It is now well established that the rate of gastric emptying and small-intestinal carbohydrate absorption are major determinants of postprandial glycaemia in healthy subjects(Reference Horowitz, Edelbroek and Wishart1) and patients with type 1(Reference Ishii, Nakamura and Kasai2) and type 2 diabetes(Reference Jones, Horowitz and Carney3), such that gastric emptying accounts for at least 35 % of the variance in the initial rise in blood glucose, as well as influencing the incretin hormone (glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide) responses, after oral glucose(Reference Horowitz, Edelbroek and Wishart1, Reference Jones, Horowitz and Carney3, Reference Nauck, Niedereichholz and Ettler4). Even relatively minor variations in the rate of gastric emptying can have a marked impact on the glycaemic response to carbohydrate(Reference O'Donovan, Doran and Feinle-Bisset5), particularly as the relationship of glycaemia with small-intestinal carbohydrate delivery is non-linear(Reference Pilichiewicz, Chaikomin and Brennan6).
Cyclodextrins are cyclic oligosaccharides composed of six to eight glucose monomers(Reference Raben, Andersen and Karberg7) that inhibit pancreatic amylase activity(Reference Buckley, Thorp and Murphy8), are poorly digested in the small intestine(Reference Flourie, Molis and Achour9) and inhibit the hydrolysis of complex carbohydrates(Reference Buckley, Thorp and Murphy8). α- and β-Cyclodextrins have been reported to reduce the postprandial glycaemic(Reference Raben, Andersen and Karberg7, Reference Buckley, Thorp and Murphy8), insulinaemic(Reference Raben, Andersen and Karberg7) and glucose-dependent insulinotropic polypeptide responses(Reference Raben, Andersen and Karberg7) to a starch meal. For example, in healthy young males, Raben et al. (Reference Raben, Andersen and Karberg7) reported that peak blood glucose and plasma insulin and glucose-dependent insulinotropic polypeptide concentrations were less when a potato starch meal was enriched with 2 % β-cyclodextrin, while plasma glucagon-like peptide-1 levels remained essentially unchanged. Subsequently, Buckley et al. (Reference Buckley, Thorp and Murphy8) reported, also in healthy subjects, that the area under the blood glucose curve after the consumption of white rice was reduced by approximately 50 % by 10 g of α-cyclodextrin. A limitation of these studies(Reference Raben, Andersen and Karberg7, Reference Buckley, Thorp and Murphy8) was that gastric emptying was not measured; hence, it remains to be determined whether the observed effects on postprandial glycaemia were related to the effects on gastric emptying and/or intestinal glucose absorption.
The aims of the present study were to determine the effects of α-cyclodextrin on gastric emptying of, and the glycaemic response to, an oral sucrose load in healthy older subjects. By selecting sucrose, a disaccharide hydrolysed by the intestine, rather than pancreatic enzymes, any effect(s) of α-cyclodextrin would not reflect the inhibition of amylase. The broad hypothesis was that α-cyclodextrin would attenuate the glycaemic response by slowing both gastric emptying and intestinal carbohydrate absorption.
Materials and methods
Ethical approval and informed consent
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by the Research Ethics Committee of the Royal Adelaide Hospital (Adelaide, SA, Australia). Written informed consent was obtained from all subjects.
Subjects
In the present study, ten healthy older subjects (seven males and three females), with a median age of 70 (range 68–76) years and BMI of 26·9 (range 23·2–32·4) kg/m2 recruited by advertisement, were studied. The number of subjects studied was based on data from a previous study, which detected a reduction in the glycaemic response to a rice meal after α-cyclodextrin when compared with the control(Reference Buckley, Thorp and Murphy8). All subjects were non-smokers, and none had a history of gastrointestinal disease or surgery, diabetes, significant respiratory, renal, hepatic or cardiac disease, chronic alcohol abuse or epilepsy, nor was taking medication known to influence gastrointestinal function.
