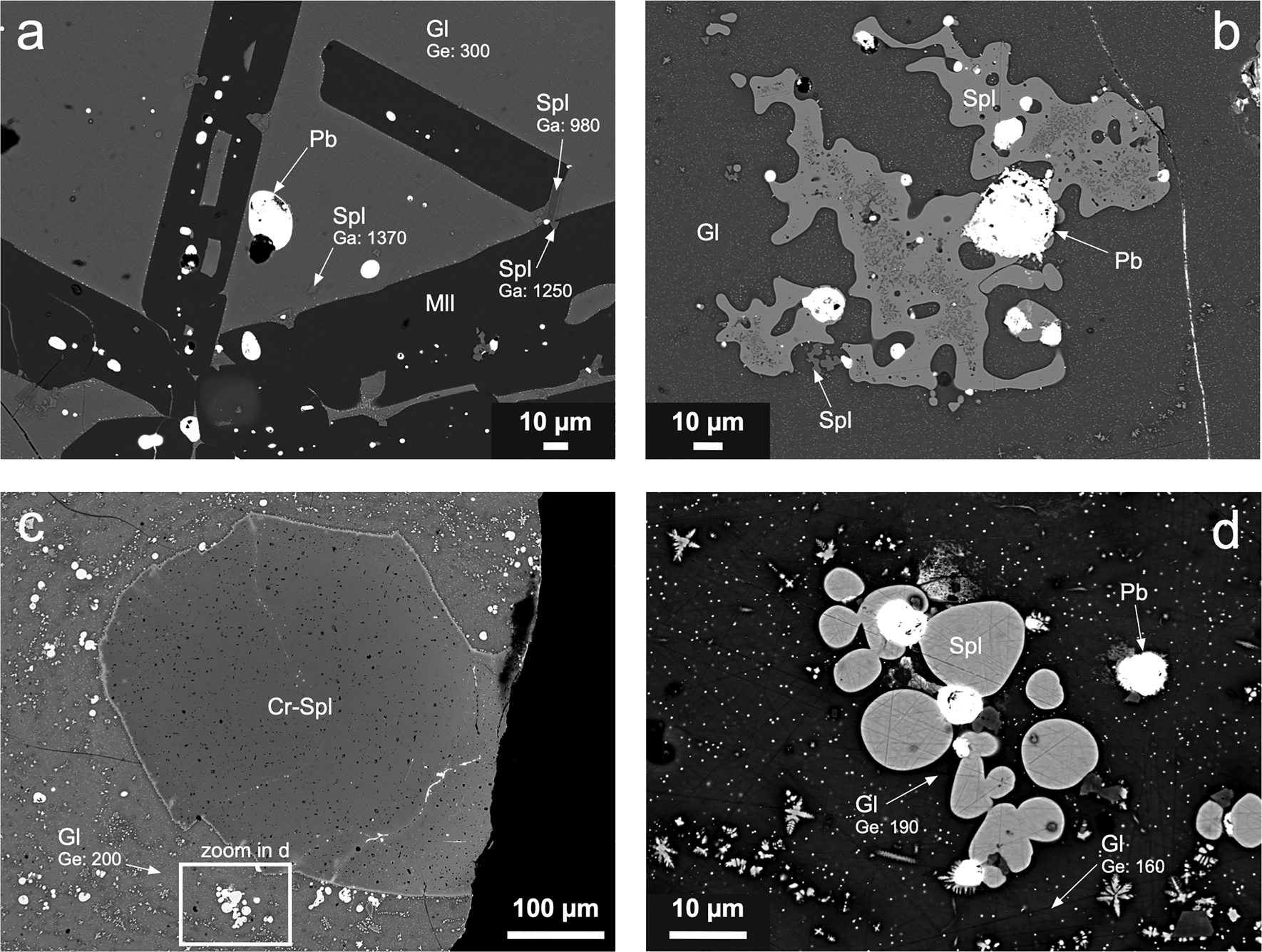
Fig. 4. Scanning electron micrographs of granulated slag samples. (a) Large melilite (Mll) crystals, Pb droplets, and euhedral spinels (Spl) within a glassy matrix (Gl) (sample T17-2). (b) Spinel blebs associated with Pb droplets embedded in glass containing submicrometric spinel crystals and metallic droplets (sample T18-2). (c) Large Cr-bearing spinel embedded in glass (sample T18-2). (d) Oval blebs of spinel and Pb droplets embedded in Ge-bearing glass (sample T18-2). Phase abbreviations according to Warr (Reference Warr2021). Gallium and germanium concentrations (in ppm) in individual phases are indicated.
