Iodine, a trace element that is necessary for human health, is an important component of thyroid hormone (TH) synthesis(Reference Ma, Venn and Manning1). TH is essential for growth and development, energy metabolism and neuronal development(Reference Taylor, Albrecht and Scholz2). Both iodine deficiency and iodine excess can cause thyroid dysfunction(Reference Zimmermann and Boelaert3). Salt iodisation is a public health intervention intended to control iodine deficiency, but regular salt iodisation policies need to be monitored to prevent iodine deficiency and iodine excess(Reference Zimmermann, Jooste and Pandav4,Reference Zimmermann5) . Iodine deficiency has historically been a problem in China, and in 1996, China began following the Universal Salt Iodization regulations. Since then, Chinese residents have experienced two periods of iodine excess: a 6-year period from 1997 to 2002 and a 10-year period from 2003 to 2012. In 2006, our research group reported the results of a 5-year prospective follow-up study of three regions with differing iodine nutritional status. We found that thyroid-stimulating hormone (TSH) levels increased with an increase in iodine intake(Reference Teng, Shan and Teng6). In our latest national cross-sectional study, we confirmed that serum TSH concentrations significantly increased with increases in iodine intake(Reference Zhao, Teng and Shi7).
TSH is synthesised and secreted by pituitary TSH cells (basophilic cells). TSH production and secretion are mainly regulated by thyrotropin-releasing hormone (TRH) which is synthesised in the hypothalamic and is negatively regulated by TH(Reference Joseph-Bravo, Jaimes-Hoy and Uribe8). Stimulus factors induce the release of TRH–TSH and may co-ordinately increase TRH transcription. Each step of the HPT axis is controlled my multiple factors and inputs.
Type 2 deiodinase (Dio2) is a member of the iodothyroxine deiodinase family and is a Se-containing protease. Dio2 catalyses the deiodination of exocyclic iodine, which converts thyroxine (T4) into the more active triiodothyronine (T3) and alters the availability of local T3(Reference Bianco9). The production of T3 catalysed by Dio2 occurs inside the cell and increases TH signals, while a decrease in Dio2 activity or reduction of Dio2 gene expression causes local hypothyroidism(Reference Werneck de Castro, Fonseca and Ueta10). The biological activity of TH is regulated by Dio2(Reference Bianco, Dumitrescu and Gereben11). The outer ring deiodination of Dio2 is mainly accomplished by ubiquitination-mediated protein degradation(Reference Gereben, McAninch and Ribeiro12,Reference Bianco and Kim13) . Protein ubiquitination is a post-translational modification that directs a protein to a controlled degradation pathway(Reference Swatek and Komander14). Regulation by ubiquitination not only mediates protein degradation but also temporarily inactivates enzyme activity by altering its conformation(Reference Sagar, Gereben and Callebaut15).
Previous studies have shown that iodine excess impairs the pituitary–thyroid axis in rodents(Reference Li and Carayanniotis16,Reference Li, Jiang and Shan17) , but the mechanism of TSH elevation caused by iodine excess is still not very clear. Iodine deficiency is a global problem, the current research on iodine nutritional status is mainly concentrated in the pituitary and thyroid gland(Reference Calil-Silveira, Serrano-Nascimento and Laconca18,Reference Zhang, Jiang and Han19) , but the effects of iodine excess in the hypothalamus have not been elucidated(Reference Basalaeva20,Reference Aceves, Anguiano and Delgado21) ; both iodine excess and iodine deficiency can cause TH changes, and the regulation of TH is co-regulated by the hypothalamus pituitary thyroid axis, so the effect of iodine on hypothalamus deserves more attention and research. Based on the results of epidemiological studies on the effects of excess iodine on TH and the role of Dio2 in the hypothalamic–pituitary–thyroid axis (HPT), in the present study, we used a rat model to investigate the underlying mechanisms by which chronic excess iodine intake results in elevated TSH and TRH secretion in the hypothalamus.
Methods and materials
Animals, diet and sample collection
A total of 140 male Wistar rats (Vital River Laboratories, Beijing, China), weighing 120 (sd 20) g and aged about 4 weeks, were used in this study, due to their low incidence of autoimmune disorders. The rats were kept in temperature-controlled rooms with a 12 h light–12 h dark cycle schedule in the SPF Laboratory Animal Center at the China Medical University (Shenyang, China). The rats were randomised into five groups, and each group was fed a different dose of iodine. Iodine doses were as follows: normal iodine (NI, the same iodine intake as regions with adequate iodine intake) (n 7), 3-fold (3HI) (n 7), 6-fold (6HI) (n 7), 10-fold (10HI) (n 7) and 50-fold higher iodine intake (50HI) (n 7). Based on our previous epidemiological studies, the 3HI and 6HI high iodine groups received equivalent dietary iodine comparable to iodine statuses of ‘more than adequate’ and ‘excessive’, respectively(Reference Teng, Shan and Teng6). The dosages for the 10HI and 50HI groups were established by identifying different tolerance levels for iodine excess between rat and human subjects(Reference Zhang, Jiang and Han19). All rats were fed normal chow (AIN-93). The average iodine content of AIN-93 and tap water in Shenyang is 200 µg/kg and 5 µg/l, respectively. The average amount of food and water intake for each rat was 20 g/d and 30 ml/d, respectively.
