Historical background
Over recent years, new data from cohort studies, randomised clinical trials (RCT) and meta-analyses have changed the view regarding the association of fatty acids (FA) with CHD. This has not ended controversies about dietary fat guidelines in the USA and Europe(Reference Nestle1,Reference Harcombe, Baker and DiNicolantonio2) , and has even led to catchy headlines in some newspapers/magazines. Our objective, in this critical narrative review, is to try to answer this question: do recommendations about specific FA intake remain useful today for primary/secondary CHD prevention? This is not provocative and the response we propose aims to be pragmatic for clinical practice and/or prevention messages. We chose to analyse non-exhaustive past and recent data, which we hope will better help to understand the controversies and to answer that question.
Historically, three groups of studies/observations have been the source of interest regarding the association of intake of different types of FA with CHD risk: the Seven Countries Study led by Keys for both the potential deleterious role of SFA, and the protective role of the Mediterranean diet (MedDiet), Bang & Dyerberg’s studies for the possible protective role of long-chain (LC) n-3 PUFA, and the epidemiological observation of a very low rate of CHD in Japanese whose traditional diet (JapDiet) was very low in fat.
Seven Countries Study
Ancel Keys and co-workers carried out the Seven Countries Study, which included, between 1958 and 1964, 12 763 healthy men aged 40–59 years. They were enrolled in one of the sixteen cohorts from seven countries. Eleven cohorts were formed from men living in rural areas of Finland, Greece, Italy, Yugoslavia and Japan, two from railroad employees living in USA and Italy, one from men working in a large cooperative in Serbia, one from university professors in Belgrade, and one from men living in a small market town in the Netherlands. After a 15-year follow-up, the rate of CHD was thirty-two times higher in East Finland than in Crete(Reference Keys, Menotti and Karvonen3). The Seven Countries Study was an ecological study that means that only the data from average populations, and not individual data, were used to establish the correlations. Differences in mean age, blood pressure, serum cholesterol and smoking habits explained 80 % of the variance of CHD deaths between cohorts. All death rates were positively related to total dietary energy percentage of SFA on the one hand, and negatively to that of MUFA, oleic acid and the MUFA:SFA ratio on the other hand. No association was found with PUFA intake. All-cause and CHD deaths were low in cohorts with olive oil (OO) as the main dietary fat. Fifteen-year age-standardised CHD death rates per 10 000 was 2·3 times lower in rural Mediterranean areas (Dalmatia, Monte Giorgio, Crevalcore, Crete and Corfu) than in their rural counterparts in non-Mediterranean Europe (East and West Finland, Suwonia and Velika Krsna). After a 25-year follow-up, 47 % of men included had died(Reference Kromhout, Menotti and Bloemberg4). Dietary samples had been taken at the beginning of the study and analysed 25 years later in 1987 from equivalent food composites. This analysis revealed a very huge difference in the amount of SFA consumed between cohorts at entry in the study (3·8 % of total energy intake (TEI) in Japan cohort v. 22·7 % in East Finland). The average intake of all major SFA as a whole was strongly positively associated with CHD deaths (r 0·88; P<0·001) as well as individually lauric, myristic, palmitic and stearic acids (r 0·81 to 0·86; all P<0·001). C18 : 1 trans-fatty acid (TFA) intake was also associated with CHD mortality (r 0·78; P<0·001). The association with dietary cholesterol was weaker (r 0·55; P<0·05). In multivariate analysis models, only SFA, antioxidants, flavonoids and smoking were independently associated with CHD mortality rates; SFA were the major determinant explaining 73 % of the total variance. Multivariate analysis selected butter, lard + margarine and meat as significant predictors of CHD mortality rate (r 2 0·922)(Reference Menotti, Kromhout and Blackburn5). Lastly, the comparative (ecological) associations of SFA intake of the whole population from the sixteen cohorts at inclusion in the study and CHD mortality rate after 50 years of follow-up showed a very strong positive correlation (r 0·92; P<0·05) (Fig. 1) with CHD mortality, while MedDiet adequacy index was negatively correlated (r – 0·91)(Reference Kromhout, Menotti and Alberti-Fidanza6).

Fig. 1. Relationship of average population saturated fat intake at baseline and 50-year CHD death rates (from Kromhout et al. (Reference Kromhout, Menotti and Alberti-Fidanza6)). US, US railroad; EF, East Finland; WF, West Finland; ZU, Zutphen, the Netherlands; CR, Crevalcore, Italy; MO, Montegiorgio, Italy; RR, Rome railroad, Italy; DA, Dalmatia, Croatia; SL, Slavonia, Croatia; VK, Velika Krsna, Serbia; ZR, Zrenjanin, Serbia; BE, Belgrade, Serbia; KT, Crete, Greece; CO, Corfu, Greece; TA, Tanushimaru, Japan; UB, Ushibuka, Japan.