In the massive slags, spinel is the first crystallising phase, followed by melilite, which often contains wüstite exsolutions (Fig. 3b,d,e). The crystallisation sequence continues with the formation of olivine-group phases and residual glass (Fig. 3b,d), which may also contain small wüstite dendrites (Fig. 3f). The silicate matrix contains numerous droplets of metallic/sulfide phases: (1) larger inclusions (<100 μm in size) of complex composition and (2) small droplets (often less than several μm in size), embedded in the glass and other phases, and composed mostly of metallic Pb or galena (PbS) (Fig. 1a,b,d,f). The large inclusions are commonly composed of various sulfides (chalcocite, Cu2S, and chalcocite–galena symplectite, sphalerite/wurtzite, ZnS, and intermetallic phases such as westerveldite, FeAs) (Fig. 1a).
Granulated slags exhibit much simpler phase compositions and textures, indicating quenching of the slag melt. Glass is a dominant phase in the samples, and it often contains submicrometre crystals of spinel-group phases and inclusions of metallic Pb (Fig. 4). Spinels are quite common: they either form tiny euhedral crystals embedded in glass or melilite (<10 μm in size) (Fig. 4a) or larger blebs (up to ~100 μm in size) (Fig. 4b–d). Large Cr-bearing spinel crystals are relatively rare (Fig. 4c). Melilite forms skeletal crystals indicating rapid cooling of the melt (Fig. 4a).
Crystal chemistry and Ga and Ge partitioning in individual phases
Despite our expectations that Ga and Ge may be bound in sulfides (e.g. sphalerite; Johan, Reference Johan1988; Saini-Eidukat et al., Reference Saini-Eidukat, Melcher and Lodziak2009; Frenzel et al., Reference Frenzel, Hirsch and Gutzmer2016; Mondillo et al., Reference Mondillo, Herrington, Boyce, Wilkinson, Santoro and Rumsey2018a), our EPMA data indicated that the slag sulfides contain no Ga or Ge in concentrations above the detection limit of the method. In addition, the small size of the sulfide and metallic inclusions precluded trace-element analysis by LA-ICP-MS. The EPMA results for individual silicate and oxides phases are given in Tables 2–4, and the LA-ICP-MS data are reported in Table 5.
Table 2. Selected electron microprobe analyses of spinels.
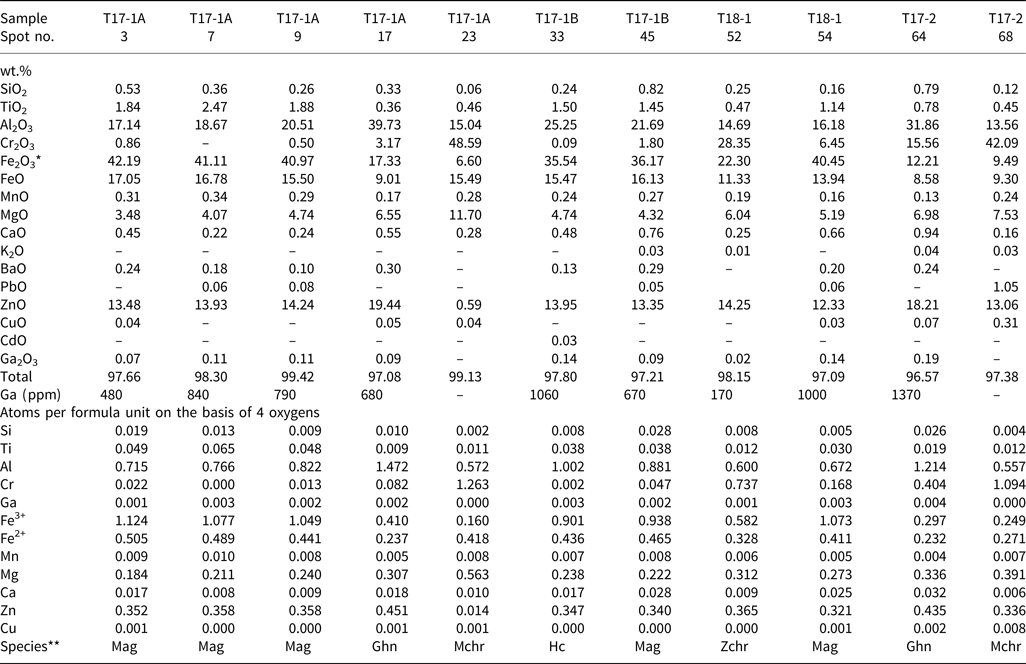
*Fe2O3/FeO were calculated using the End-Members Generator (EMG, version 8.0) software by Ferracutti et al. (Reference Ferracutti, Gargiulo, Ganuza, Bjerg and Castro2015).