Rats were kept in metabolic cages for 2 d prior to euthanasia. After treatment, the rats were anaesthetised and then euthanised at 4, 8, 12 and 24 weeks after iodine administration by decapitation. Brains were collected on dry ice and stored at −80°C. Tissue sections were used for immunohistochemistry and immunofluorescence, and RNA was extracted from hypothalamus tissue. Blood samples were collected and centrifuged at 600 g at 5°C for 10 min to evaluate serum TSH, T4, T3 and TRH. All animal experiments were approved by the Animal Ethics Committee of China Medical University (No. KT2021223).
Measurement of thyroid hormone and thyrotropin-releasing hormone
Serum TSH, TT4 and TT3 were measured using a chemiluminescent method, according to the manufacturer’s instructions (Diagnostic Products Corporation). The between-run CV and within-run CV were < 5 %. The detection range for TT4 and TT3 concentrations was 66–181 nmol/l and 1·3–3·1 nmol/l, respectively. The analytical sensitivity for TSH was 0·005 mµ/l. The between-run CV and within-run CV were < 10 %. Serum TRH levels were assessed by ELISA, TRH ELISA kits (Cloud-Clone Corp.). Briefly, the prepared reagents, samples and standards were added to a 96-well plate and allowed to react at 37°C for 30 min. Then, the plate was washed five times, the enzyme-labelled reagent was added and the assay plate was incubated at 37°C for 30 min. Next, the plate was washed another five times, and the colour development solution was added and colour allowed to develop at 37°C for 10 min. Finally, the stop solution was added, and the OD value at 450 nm wavelength was measured within 15 min to calculate concentration. The TRH kit detection range was reported at 6·17–500 pg/ml.
Real-time quantitative RT-PCR
To collect hypothalamus tissue, the brain area was separated and brain tissue was acclimated to −10°C. The hypothalamic tissue was determined by orientation with the centre point between the grey nodule and the optic chiasm as the centre: the anterior boundary is the anterior edge of the optic chiasm; the posterior boundary is the posterior edge of the papillary body; the temporal sulcus on both sides, about 4 mm wide; the depth is about 2 mm, and the length is about 4 mm(Reference Müller, Kröger and Jöhren22). Total RNA was extracted from rat hypothalamus using TRIzol Reagent (Invitrogen) and then reverse-transcribed into cDNA using a PrimeScript RT Master Mix (TaKaRa). Reactions were performed using SYBR Premix Ex Taq (TaKaRa) using a LightCycler 480 instrument (Roche Molecular Biochemicals). Glyceraldehyde 3-phosphate dehydrogenase was used as the internal reference gene. The forward and reverse primer sequences for Dio2 and glyceraldehyde 3-phosphate dehydrogenase were designed and synthesised by TaKaRa or Sangon Biotech (Shanghai, China) (Table 1).
Table 1. Primer sequences

Dio2, Type 2 deiodinase; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase.
Tissue immunohistochemistry
Brain tissues were placed in a cryomold, covered with ornithine carbamyl transferase (Tissue-Tek) and snap-frozen on dry ice. Serial 10-mm thick coronal sections were cut on a cryostat (Leica CM3050 S; Leica Microsystems GmbH) and adhered to SuperFrost glass slides (Haimen). The tissue sections were stored at −80°C until immunohistochemistry was performed. Sections were fixed by immersion in 4 % paraformaldehyde for 1 h at room temperature, and endogenous peroxidase was quenched with 3 % H2O2 for 10 min. All sections were washed with 0·01 mm PBS (Zhongshan Jinqiao) (pH 7·2–7·4) and incubated overnight at 4°C with primary antibody (monocarboxylate transporter 8 (MCT8), 1:200, ab214446; Abcam). The binding of antibodies was detected using a two-step immunohistochemistry detection reagent (Zhongshan Jinqiao). The sections were stained with haematoxylin (Sigma, H9627) and eosin (Sigma, E4009-5G) and then examined by using a light microscope (BX51/BX52; Olympus). The stained cells in brain sections were quantified using Image J software (National Institutes of Health).