Bang & Dyerberg studies in Inuits
The interest for the CHD protective effect of LC n-3 PUFA arose from the Bang & Dyerberg hypothesis in the 1970s that the lower prevalence of CHD among Inuits from Greenland compared with a Danish population might be explained by the high content in LC n-3 PUFA of their traditional food, leading, via the generation of A3 thromboxane and prostacyclin, to a lower platelet aggregability and vasodilatation. During the 1970s, the age-adjusted CHD mortality among Greenland Inuit males aged 45–65 years was 5·3 % as compared with 40·4 % in the USA. Bang & Dyerberg, plus a laboratory technician (Nielsen), made their first sled expedition in 1970 to Greenland. They found that among Inuit hunter–fishermen and their wives (aged 40 years and over) living in the Umanaq district of Igdlorssuit village, 500 km north of the Arctic Circle (latitude 71°N), all plasma lipoprotein concentrations were lower than those of Danes, except for HDL-cholesterol concentrations, which were higher(Reference Bang, Dyerberg and Nielsen7). In addition, plasma lipoprotein concentrations were higher in Inuit women who had emigrated and lived in Denmark than in those still living in Greenland(Reference Bang, Dyerberg and Nielsen7). This argued more in favour of a protective dietary environmental factor than in a genetic one. The n-6 PUFA plasma concentrations of Inuits were 50 to 30 % lower than those of Danes while LC n-3 PUFA concentrations were five to ten times higher. These differences were not observed between Inuits living in Denmark and Danes; hence, the conclusion that a different content in FA of food consumed probably explained the differences(Reference Dyerberg, Bang and Hjorne8). Total LC n-3 PUFA intake accounted for 13·1 % of total FA among Inuit v. 0·8 % among Danes, 18 : n-6 accounted for 5 v. 10 %, and 18 : 3n-3, respectively: 0·6 v. 2 %(Reference Bang, Dyerberg and Hjøorne9). The difference in FA composition was due to the high consumption of marine products (rich in LC n-3 PUFA) by Inuits v. a high consumption of dairy products by the Danes(Reference Bang, Dyerberg and Sinclair10). Another aspect highlighted by Bang & Dyerberg was the low platelet aggregation and long bleeding time in Inuits(Reference Dyerberg and Bang11). In their 1980 article(Reference Bang, Dyerberg and Sinclair10), the authors pointed out that the factors that could explain the lower incidence of CHD among Inuits, even though they were very heavy smokers, were: a protective lipid profile, lower platelet aggregation, low prevalence of obesity, hypertension and lack of diabetes. This perfectly reflected the knowledge of that time! The anti-thrombotic effects of LC n-3 PUFA – EPA increases the production of inactive thromboxane A3 (TBXA3), decreases that of thromboxane A2 (TBXA2), increases concentrations of anti-thrombin III, deformability of erythrocytes, and decreased blood viscosity – were subsequently confirmed by other studies(Reference Bang12). These biochemical alterations led Dyerberg & Bang to hypothesise that the anti-thrombotic effect of LC n-3 PUFA probably explained the lower incidence of myocardial infarction (MI) in Greenland Inuits(Reference Dyerberg and Bang13). In a 1990 review(Reference Bang12), Bang used the three following observations from scientific literature as arguments in favour of the potential protective role of LC n-3 PUFA towards MI: (a) Nelson’s 1972 work(Reference Nelson14) in eighty patients showing that 16–19 years following a CHD event, mortality was lower among those who consumed fish compared with those who never consumed it; (b) the observation during the Second World War of a significant decrease in thrombo-embolic pathologies in Norwegians whose intakes of EPA were of 4–5 g/d, due to a sharp increase in fish consumption at the expense of dairy products, eggs and meat whose availability was significantly decreased(Reference Bang and Dyerberg15); and (c) the confirmation of Bang & Dyerberg data in Japanese fishermen (consuming 250 g/d of fish, equivalent to 2·5 g/d EPA) compared with control farmers (90 g/d of fish equivalent to 0·9 g/d EPA), showing higher EPA/DHA plasma concentrations in fishermen associated with lower platelet aggregation(Reference Hirai, Hamazaki and Terano16).