** Species identification according to spinel supergroup nomenclature and classification (Bosi et al., Reference Bosi, Biagioni and Pasero2019). Abbreviations: Hc – hercynite (FeAl2O4), Ghn – gahnite (ZnAl2O4), Mag – magnetite (Fe2+Fe3+2O4), Mchr – magnesiochromite (MgCr2O4), Zchr – zincochromite (ZnCr2O4).
Table 3. Selected electron microprobe compositional data for melilite- and olivine-group phases.

‘–’ not detected; *abbreviations: Hdy – hardystonite (Ca2ZnSi2O7); Åk – åkermanite (Ca2MgSi2O7); FeÅk – ‘ferroåkermanite’ (Ca2FeSi2O7); Gh – gehlenite (Ca2Al2SiO7); Aåk – alumoåkermanite (CaNaAlSi2O7; large cations K, Pb and Ba were associated with this end-member); Lrn – larnite (Ca2SiO4); Fo – forsterite (Mg2SiO4); Fa – fayalite (Fe2SiO4); and Tep – tephroite (Mn2SiO4).
Table 4. Selected electron microprobe compositional data for glass.

‘–’ not detected; *mean calculated for N = 40 analyses, except for CuO (N = 37), GeO2 and Ge (N = 16), Na2O (N = 13), Cr2O3 (N = 12) and CdO (N = 4).
Table 5. LA-ICP-MS data for Ga and Ge concentrations in individual phases (mean values or ranges of means).
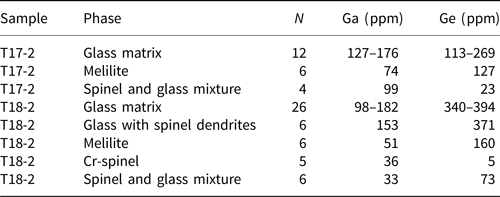
The EPMA showed that the oxyspinels are the key hosts of Ga in the slag. However, large variability in their compositions has been found (Table 2). The Zn–Fe–Al spinels, which according to classification (Bosi et al., Reference Bosi, Biagioni and Pasero2019) correspond to gahnite, (ZnAl2O4), hercynite (FeAl2O4) and magnetite (Fe2+Fe3+2O4) and form small euhedral crystals and rims (Fig. 3a,b,d,e), are Ga-rich and contain between 480 and 1370 ppm Ga. The most Ga-rich spinel was identified as gahnite with a chemical formula corresponding to (Zn0.453Mg0.336Fe2+0.232Al1.214Cr0.404Fe3+0.297)O4, and Ga content in this spinel accounted for 0.004 apfu. In contrast, Cr-bearing spinels forming larger crystals (Figs 3b and 4c) exhibit low Ga concentrations (zincochromite, ZnCr2O4, analysis 52 in Table 2, 170 ppm Ga) or are Ga-free (magnesiochromite, MgCr2O4, analyses 23 and 68 in Table 2). The LA-ICP-MS analyses of the large Cr-bearing spinel crystals indicate mean concentrations of 36 ppm Ga and 5 ppm Ge and the magnetite-dominant spinels within the glass exhibit on average 33–99 ppm Ga and 23–73 ppm Ge (the latter element probably being related to a Ge-bearing glass phase in the sample analysed in a mixture with the spinel) (Table 5).
In addition, we also detected Ga via EPMA in some wüstite dendrites (up to 190 ppm Ga [analyses not shown]; Fig. 3a) and rarely in melilite (90 ppm Ga; analysis 60 in Table 3) in the crystalline slags. The LA-ICP-MS analyses indicated that the melilites in granulated slags exhibit 51–74 ppm Ga and 127–160 ppm Ge. Interestingly, no Ge was detected in melilite by EPMA (detection limit was 90 ppm Ge) and LA-ICP-MS results with values above 100 ppm Ge indicates that some Ge-bearing glass inclusions might have been analysed together with the melilite matrix.