Tissue immunofluorescence
Brain tissues were analysed by immunofluorescence microscopy. Brain tissue frozen sections were dried at room temperature, baked in an oven at 37°C for 10–20 min and placed in 4 % paraformaldehyde for fixation. Brain tissue frozen sections were placed in a repair box filled with EDTA antigen repair buffer (pH 9·0) for antigen retrieval in a microwave oven. Tissues were covered uniformly with 3 % BSA and were blocked for 30 min at room temperature. Tissue sections were incubated in primary antibody overnight at 4°C, incubated with secondary antibody at room temperature for 2 h and then were treated with 4',6-diamidino-2-phenylindole counterstain. The primary antibody used was goat anti-Dio2 (1:200; ab77481). The secondary antibody used was anti-goat (1:300; Servicebio; GB21404). The stained cells in brain sections were quantified using Image J software (National Institutes of Health).
Measurement of type 2 deiodinase activity
Dio2 activity was measured in hypothalamus tissue, as described previously(Reference Curcio-Morelli, Gereben and Zavacki23). First, T4 was labelled. Briefly, 0·5 μg T4 was placed into 10 μl 0·01 N phosphate buffer, and 125 Ina, 1 Mci and 50 μg chloramine-T were added at the same time and reacted at 4°C for 30 s. The markers were placed on a SEPHADEX G-25 column (Sigma, G2580) and allowed to pass through the column and then were washed off with 0·01 N phosphate buffer at a rate of 0·2 ml/min. The first peak represented the 125I-T4. The labelling working solution was 0·01 NPB (0·05 % BSA) dilution label, with a concentration of 40 000 CPM/100 μl. Marking rate: 87 %, radiochemical purity: 95 %. In unit time, 125I-T4 is used as the substrate. Dio2 activity converts the 125I-T4 in the homogenate into T3 and releases 125I into the supernatant. The supernatant is separated using a separating agent, and the CPM is measured in the precipitate. Finally, the CPM data are used to calculate Dio2 activity, according to the established formula(Reference Kwakkel, van Beeren and Ackermans24).
Statistical analysis
We estimated that a sample size of five rats per group was required in order to have more than 90 % probability to detect a significant between-group difference. The power to detect this difference was calculated based on two-way ANOVA with a significance level of 5 %. Sample size estimation was performed using G*Power 3.1.9.7 programme. The effect size for the power calculation was 0·4.
Data were analysed by one-way ANOVA using SPSS 20 software and are expressed as mean and standard deviation. Bonferroni post hoc test and R-E-G-W-Q (Q) were used to assess the statistically significant differences between more than three treatment groups. A P-value of P < 0·05 was considered to indicate a significant difference.
Results
Effects of chronic iodine excess on T3, T4 and thyroid-stimulating hormone serum concentrations
Compared with NI, the levels of T3 and T4 were not significantly changed (Fig. 1(a) and (b)). TSH levels were increased in the iodine intake groups. There was a non-significant increase in TSH in the high iodine intake groups compared with the control group at 4 weeks. Compared with the NI group, the 10HI and 50HI groups had significantly increased TSH at 8 weeks (Fig. 1(c)). Compared with the NI group, the 6HI, 10HI and 50HI groups had significantly increased TSH at 12 and 24 weeks (Fig. 1(c)).
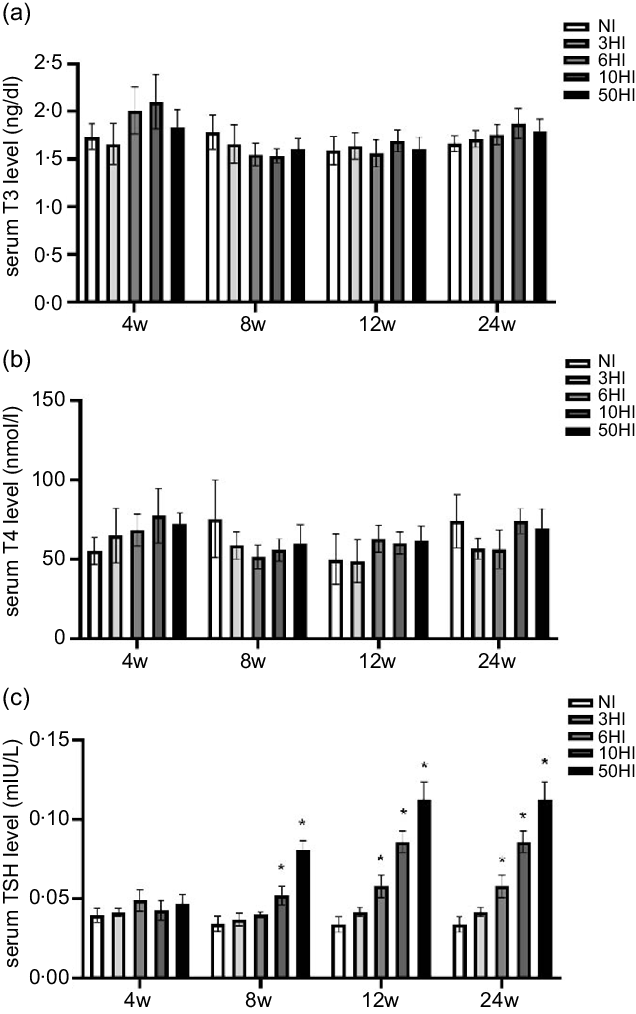
Fig. 1. Serum thyroid-stimulating hormone (TSH) levels increased in rats exposed to chronic iodine excess; no significant differences were seen in triiodothyronine (T3) and thyroxine (T4) levels. Data are expressed as mean values and standard deviation. Serum T3 levels were evaluated in rats chronically exposed to different concentrations of iodine excess for 4 weeks, 8 weeks, 12 weeks and 24 weeks (a). Serum T4 levels were evaluated in rats chronically exposed to different concentrations of iodine excess for 4 weeks, 8 weeks, 12 weeks and 24 weeks (b) (n 5 rats per group). Serum TSH levels were evaluated in rats chronically exposed to different concentrations of iodine excess for 4 weeks, 8 weeks, 12 weeks and 24 weeks (C). *P < 0·05.