Very low incidence of CHD in Okinawan and Japanese populations
At the end of Second World War, individuals living in Okinawa prefecture (Ryukyu Islands) had the longest duration of life in the world. In 1995, death from CHD was 35 % lower in Okinawa than in Japan and 83 % lower than in the USA. This longevity, resulting mainly from lower incidence of CHD and cancers, has been attributed to their lifestyle and more specifically to their very specific diet resulting from multiple historic influences from Japan, continental China and countries of South-East Asia(Reference Sho17). Dietary surveys from 1879(Reference Toyokawa18), 1919(Reference Nagata19) and 1949(20,21) are available. The survey from 1879 showed that everyday diet was made of 93 % of sweet potato, the remaining being made of minor grains, wheat, millet, barnyard grains, sugar-beet and rice, which left not much place for fat. The survey from 1919 showed that 93 % of energy intake was from carbohydrates (CHO), 7·1 % from proteins and only 1·7 % from fat. The survey from 1949 showed that CHO accounted for 85 % of energy (79 % in other Japan areas), proteins for 9 % (13 % in other Japanese areas) and fat for 6 % only (8 % in other Japan areas). The composition of fat intake was 30·8 % SFA, 30 % MUFA and 40 % PUFA. Because of the Westernisation that began in the 1950s, the incidence of CHD has increased among the Okinawans, indirectly confirming the very likely protective role of their traditional diet and lifestyle in their low incidence of CHD and high longevity. In 2018, Japanese women had the longest (87·32 years) life span in the world and men had the third longest (81·25 years) life span in the world(22) with consistently low morbidity and mortality from CHD(Reference Wong, Brown and Lau23). Japanese are not genetically protected from CHD risk factors. Indeed, the N-Hon-San study showed that Japanese immigrants living in Hawaii and San Francisco Bay Area and Honolulu had higher plasma cholesterol and CHD incidence than those living in Hiroshima and Nagasaki(Reference Marmot, Syme and Kagan24,Reference Benfante25) . As underlined by Tada et al. (Reference Tada, Maruyama and Koba26) in their review, total cholesterol in Japanese is now reaching a level similar to that of Americans, but the incidence of CHD in Japanese remains approximately one-fifth to one-third of that in Americans. Altogether, this supports evidence that dietary habits, beyond a low-fat diet, and lifestyle influence CHD rates in Japanese and not genetic background.
In summary, the Seven Countries Study reported a very strong ecological association between SFA intake and CHD mortality in middle-aged men followed for 50 years. This was also the first study highlighting the possible/probable protective effect of a MedDiet pattern and CHD mortality (see the Seven Countries Study section). Bang & Dyerberg’s pioneering works led to their hypothesis that the low incidence of MI in Inuits living in Greenland could be related to their high consumption of marine products rich in LC n-3 PUFA that favoured a less atherogenic lipid profile and lower platelet aggregation, without neglecting the role of their high physical activity during the 1970s. As to Okinawans and Japanese, although their traditional diet (JapDiet) is very low in total fat intake and high in LC n-3 PUFA, it is probably the whole diet, more than these amounts of specific FA, that explains their low incidence of CHD.
In the next sections, we critically analyse separately the data about the association of TFA, SFA, MUFA, plant PUFA and LC n-3 PUFA intake with CHD. The association is presented as relative risk (RR).
Trans-fatty acids and CHD
Industrially produced TFA, formed during partial hydrogenation of vegetable oils, increase CHD risk(Reference Mozaffarian, Katan and Ascherio27–Reference Zhu, Bo and Liu29), which is now widely accepted, so that it does not deserve any more comment, except that they must be completely suppressed as soon as possible from global food supplies as proposed by the REPLACE action package released by the WHO(30). In 2015, the Food & Drug Administration concluded that TFA were not generally recognised as safe and decided they must be removed from industrial products by 1 January 2021(Reference United States and Drug31). The main mechanisms advocated for their deleterious effects are an increase in LDL-cholesterol and decrease in HDL-cholesterol concentrations, an increase in inflammation and an induction of endothelial dysfunction(Reference Mozaffarian, Katan and Ascherio27).