Protocol
Each subject was studied on two occasions, on which they attended the Department of Nuclear Medicine, Positron Emission Tomography and Bone Densitometry at 08.30 hours following an overnight fast (10·5 h for solids and 8·5 h for liquids)(Reference Gentilcore, Bryant and Wishart10). A cannula was placed in a left antecubital vein for blood sampling, and subjects were seated with their back against a gamma camera. Each subject rested comfortably in the sitting position for about 30 min(Reference Gentilcore, Bryant and Wishart10). At t = − 2 min, subjects consumed a drink comprising 100 g sucrose dissolved in water (total volume of the drink 300 ml) and labelled with 20 MBq 99m Tc-sulphur colloid (Royal Adelaide Hospital Radiopharmacy, Adelaide, SA, Australia). On one of the days, 10 g α-cyclodextrin (Cavamax®; Wacker Fine Chemicals, Adrian, MI, USA) were added to the sucrose before being dissolved in water (total volume 300 ml). The two studies were separated by at least 7 d and performed in a double-blind, randomised order. Gastric emptying, blood glucose and serum insulin were measured. At t = 300 min, the intravenous cannula was removed, and subsequently, the subject was allowed to leave the laboratory. On both study days, subjects were given a light meal before leaving the laboratory.
Measurements
Gastric emptying and intragastric distribution
Subjects consumed the drink within 2 min, and the time of drink completion was considered to be t = 0 min. Radioisotopic data were acquired for 300 min (60 s frames for the first 60 min and 3 min frames thereafter)(Reference Gentilcore, Bryant and Wishart10). Data were corrected for subject movement, radionuclide decay and γ-ray attenuation(Reference Gentilcore, Bryant and Wishart10). Regions of interest were drawn around the total stomach, which was subsequently divided into the proximal and distal stomach regions, and gastric emptying curves (expressed as percentage retention over time) were derived. The amounts of the drink remaining in the total, proximal and distal stomach at 15 min intervals between t = 0 and 300 min were calculated. The 50 % gastric emptying time (T 50) was also determined(Reference Gentilcore, Bryant and Wishart10).
Blood glucose and serum insulin concentrations
Venous blood samples (approximately 7·5 ml) were obtained immediately before the drink (i.e. t = − 2 min) and at 15 min intervals between t = 0 and 300 min(Reference Gentilcore, Bryant and Wishart10). Blood glucose concentrations were determined immediately using a portable blood glucose meter (Medisense Precision Q.I.D™ System, Abbott Laboratories; Medisense Products, Inc., Bedford, MA, USA)(Reference Gentilcore, Bryant and Wishart10). Blood samples for serum insulin were collected in ice-chilled serum tubes with clotting activator and stored at − 70°C for subsequent analysis, and insulin was measured on samples collected at 30 min intervals between t = 0 and 300 min. Insulin concentrations were measured by ELISA (Diagnostics Systems Laboratories, Inc., Webster, TX, USA). Sensitivity was 1·8 pmol/l, intra-assay CV was 2·6 % and inter-assay CV was 6·2 %(Reference O'Donovan, Doran and Feinle-Bisset5).
Statistical analysis
Data were evaluated using repeated-measures two-way ANOVA, with ‘treatment’ and ‘time’ as within-subject factors. Gastric emptying, blood glucose and serum insulin concentrations were analysed as absolute values. Data were analysed from t = 0 to 300 min to determine the effects (‘treatment’ and ‘time’) of sucrose and α-cyclodextrin. In the event that the ANOVA demonstrated a statistically significant ‘treatment × time’ interaction, post hoc tests were used to examine point-by-point comparisons between treatments, with significance corrected for multiple comparisons. In addition, based on our previous studies(Reference Horowitz, Edelbroek and Wishart1, Reference Jones, Horowitz and Carney3), blood glucose concentration at 60 min was selected, a priori, for analysis. One-way ANOVA was used to analyse the effects of ‘time’ on blood glucose and serum insulin concentrations. Incremental areas under the curve (iAUC) were calculated using the trapezoidal rule for blood glucose and serum insulin from t = − 2 to 300 min. For gastric emptying (total, proximal and distal), total areas under the curve (AUC) were calculated from t = 0 to 300 min. All outcomes were analysed using paired t tests. Relationships between variables were assessed using linear regression analysis. All analyses, unless otherwise stated, were performed using Statview (version 5.0; Abacus Concepts, Berkeley, CA, USA) and SuperANOVA (version 1.11; Abacus Concepts). iAUC and AUC analyses were performed by a professional statistician using SPSS version 18 (SPSS, Inc., Chicago, IL, USA). Data are presented as means with their standard errors. A P value < 0·05 was considered significant in all analyses.