Effects of chronic iodine excess on thyrotropin-releasing hormone serum concentrations
We compared differences in serum TRH levels between the four iodine intervention groups and the normal control group at four intervention periods. In response to an increase in iodine, the serum TRH levels in Wistar rats exhibited a declining trend at various time points. The serum TRH levels in each iodine intervention group were significantly different from those in the normal control group. At 4 and 8 weeks, the serum TRH levels of rats in the intervention groups showed decreasing trend compared with the control group and there were significant differences in the TRH levels in the 10HI and 50HI iodine groups at 4 and 8 weeks (Fig. 2). The serum TRH levels of rats in the iodine intervention groups at 12 weeks were decreased compared with the control group, and the difference between the 6-fold iodine treatment group was significant (Fig. 2). At 24 weeks, there were significant differences in TRH levels in the 3HI group, 6HI group and 50HI group compared with the normal control group (Fig. 2).
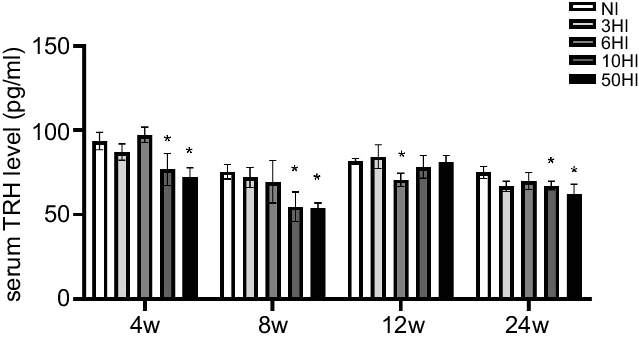
Fig. 2. Serum thyrotropin-releasing hormone (TRH) increased in rats exposed to chronic iodine excess. Data are expressed as mean values and standard deviation. Serum TRH levels were evaluated in rats chronically exposed to different concentrations of iodine excess for 4 weeks, 8 weeks, 12 weeks and 24 weeks (n 5 rats per group). *P < 0·05.
High iodine intake increases type 2 deiodinase protein expression and type 2 deiodinase activity, but not type 2 deiodinase mRNA level, in hypothalamus
Examination of brain tissue with immunofluorescence showed that increased iodine increased Dio2 expression levels in Wistar rats at all time points. The expression of Dio2 in the hypothalamus showed an increasing trend in each experimental group at 4 weeks, and there were significant differences between the 3HI, 6HI, 10HI and 50HI groups compared with the normal control group (Fig. 3(b)). The expression of Dio2 in the hypothalamus exhibited an increasing trend in each experimental group, and there were significant differences between the 3HI, 6HI, 10HI and 50HI groups compared with the NI group (Fig. 3(b)). At 12 weeks of iodine intervention, Dio2 expression in the hypothalamus showed an increasing trend in each experimental group and there were significant differences between the 3HI, 6HI and 50HI groups compared with the NI group (Fig. 3(b)). At 24 weeks of iodine intervention, Dio2 expression in the hypothalamus showed an increasing trend in each experimental group and Dio2 expression in the 50HI group was significantly different compared with the NI group (Fig. 3(b)). Representative immunofluorescence images of hypothalamic Dio2 from each iodine intervention group at 4 weeks, 8 weeks, 12 weeks and 24 weeks are shown (Fig. 3(a)). Compared with the NI group, the expression of Dio2 mRNA was unchanged in the 3NI, 6NI, 10NI and 50NI intervention groups at 4 weeks, 8 weeks, 12 weeks and 24 weeks (Fig. 3(c)).