Some Ge was detected in olivine-group phases in the massive slags (olivine and monticellite; 140–150 ppm Ge), but glass is the principal host for Ge, especially in the granulated samples, which are richer in this element compared to massive slags (Tables 1, 3 and 4). Germanium concentrations in glass as detected by the EPMA range from <90 to 470 ppm (Table 4; Fig. 4). The LA-ICP-MS analyses of the glass matrices in the granulated slags indicated that the Ga and Ge concentration ranges are 98–182 ppm and 113–394 ppm, respectively (Table 5). However, many of the glass analyses obtained by LA-ICP-MS probably corresponded to mixtures with other phases because the glass phase contains submicrometric spinel dendrites and the laser spots were significantly larger (Supplementary Fig. S2).
Discussion
The phase assemblages and phase proportions in slags depend on the melt compositions, and melting and cooling conditions (Piatak et al., Reference Piatak, Ettler, Hoppe, Piatak and Ettler2021). The position of bulk slag compositions and glass analyses in the ternary SiO2–CaO–FeO diagram (Supplementary Fig. S3) indicates that the melting temperatures ranged from 1150 to 1400°C, similarly to other non-ferrous smelting slags (Vítková et al., Reference Vítková, Ettler, Johan, Kříbek, Šebek and Mihaljevič2010; Warchulski et al., Reference Warchulski, Gaweda, Kadziolka-Gawel and Szopa2015; Piatak et al., Reference Piatak, Ettler, Hoppe, Piatak and Ettler2021). The mineralogical compositions of the studied Ga- and Ge-bearing slags with dominant glass, spinel-group phases, melilite and olivine are consistent with the descriptions of the old slags produced from smelting in reverberatory and blast furnaces that were in operation in Tsumeb until 1996 (Ettler et al., Reference Ettler, Johan, Kříbek, Šebek and Mihaljevič2009a,b; Jarošíková et al., Reference Jarošíková, Ettler, Mihaljevič, Kříbek and Mapani2017). The orders of the phase formation are spinel > melilite > olivine > glass for massive slags and spinel > melilite > glass for granulated slags. This is consistent with observations from other polymetallic slags (Ettler et al., Reference Ettler, Legendre, Bodénan and Touray2001; Ettler et al., Reference Ettler, Johan, Kříbek, Šebek and Mihaljevič2009a,Reference Ettler, Johan, Kříbek and Nolteb; Jarošíková et al., Reference Jarošíková, Ettler, Mihaljevič, Kříbek and Mapani2017). The occurrence of melilite in the mineral assemblages indicates that the slag melt was rich in Ca (Warchulski et al., Reference Warchulski, Gaweda, Janeczek and Kadziolka-Gawel2016; Piatak et al., Reference Piatak, Ettler, Hoppe, Piatak and Ettler2021). Interestingly, clinopyroxene [Ca(Mg,Fe)Si2O6], a typical phase observed in many Cu and Pb–Zn slags (Lottermoser, Reference Lottermoser2002; Vítková et al., Reference Vítková, Ettler, Johan, Kříbek, Šebek and Mihaljevič2010; Kierczak and Pietranik, Reference Kierczak and Pietranik2011; Warchulski et al., Reference Warchulski, Gaweda, Kadziolka-Gawel and Szopa2015, Reference Warchulski, Gaweda, Janeczek and Kadziolka-Gawel2016), was not observed in the studied samples. Clinopyroxene was only detected in some Cu smelting slags from Tsumeb produced by the Ausmelt/TSL [Top-Submerged Lance] furnaces operating in Tsumeb until present times (Jarošíková et al., Reference Jarošíková, Ettler, Mihaljevič, Kříbek and Mapani2017; Lohmeier et al., Reference Lohmeier, Lottermoser, Schirmer and Gallhofer2021). This fact and the chemical compositions showing the predominance of Pb over Cu (Table 1), indicate that the Ga- and Ge-slags studied correspond to materials issued from the processing of the Cu-depleted portion of the tennantite [(Cu,Fe)12As4S13]-dominated Cu–Pb ores, being the major source of Ga and Ge, which were mined from levels 34 to 46 of the Tsumeb deposit (Frondel and Ito, Reference Frondel and Ito1957; Gebhard, Reference Gebhard1999; Melcher, Reference Melcher2003). These valuable elements probably do not originate from the fuel because our unpublished analyses show that their concentrations in the hard coal used in the Tsumeb smelter in 2012 were relatively low: 22.6 ± 0.16 ppm Ga and 33.1 ± 0.29 ppm Ge.