SFA and CHD
Cohort studies
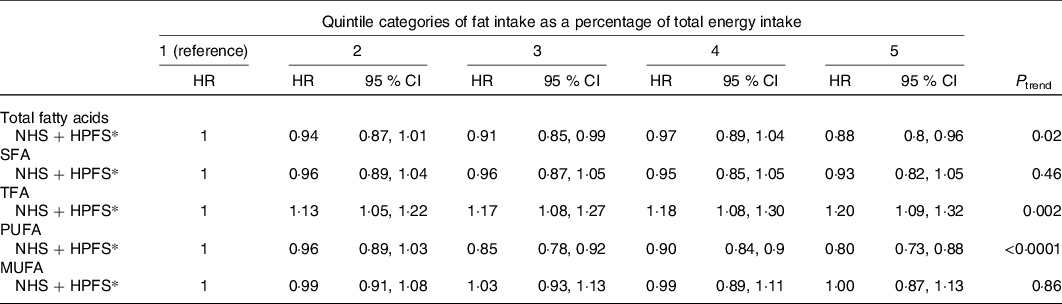
Zong et al. (Reference Zong, Li and Wanders32) included 81 557 women from the Nurses’ Health Study (NHS) and 51 529 men from the Health Professionals Follow-up Study (HPFS) with 24–28 years of follow-up. Association with CHD was analysed according to quintiles of SFA intake. The sum of LC SFA (12 : 0 to 18 : 0; major SFA of the diet) was positively related to CHD for each cohort as well as for the pooled cohorts (RR 1·18; 95 % CI 1·09, 1·28; P trend < 0·001). RR was 1·04 (95 % CI 0·96, 1·12; NS) for short-medium-chain SFA. RR for each of LC SFA were all significant (1·07 for 12 : 0, 1·13 for 14 : 0, 1·18 for 16 : 0 and 1·18 for 18 : 0) (Table 1). It is noteworthy that a recent meta-analysis of sixteen studies(Reference Neelakantan, Seah and van Dam33) shows that coconut oil (rich in short-medium-chain SFA) increases LDL-cholesterol by 10·47 mg/dl (0·27 mmol/l) and HDL-cholesterol by 4·00 mg/dl (0·10 mmol/l) as compared with non-tropical vegetable oils, leading the authors to conclude that it is not a healthy oil. The study of association of SFA with CHD by comparing their foods sources (by example meats v. dairy or different dairy sources between them) is confounded by the other constituents of these foods as recently reviewed(Reference Wu, Micha and Mozaffarian34), which precludes a clear conclusion about a direct association of their content in SFA with CHD. The Prospective Urban Rural Epidemiology (PURE) cohort(Reference Dehghan, Mente and Zhang35) included 135 335 participants, aged 35–70 years from eighteen countries (three high-income, eleven middle-income and four low-income); the USA and European countries except Sweden were not included. After 7·4 years of follow-up, the higher quintile of SFA intake was not associated with MI or CHD mortality. The National Institutes of Health-American Association of Retired Persons (AARP) Diet and Health Study cohort(Reference Zhuang, Zhang and He36) included 521 120 participants aged 50–71 years (16 years of follow up). The highest quintile of SFA intake was associated with CHD mortality (RR 1·29; 95 % CI 1·25, 1·33; P trend < 0·0001).
Table 1. Relative risks of intake of individual SFA and risk of CHD in the Nurses’ Health Study (NHS; 1984–2010) and Health Professionals Follow-up Study
(HPFS; 1986–2010) (adapted from Zong et al. (Reference Zong, Li and Wanders32))
(Relative risks (RR) and 95 % confidence intervals)

* Multivariable model (for more details, see Nelson(Reference Nelson14)).
Meta-analyses
Skeaff & Miller(Reference Skeaff and Miller37), in 2009, found no association of SFA intake with CHD death (eight cohorts) (RR 1·14; 95 % CI 0·82, 1·60) or CHD events (five cohorts) (RR 0·93; 95 % CI 0·83, 1·05). Siri-Tarino et al. (Reference Siri-Tarino, Sun and Hu38), in 2010, included twenty-one prospective cohorts (347 747 subjects; 5–23 years of follow-up; 11 006 CHD events or stroke). SFA intake was not associated with CHD (sixteen cohorts; RR 1·07; 95 % CI 0·96, 1·19). There was heterogeneity among studies, not explained by age, sex, sample size, duration of follow-up, medical record review and study quality score. Chowdhury et al. (Reference Chowdhury, Warnakula and Kunutsor39) performed, in 2014, a meta-analysis of thirty-two observational studies (512 420 subjects, 5–23 years of follow-up, 15 945 CHD events). They found no association of SFA with CHD, comparing the highest tertile of intake with the lowest (RR 1·03; 95 % CI 0·98, 1·07). TFA were significantly associated with a higher risk (RR 1·16; 95 % CI 1·06, 1·27) and LC n-3 PUFA with a lower risk (RR 0·87; 95 % CI 0·78, 0·97). The conclusion of Chowdhury et al. (Reference Chowdhury, Warnakula and Kunutsor39) was: ‘Current evidence does not clearly support cardiovascular guidelines that encourage high consumption of PUFA and low consumption of total saturated fats’. That conclusion led Mark Bittman to write an article in The New York Times entitled ‘Butter is back’(Reference Bittman40) and, 3 months later, Brian Walsh to cover ‘Eat Butter. Scientists labelled fat the enemy. Why they were wrong’ on the front page of Time magazine(Reference Walsh41). Zhu et al. (Reference Zhu, Bo and Liu29) performed, in 2019, a meta-analysis including fifty-six cohorts. Highest v. lowest SFA intake was not associated with CHD (RR 0·97; CI 0·93, 1·02). There was a significant heterogeneity, poorly explained by all covariates explored in meta-regression.