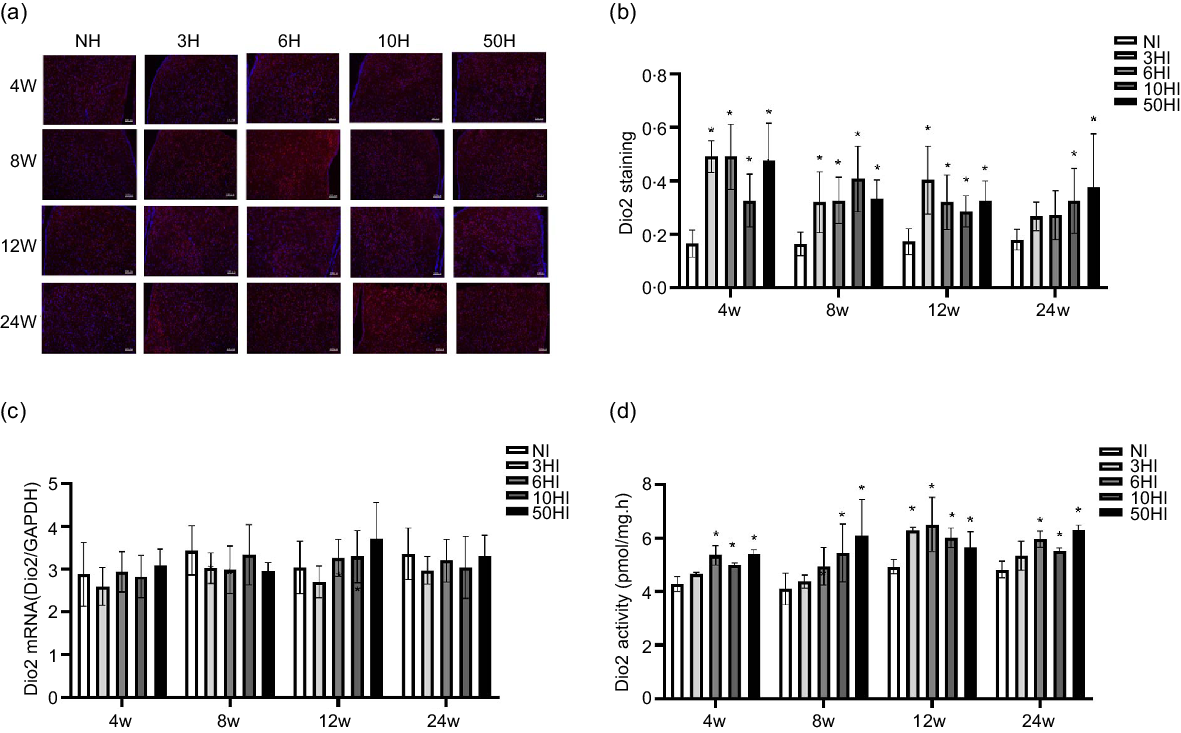
Fig. 3. High iodine intake increased type 2 deiodinase (Dio2) protein expression and activity, but not Dio2 mRNA levels in rat hypothalamus. Data are expressed as mean values and standard deviation. Dio2 protein expression was evaluated in rats chronically exposed to different concentrations of iodine excess for 4 weeks, 8 weeks, 12 weeks and 24 weeks (b). Representative immunofluorescence pictures of hypothalamus Dio2 from rats chronically exposed to different concentrations of iodine excess at 4 weeks, 8 weeks, 12 weeks and 24 weeks (a); scale bar: 100 μm; Quantification of Dio2 positive cells in hypothalamus (n 5 rats per group). Hypothalamus Dio2 mRNA levels in rats exposed to different concentrations of iodine excess at different time points (c) (n 5 rats per group). Dio2 activity of rats chronically exposed to different concentrations of iodine excess for 4 weeks, 8 weeks, 12 weeks and 24 weeks (D) (n 6 rats per group). *P < 0·05.
In order to determine the effects of iodine intake on Dio2 activity, we evaluated hypothalamic Dio2 activity. The Dio2 activity in the hypothalamus of Wistar rats increased in each experimental excess iodine group at 4 weeks, 8 weeks, 12 weeks and 24 weeks (Fig. 3(d)).
High iodine intake increases monocarboxylate transporter 8 protein expression in the hypothalamus
To explore possible mechanisms of TRH reduction due to excess iodine intake, we examined the expression of MCT8 in the hypothalamus. Examination of brain tissue by immunohistochemistry showed that increasing iodine intake resulted in an increase in MCT8 expression levels in Wistar rats at all time points. Compared with the control group, the expression of MCT8 was increased in the 10NI and 50NI intervention groups at 4 weeks (Fig. 4(b)) and 12 weeks (Fig. 4(b)). Compared with the NI group, the expression of MCT8 was increased in the 6NI, 10NI and 50NI intervention groups at 8 weeks (Fig. 4(b)). Compared with the control group, the expression of MCT8 was increased in the 3NI, 6NI, 10NI and 50HI intervention groups at 8 weeks and 24 weeks (Fig. 4(b)). Representative immunohistochemistry images of hypothalamus MCT8 in all intervention groups at different time points are shown (Fig. 4(a)).