The concentrations of Ga (135–156 ppm) and Ge (128–441 ppm) in the slags studied (Table 1) correspond well to values reported previously for some of the Tsumeb slags: 47–194 ppm Ga (average: 102 ppm, N = 21; Lohmeier et al., Reference Lohmeier, Lottermoser, Schirmer and Gallhofer2021), 200 ppm Ga and 183 ppm Ge (Höll et al., Reference Höll, Kling and Schroll2007), and 220–280 ppm Ga and 350–690 ppm Ge (average: 245 ppm Ga, 455 ppm Ge, N = 4; Chirkst et al., Reference Chirkst, Chistyakov and Cheremisina2008). Other similar slags from Lubumbashi in the Democratic Republic of Congo exhibit concentrations of 100 ppm and 250 ppm Ge on the margin and in the core of the slag dump, respectively (Melcher and Buchholz, Reference Melcher and Buchholz2012).
The results presented here indicate that Ga is hosted primarily in spinel-group phases. According to the spinel supergroup nomenclature and classifications (Bosi et al., Reference Bosi, Biagioni and Pasero2019), the general formula of the oxyspinel subgroup is A2+B3+2O4. It is probable that Ga occurs as Ga3+ and substitutes for other trivalent cations due to similar ionic radii (Ga3+: 0.62 Å, Cr3+: 0.62 Å, Fe3+: 0.55 Å, Al3+: 0.54 Å in octahedral coordination; Shannon, Reference Shannon1976). However, it is bound primarily in Zn–Fe–Al spinels (up to 1370 ppm Ga) (Table 2, Fig. 3). Interestingly, Ga was not substantially bound in Cr-bearing spinels (Table 2, Fig. 3). A recent study compared the Ga binding preferences in spinels from high-Al and high-Cr chromitites and found a similar phenomenon (Eliopoulos and Eliopoulos, Reference Eliopoulos and Eliopolous2019). Gallium concentrations were substantially higher in Al-rich spinels than in Cr-bearing spinels, because at magmatic conditions, Ga3+ shows a strong preference on tetrahedral sites where it can substitute for Al3+, which can occupy both octahedral and tetrahedral sites in contrast to Cr3+, which only occupies octahedral sites in the spinel structure (Eliopoulos and Eliopoulos, Reference Eliopoulos and Eliopolous2019). There is quite limited information about Ga content in phases produced from pyrometallurgy in the available literature. Avarmaa et al. (Reference Avarmaa, Yliaho and Taskinen2018) studied Ga distribution during the secondary copper smelting and found, in agreement with our results, that Ga dissolves 3–4 times more in the spinel phase than in the silicate slag. Natural slags formed by fusion and recrystallisation during the burning of coal seams described by Kruszewski (Reference Kruszewski and Stracher2018) represent another analogy. Interestingly, Ga was not substantially partitioned into spinel phases in these materials; only one microprobe analysis indicated 222 ppm Ga in magnesioferrite, MgFe2O4 (Kruszewski, Reference Kruszewski and Stracher2018). Whereas our LA-ICP-MS results indicate that Ga contents in melilite are relatively low (<74 ppm) (Table 5), Kruszewski (Reference Kruszewski and Stracher2018) reported 98–572 ppm Ga melilite-group phases from slags originating from coal burning. Other Ga-bearing phases were also found in these peculiar materials: chlormayenite, Ca12Al14O32[□4Cl2] (up to 3.17 wt.% Ga), srebrodolskite, Ca2Fe3+2O5 (up to 772 ppm Ga), anorthite, CaAl2Si2O8 (up to 301 ppm Ga), hematite, Fe2O3 (up to 210 ppm Ga), indialite, Mg2Al2Si5O18 (up to 220 ppm Ga) and ye'elimite, Ca4Al6(SO4)O12 (up to 580 ppm Ga) (Kruszewski, Reference Kruszewski and Stracher2018).