Results
We recruited thirteen people, of which ten completed the study. Of the thirteen subjects, three were unwilling to continue with the study after experiencing diarrhoea on completion of the first visit. In the remaining subjects, the studies were generally well tolerated. Loose stools were reported by three of the ten subjects after α-cyclodextrin (i.e. from t = 300 min). In all cases, these symptoms were mild and had resolved spontaneously within 7 h of the completion of each study. No subject reported a difference in the taste of the drink between the study days.
Gastric emptying
Total stomach
On both study days, gastric emptying of glucose was non-linear and approximated a monoexponential pattern. There was a significant treatment × time interaction (P < 0·002) for gastric emptying so that intragastric content was slightly less (P < 0·05) between t = 135 and 195 min, after control, compared with α-cyclodextrin. However, there was no difference between the AUC for gastric emptying after control than after α-cyclodextrin (P = 0·22), nor any difference in the T 50 between the 2 d (control: 91·7 (sem 10·7) min than after α-cyclodextrin: 104·9 (sem 19·4) min; P = 0·37; Fig. 1(a)).
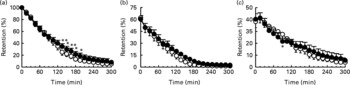
Fig. 1 Effects of control (○) and α-cyclodextrin (10 g) (●) treatments on (a) total gastric emptying and (b) proximal and (c) distal stomach retention of a 300 ml drink containing 100 g sucrose in ten healthy older subjects. Data are means, with their standard errors represented by vertical bars. Mean value was significantly different from that of the control treatment: *P < 0·05, **P < 0·01.
Intragastric distribution
There was no difference in proximal stomach retention between the two study days (Fig. 1(b); P = 0·14) nor between the AUC for proximal retention after control than after α-cyclodextrin (P = 0·12). In contrast, there was a significant treatment × time interaction for the distal stomach (P < 0·005), so that retention of the drink was slightly greater after control than after α-cyclodextrin at t = 90 min (P < 0·05). However, there was no significant difference between the AUC for distal stomach retention after control than after α-cyclodextrin (Fig. 1(c); P = 0·79).
Blood glucose concentrations
There was no significant difference in baseline (i.e. t = − 2 min) blood glucose between the two study days (control v. α-cyclodextrin: 6·3 (sem 0·2) mmol/l for both; P = 0·87) (Fig. 2(a)). There was a rise in blood glucose after the drink on both days (P < 0·0001 for both), which was evident from t = 15 min following both the control and α-cyclodextrin (P = 0·0001 for both). Peak blood glucose concentrations were not different after control (10·3 (sem 0·6) mmol/l) v. α-cyclodextrin (10·3 (sem 0·5) mmol/l; P = 0·88). However, there was a significant ‘treatment × time’ interaction (P < 0·0001) for blood glucose concentrations so that at t = 60 min, blood glucose was slightly greater (P < 0·05) and at t = 180 and 210 min slightly less (P < 0·005) after control when compared with α-cyclodextrin. There was no difference between the iAUC for blood glucose after control than after α-cyclodextrin (P = 0·87). At t = 300 min, there was a trend for blood glucose to be less than baseline after control (P = 0·07), but not after α-cyclodextrin (P = 0·17).

Fig. 2 Effects of control (○) and α-cyclodextrin (10 g) (●) treatments on (a) blood glucose and (b) serum insulin concentrations following a 300 ml drink containing 100 g sucrose in ten healthy older subjects. Data are means, with their standard errors represented by vertical bars. Mean value was significantly different from that of the control treatment: *P < 0·05, **P < 0·001.