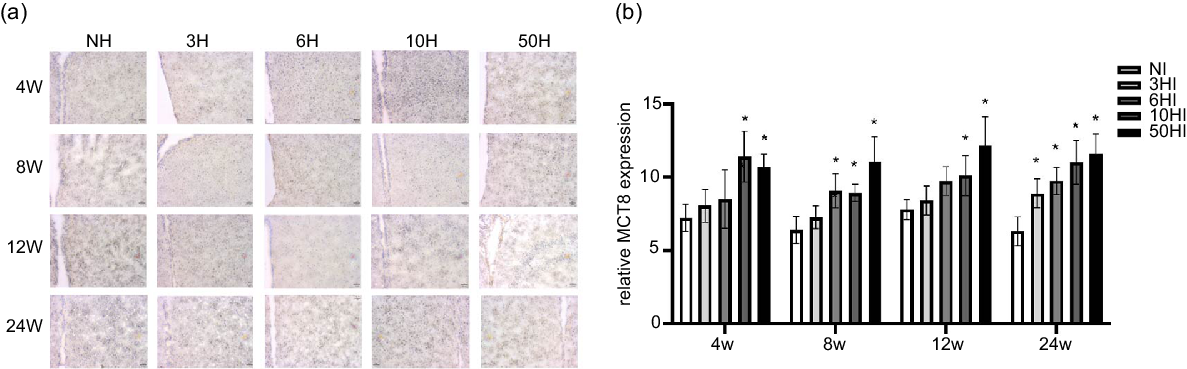
Fig. 4. High iodine intake increased monocarboxylate transporter 8 (MCT8) protein expression in hypothalamus. Data are expressed as mean values and standard deviation. MCT8 protein expression of rats chronically exposed to different concentrations of iodine excess for 4 weeks, 8 weeks, 12 weeks and 24 weeks (b). Representative immunohistochemistry images of hypothalamus MCT8 from rats exposed to different concentrations of iodine excess at different time points (a); scale bar: 51 μm; Quantification of MCT8 positive cells in hypothalamus (n 5 rats per group). *P < 0·05.
Discussion
The current study aimed to study the regulatory role of hypothalamus Dio2 in a model of TSH elevation induced by chronic iodine excess in Wistar rats. Long-term chronic iodine excess and excessive iodine intake can lead to increased TSH levels and increased incidence of subclinical hypothyroidism(Reference Teng, Shan and Teng6). The 2011 National Iodine Deficiency Disease Monitoring Center survey also demonstrated a significant positive correlation between iodine intake and serum TSH levels. The prevalence of clinical hypothyroidism and subclinical hypothyroidism in areas with excessive iodine is significantly higher than in areas with sufficient iodine intake(Reference Du, Gao and Meng25). Chronic iodine overdose in our Wistar rat model induced an increase of serum TSH and did not affect the levels of T3 and T4 (Fig. 1(a)–(c)). Using this model, we had previously reported that levels of pituitary Dio2 protein decreased, while the mRNA content remained unchanged(Reference Zhang, Jiang and Han19). TSH is secreted in the pituitary gland, it establishes a negative feedback regulation with T3 and T4 levels, which requires the coordinated participation of the hypothalamus, pituitary and thyroid gland, and Dio2 plays an important role in converting T4 to T3. Our data suggest that changes in Dio2 expression and activity are important in mediating the increase of TSH caused by iodine excess. In the central nervous system, Dio2 is not only expressed in the pituitary but also abundantly expressed in hypothalamic astrocytes(Reference Guadaño-Ferraz, Obregn and St Germain26,Reference Tu, Kim and Salvatore27) . In order to determine the relative contribution of the Dio2 pathway in the HPT feedback loop, two studies developed models of pituitary and astrocyte tissue-specific Dio2 knockout mice (Pit-Dio2 KO mice and astro-Dio2 KO mice, respectively)(Reference Werneck de Castro, Fonseca and Ueta10,Reference Fonseca, Correa-Medina and Campos28) . These studies demonstrated that the serum T3 levels of Pit-Dio2 KO mice were normal, the thyroid function was normal and that while serum TSH levels increased about 3-fold, the biological activity of TSH was decreased by 40 %(Reference Fonseca, Correa-Medina and Campos28). Although the expression of Dio2 in the brain is almost completely lost in astro-Dio2 KO mice, the content of monocyte Dio2 in astro-Dio2 KO mice is sufficient to maintain the T4-dependent negative feedback loop and maintain thyroid content(Reference Fonseca, Correa-Medina and Campos28). These findings are consistent with the hypothesis that the hypothalamus and pituitary Dio2 co-ordinately regulate the expression of TSH and demonstrate that the Dio2 pathway in the hypothalamus and pituitary contributes to the feedback loop.