Germanium is found mainly in glass (up to 470 ppm), although some concentrations were also detected in olivine-group phases (EPMA) and melilite (LA-ICP-MS) (Tables 3–5). Saini-Eidukat et al. (Reference Saini-Eidukat, Melcher, Göttlicher and Steininger2016) demonstrated using the X-ray absorption fine structure (XAFS) spectroscopy that Ge in Ge-bearing willemite (Zn2SiO4) is present as Ge4+, is four-fold coordinated with oxygen and substitutes for Si4+ due to similarities with the ionic radius (Ge: 0.39 Å and Si: 0.26 Å according to Shannon, Reference Shannon1976). We assume that the same mechanism is responsible for Ge preferential binding in glass and silicates in the slag materials studied. Due to the known geochemical affinity of Ge towards Fe3+, it can also be hosted in secondary phases such as Fe(III) oxides and hydroxides (goethite, FeOOH or hematite, Fe2O3) in many deposits (Melcher and Buchholz, Reference Melcher, Buchholz and Gunn2014; Mondillo et al., Reference Mondillo, Herrington, Boyce, Wilkinson, Santoro and Rumsey2018a,Reference Mondillo, Arfè, Herrington, Boni, Wilkinson and Mormoneb). However, our slag samples are relatively unweathered, and practically no secondary Fe(III) oxides and hydroxides have been identified. In slag originating from coal burning, Kruszewski (Reference Kruszewski and Stracher2018) reported a Ge-bearing cuspidine (Ca4Si2O7(F,OH)2; up to 941 ppm Ge), but nothing similar was observed in our samples. However, Kruszewski (Reference Kruszewski and Stracher2018) also mentions that some melilites from these slag-like materials can contain up to 275 ppm Ge, which is comparable to our results (cf. Table 5).
The results obtained have important implications for the potential hydrometallurgical recovery of Ga and Ge from slags. Given that Ga is mostly found in spinel crystals <10 μm and Ge is concentrated in the glass matrix, forming a major part of the granulated slags volumetrically, ultrafine milling is needed to liberate Ga- and Ge-hosting phases for interaction with the leaching solution. Chirkst et al. (Reference Chirkst, Chistyakov and Cheremisina2008), in their first attempt to recover Ge from Tsumeb slags, milled the materials to <70 μm, which seems to be insufficient for the parallel liberation of Ga-bearing spinels from the samples reported here. More experimental work is needed to understand the optimum conditions for comminution of the slags (cf. Ettler et al., Reference Ettler, Jarošíková, Mihaljevič, Kříbek, Nyambe, Kamona and Mapani2020 and references therein), extraction, and recovery of Ga and Ge from these materials.
Conclusions
Old metallurgical slags from Tsumeb, Namibia are particularly rich in Ga (135–156 ppm) and Ge (128–441 ppm). A combination of PXRD, FEG-SEM-EPMA and LA-ICP-MS indicated that Ga and Ge have a different deportment in the slag phases. Gallium was hosted predominantly in Zn–Fe–Al spinels forming euhedral crystals <10 μm in size, which are enclosed in the silicate matrix (concentrations up to 1370 ppm Ga). In contrast, Ge was bound mostly in the silicate glass and, to a lesser extent, in other silicates (melilite and olivine-group phases). Germanium concentrations in the glass, being the dominant phase in granulated slags, vary in the range of 113–470 ppm. The results indicate that ultrafine milling is needed to liberate Ga- and Ge-hosting phases before potential extraction and recovery of Ga and Ge from the slag material. In addition, the highly variable concentrations observed indicate strict grade control is needed to optimise Ga and Ge recovery.
Acknowledgements
This study was supported by the Czech Science Foundation project (GAČR 19-18513S) and received institutional funding from the Center for Geosphere Dynamics (UNCE/SCI/006). We thank numerous colleagues for their support in the laboratories: Věra Vonásková, Lenka Jílková and Petr Drahota (PXRD). Hans Nolte, Pierre Reinecke and Mbabuu Trust of Dundee Precious Metals Tsumeb (DPMT) are thanked for providing us the old slag samples from Tsumeb. The thorough reviews of the Associate Editor and two anonymous reviewers helped to improve the original version of the manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2021.83