Serum insulin concentrations
There was no significant difference in baseline (i.e. t = − 2 min) serum insulin between the two study days (control v. α-cyclodextrin: 66·7 (sem 13·9) v. 72·9 (sem 13·9) pmol/l; P = 0·47) (Fig. 2(b)). There was a rise in serum insulin after the drink on both days (P < 0·0001 for both), which was evident from t = 30 min (the first time point at which insulin was measured) following both the control and α-cyclodextrin (P = 0·0001 for both). Peak serum insulin concentrations were not different after control (839 (sem 150) pmol/l) than after α-cyclodextrin (770·9 (sem 137·5) pmol/l; P = 0·22). However, there was a significant ‘treatment × time’ interaction (P < 0·0001) for serum insulin concentrations. At t = 90 and 120 min, serum insulin was modestly greater (P < 0·0005) after control compared with α-cyclodextrin. There was a trend for a significant difference between the iAUC for serum insulin after control than after α-cyclodextrin (P = 0·09), representing a mean relative decrease in the iAUC of 17·0 % after α-cyclodextrin, compared with control. At t = 300 min, there was no difference in serum insulin from baseline after control (P = 0·26) or α-cyclodextrin (P = 0·81).
Relationships between changes in blood glucose concentration with gastric emptying
When data from the two study days were pooled, the magnitude of the rise in blood glucose from baseline was inversely related to the intragastric retention of the drink (e.g. percentage retention at t = 45 min; r − 0·46, P < 0·04; data not shown).
Discussion
The present study indicates that in healthy older subjects, α-cyclodextrin, when given acutely in a dose of 10 g, has modest effects to slow gastric emptying of, and modify the glycaemic and insulinaemic responses to, oral sucrose. Given that the digestion of sucrose is independent of the action of pancreatic amylase, these effects are not attributable to the inhibition of amylase.
α-Cyclodextrin has been reported to attenuate the blood glucose(Reference Raben, Andersen and Karberg7, Reference Buckley, Thorp and Murphy8) and serum insulin(Reference Raben, Andersen and Karberg7) responses to starch meals, the digestion of which is dependent on amylase. When α-cyclodextrin was included in the sucrose drink, we found that there was a slight lowering of blood glucose at 60 and 75 min, and that blood glucose concentrations were subsequently slightly greater at 180, 195 and 210 min. However, α-cyclodextrin had no effect on the initial rise, peak blood glucose concentration or overall iAUC. In contrast to the present study, Raben et al. (Reference Raben, Andersen and Karberg7) and Buckley et al. (Reference Buckley, Thorp and Murphy8) reported reductions in the iAUC for blood glucose of up to 50 % in response to α-cyclodextrin. The discordance between their observations(Reference Raben, Andersen and Karberg7, Reference Buckley, Thorp and Murphy8) and our own may reflect differences in the test meals and/or doses of α-cyclodextrin used. In the present study, serum insulin was reduced by α-cyclodextrin at 90 and 120 min, and the observed initial lowering of glucose induced by α-cyclodextrin, albeit small, is not accounted for by stimulation of insulin secretion. This is consistent with previous reports with starch meals that α-cyclodextrin does not stimulate either insulin(Reference Buckley, Thorp and Murphy8) or incretin hormones postprandially(Reference Raben, Andersen and Karberg7), and given that a sucrose drink was used, must be indicative of a minor reduction in the rate of small-intestinal glucose absorption. We have previously shown that modest variations in glycaemia have the potential to have a relatively greater effect on insulinaemia(Reference Horowitz, Edelbroek and Wishart1). Hence, the reduction in the subsequent insulin response induced by α-cyclodextrin may be attributable to the preceding decrease in glucose.