Dio2 is expressed in many tissues and organs, and the Dio2 pathway plays an important role in regulating local T3 content(Reference Gereben, Zeöld and Dentice29). In the brain and in brown adipose tissue, TH signal transduction depends on plasma T3 and on T3 produced locally through the Dio2 pathway(Reference Freitas, Gereben and Castillo30). T3, which depends on Dio2, is present in astrocytes and regulates the expression of T3 response genes in nearby neurons through a paracrine mechanism(Reference Freitas, Gereben and Castillo30). T3 levels can be adjusted according to the availability of T4(Reference Gereben, Goncalves and Harney31). For example, when serum T4 levels are low, or in conditions of iodine deficiency or hypothyroidism, the accumulation of Dio2 in cells will increase the conversion of T4 to T3. In contrast, because T4 can inactivate Dio2, when T4 levels are high, the TH signal may be weakened. Our results show that the levels of Dio2 protein and Dio2 activity were increased in the hypothalamus of rats under chronic iodine excess, while Dio2 mRNA levels were unchanged (Fig. 3). In our previous study, we found that excessive iodine can lead to decreased D2 expression in the pituitary(Reference Li, Jiang and Shan17,Reference Zhang, Jiang and Han19) . From that study, we found that excessive iodine can also lead to increased D2 expression and activity in hypothalamic tissue. In agreement with our present results, it has been reported that Dio2 ubiquitination is not universal in all tissues and Dio2 ubiquitination sensitivity is relatively low in hypothalamic tissue, which is a key factor for TRH/TSH negative feedback regulation(Reference Werneck de Castro, Fonseca and Ueta10).
We evaluated serum TSH–TRH levels and found that serum TRH showed a downward trend as iodine intake increased (Fig. 2). It is generally accepted that TRH neurons located in the pituitary stimulating region of the paraventricular nuclei are the core regulatory regions of the HPT axis(Reference Chiamolera and Wondisford32). By participating in the negative feedback regulation of the HPT axis, T4 can affect the availability of T3 in cells through deiodinated enzymatic deiodination of central nervous system cells and can participate in the production of TRH neurons in paraventricular nuclei and in the production of TSH in the pituitary. Upon uptake into neurons through the TH transporter, T4 can supplement and participate in the regulating target genes.
TH cannot directly enter the cell, and the transport of T3 in elongated cells depends on regulation by MCT8, which plays a significant role in TH signalling. MCT8 is a TH transport protein, highly expressed in liver, kidney, heart and brain and plays an important role in the negative feedback regulation of hypothalamus TH(Reference Friesema, Visser and Borgers33). MCT8 is the most characteristic TH transporter in the brain. MCT8 recognises different TH and is expressed in various tissues and cell types, including neurons, endothelial cells, glial cells, astrocytes and monocytes(Reference Visser, Friesema and Visser34,Reference Wirth, Schweizer and Köhrle35) . TH transporters are also of clinical importance. For instance, patients with MCT8 mutations have severe X-linked psychomotor disorders and TH level disorders, especially high serum T3 levels, referred to as Allan–Herndon–Dudley syndrome(Reference Friesema, Visser and Visser36). In vitro studies have also shown that MCT8 can increase the metabolism of TH in cells. Research by Alkemade et al. demonstrated that, at the blood–hypothalamus border, MCT8 protein is present in neurons and astrocytes in the funnel-shaped nuclei of the paraventricular nucleus of the hypothalamus(Reference Alkemade, Friesema and Unmehopa37). High expression of MCT8 was also observed in monocytes in the middle layer of the third ventricle. MCT8 expressing monocytes are in close contact with CSF, the hypothalamus, and the median bulge and are involved in providing T3 to the central nervous system(Reference Lechan and Fekete38). Our study demonstrated that iodine intake can lead to increased MCT8 expression in the hypothalamus (Fig. 4). This result explains the reduction in TRH caused by iodine intervention. Excess iodine increases the expression of Dio2 in the hypothalamus, increasing the conversion of T4 to T3. T3 is transported through the MCT8 transporter in elongated cells into the hypothalamic paraventricular nucleus, the pituitary-promoting region and the synthetic TRH cell region and inhibits the production of TRH, resulting in a decrease in serum TRH content. Although the Dio2 expression in the pituitary is reduced, the conversion of T3 and T4 is also reduced, resulting in a weakening of T3's inhibitory effect on TSH and, ultimately, leading to an increase in TSH. However, in the hypothalamus, we found that excessive iodine increased the expression of Dio2 in elongated cells and increased the conversion of T3 and T4. The inhibitory effect of T3 on TRH increased, and TRH levels decreased. Therefore, the increase in pituitary TSH is not entirely due to the regulation of hypothalamic TRH (Fig. 5).
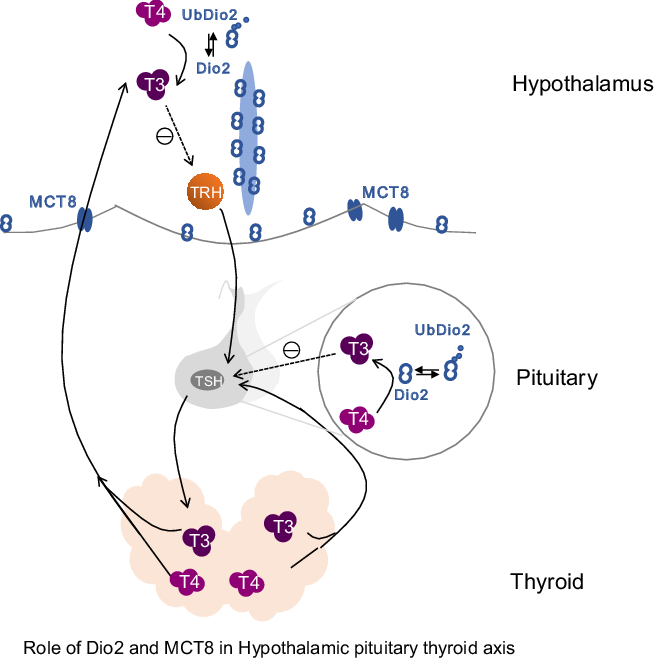
Fig. 5. Role of type 2 deiodinase (Dio2) and monocarboxylate transporter 8 (MCT8) in the hypothalamic–pituitary–thyroid axis. Dio2 is mainly expressed in the hypothalamus and pituitary in the central nervous system. This diagram includes the hypothalamic–pituitary–thyroid axis and highlights the role of Dio2 and MCT8 within the axis. The main role of Dio2 is to convert thyroxine (T4) into biologically active triiodothyronine (T3) by deiodinating the outer ring. During the conversion of T4 to T3, Dio2 works through ubiquitination and is converted into UbDio2. Thyroid hormone cannot directly enter the cell, and the transport of T3 in elongated cells depends on regulation by MCT8. Dio2 plays an important role in thyroid-stimulating hormone (TSH) negative feedback regulation.
Dio2 is the main deiodinase activated by TH, and its activity is regulated by complex mechanisms, including ubiquitination of the Dio2 protein(Reference Gereben, Goncalves and Harney31,Reference Sagar, Gereben and Callebaut39) . Ubiquitination modification is a post-translational modification and leads to a tightly regulated protein degradation pathway. Selective proteolysis is driven by the ubiquitin-proteasome system. The ubiquitin-proteasome pathway is a process in which the substrate protein is ubiquitinated and then recognised and degraded by the proteasome, including the dissociation of deubiquitinating enzymes(Reference Vembar and Brodsky40). The reverse regulation of ubiquitin is a primary regulator and terminator of protein function. Ubiquitination is a reversible, cyclical process involving multiple enzymes(Reference Vembar and Brodsky40,Reference Scheffner, Nuber and Huibregtse41) . The regulation of Dio2 by ubiquitination not only mediates its degradation but also temporarily inactivates the enzyme by changing its conformation. When Dio2 binds to its substrate T4, Dio2 is inactivated by ubiquitination followed by a conformational change. The ubiquitination of Dio2 is proportional to the concentration of T4, thereby forming a negative feedback loop in the regulation of T3 conversion. Under physiological conditions, ubiquitination and deubiquitination maintain a dynamic balance and Dio2 and inactive Dio2 (UbDio2) are in an equilibrium. Based on the increase in hypothalamus Dio2 under the iodine overdose intervention, we measured Dio2 activity and explored the role of ubiquitination in response to iodine intervention. We found that excess iodine not only increased the expression of Dio2 in the hypothalamus but also increased its activity, compared with the control group. This result shows that excess iodine reduces the ubiquitination of Dio2 in the hypothalamus. We conclude that this is the result of the regulation of the HPT. This result is also consistent with reports that Dio2 ubiquitination is not identical in all tissues and suggests that an inability of the hypothalamus to drive Dio2 ubiquitination is a pivotal element in the TRH/TSH negative feedback regulation. This allows circulating TH to function as a regulatory signal for hypophysiotropic TRH. The results of our study may provide significant guidance for human iodine intake.
In this study, we report on the effects of iodine overdose on hypothalamus tissue for the first time. Combined with previous studies, the study of iodine overdose on the HPT provides a more comprehensive view of the impact of iodine excess. However, there are some limitations in the current study; as the regulation of the HPT is a very complicated process, whether other factors are involved in addition to Dio2 remains to be explored. The study of Dio2 ubiquitination in the hypothalamus also requires more in-depth research to identify the specific mechanism of ubiquitination.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81670719) and Education Department Project of Liaoning Province (No. JCZR2020005).
Y. Q. J., W. P. T. and Z. Y. S. conceived of and designed the study; Y. S. and X. D. conducted research. Y. S. conducted the statistical analysis. All authors contributed to the acquisition, analysis and/or interpretation of data. Y. Q. J. and Y. S. drafted the manuscript. All authors revised the report and approved the final version before submission. No competing financial interests exist.
The authors declare that they have no conflicts of interest.