Gastric emptying of glucose, and other carbohydrates, is regulated at an overall rate of 4–17 kJ/min as a result of a length-dependent inhibitory feedback from receptors located throughout the small intestine(Reference Brener, Hendrix and McHugh11–Reference Moran, Ladenheim and Schwartz13). In the case of fat, protein and carbohydrate, it appears that this feedback is triggered by the digestion products, i.e. fatty acids, amino acids and monosaccharides(Reference Hunt14). The observed effect of α-cyclodextrin to slow gastric emptying, with concomitant changes in intragastric distribution, is novel. As in both healthy young and older subjects, perceptions of postprandial fullness(Reference Jones, Doran and Hveem15) and energy intake(Reference Sturm, Parker and Wishart16) are related to the content of the distal stomach (i.e. energy intake is less and fullness is greater when antral content is greater); this finding has potential implications for the regulation of appetite. Because differences in gastric emptying were only evident after approximately 135 min, the observed slowing is unlikely to be mediated by changes in the physical characteristics of the intragastric content (viscosity) and probably reflects an increase in the small-intestinal feedback secondary to the exposure of a greater length of the small intestine to sucrose. The latter appears to be the primary mechanism by which guar gum(Reference Jones, MacIntosh and Su17, Reference Russo, Stevens and Wilson18) and acarbose(Reference Gentilcore, Bryant and Wishart10) slow gastric emptying. Given that the modest glucose-lowering effect was evident before any retardation of gastric emptying, we conclude that the former is likely to reflect primarily a delay in small-intestinal carbohydrate absorption, possibly due to the inhibitory effect of α-cyclodextrin on the absorption of carbohydrate, so that carbohydrate is absorbed more distally. The viscous polysaccharide guar and some other fibres(Reference Holt, Heading and Carter19–Reference Torsdottir, Alpsten and Holm21) have similar effects. However, it should be recognised that even minor perturbations in the rate of gastric emptying can affect postprandial glycaemia substantially(Reference O'Donovan, Doran and Feinle-Bisset5).
In interpreting our observations, it should be recognised that we utilised a dose (10 g) of α-cyclodextrin that has been reported to diminish the glycaemic response to a carbohydrate (rice) meal in young, healthy subjects while inducing few, and tolerable, adverse gastrointestinal symptoms including nausea, abdominal bloating and diarrhoea(Reference Buckley, Thorp and Murphy8). While the reported effects of 10 g of α-cyclodextrin on blood glucose were modest, this dose has been shown to reduce the blood glucose response to rice to a greater extent than 5 g(Reference Buckley, Thorp and Murphy8). It remains possible that a higher dose of α-cyclodextrin may have induced greater effects on glycaemia and gastric emptying, but as three of the ten subjects reported adverse effects, it is likely that higher doses would not be well tolerated. It is also important not to extrapolate our observations to the effects of α-cyclodextrin on the gastric emptying of starch meals where amylase inhibition is of major relevance. We would speculate that gastric emptying of starch may be accelerated initially as a result of reduced intestinal feedback, but that the effects on carbohydrate absorption, and hence glycaemia, may be greater.
Acknowledgements
The authors have no conflicts of interest or financial interests to declare. The present study was supported by a Royal Adelaide Hospital/Institute of Medical and Veterinary Science Research Committee Project Grant. D. G. was supported by a Postdoctoral Fellowship (PR 07A 3309) from the National Heart Foundation of Australia. K. L. J. was funded by a National Health and Medical Research Council of Australia Career Development Award. The authors wish to thank the staff of the Department of Nuclear Medicine, Positron Emission Tomography and Bone Densitometry, Royal Adelaide Hospital, for providing radiopharmaceuticals and gamma camera time, Amtrade International Private Limited (Adelaide, SA, Australia) and Wacker Chemicals (Mulgrave, VIC, Australia), for supplying α-cyclodextrin, and Kylie Lange, Discipline of Medicine, University of Adelaide, for her involvement in the statistical analyses. The authors' contributions are as follows: D. G. was involved in the concept and design of the study, acquisition of subjects, data collection, analysis and interpretation, and preparation of the manuscript; L. V. and J. C. T were responsible for the data collection; J. M. W. performed the insulin analysis; J. D. B. and C. K. R. assisted in the preparation of the manuscript; M. H. and K. L. J. had a role in the design of the study, data analysis and interpretation, and preparation of the manuscript. Data from the present study have been presented in abstract form at the 19th IAGG World Congress of Gerontology and Geriatrics, Paris, France, 7 July 2009(Reference Gentilcore, Vanis and Rayner22).