To better clarify the nature of the association of SFA with CHD, it is quite useful to look at the effect of their partial replacement by other FA or different types of CHO.
Substitution/reduction studies of SFA and CHD
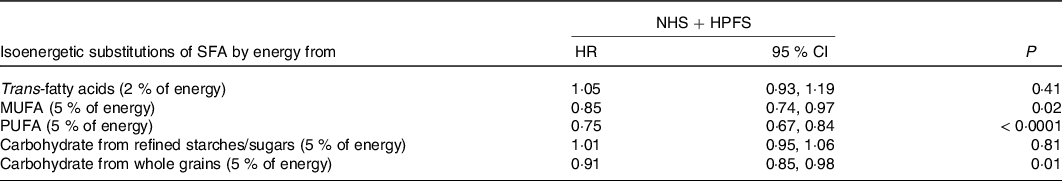
Several studies from the same group(Reference Wang, Li and Chiuve28,Reference Li, Hruby and Bernstein42,Reference Chen, Li and Sun43) assessed the effects of the different types of FA in 83 349 women from the NHS and 42 884 men from the HPFS. Comparisons were made by quintiles of fat intake. The percentage of energy intakes from total fat or specific FA intake was calculated as cumulative average up to the start of each 2- or 4-year follow-up interval (Table 2). For multivariable analyses, isoenergetic substitution models were built, which simultaneously included energy intake, percentages of energy derived from protein and specific types of fat and other potentially confounding variables. Thus, as underlined by the authors, the coefficients from these models can be interpreted as the estimated effect of substituting a certain percentage of energy from fat for equivalent energy from CHO. By using these models, TFA were associated with a 20 % increase in cardiovascular (CV) mortality (P trend < 0·001); SFA intake, when replaced by total CHO, was not related to CV mortality. PUFA and MUFA intakes were inversely associated with CV mortality (both P trend < 0·001). The replacement of 5 % of SFA by CHO from whole grains was associated with a 9 % lower risk of CHD (P = 0·01), whereas it was not if replaced with refined CHO (P >0·10) (Table 3). When 5 % of SFA intake was replaced by MUFA or PUFA, the risk of CHD decreased by 15 % (P = 0·02) and 25 % (P = 0·0001), respectively. The Women’s Health Initiative Dietary Modification Trial(Reference Prentice, Aragaki and Van Horn44), a RCT (48 835 US women, 50–79 years, follow-up 8·3 years), showed that a low-fat-diet (20 % TEI) decreased CHD by 30 % in primary prevention (RR 0·70; 95 % CI 0·56, 0·87; P = 0·003), but increased it by 47 % in secondary prevention (RR 1·47; 95 % CI 1·12, 1·93). The authors indicated that secondary prevention outcomes were skewed by post-randomisation use of cholesterol-lowering drugs. Mozaffarian et al. (Reference Mozaffarian, Micha and Wallace45), in 2010, performed a meta-analysis of eight RCT (13 614 participants; 1042 CHD events; follow-up > 1 year). Average PUFA consumption was 14·9 % TEI in intervention groups v. 5·0 % in controls. Overall, CHD risk reduction was 19 % (RR 0·81; 95 % CI 0·70, 0·95; P = 0·008), corresponding to 10 % reduced risk (RR 0·90; 95 % CI 0·83, 0·97) for each 5 % energy increase from PUFA. Hooper et al. (Reference Hooper, Martin and Abdelhamid46), in 2015, performed a meta-analysis of fifteen RCT (59 000 participants). Reducing SFA intake reduced CHD events by 17 % (RR 0·83; 95 % CI 0·72, 0·96). Harcombe et al. (Reference Harcombe, Baker and DiNicolantonio2), in 2016, performed a meta-analysis of ten RCT (62 421 participants; 1218 CHD deaths) and found no effect of reduced/modified fat intake towards CHD mortality (RR 0·98; 95 % CI 0·88, 1·08).
Table 2. Hazard ratios of CHD by intake of fatty acids as percentages of total energy (adapted from Li et al. (Reference Li, Hruby and Bernstein42))
(Hazard ratios (HR) and 95 % confidence intervals)
NHS, Nurses’ Health Study (1984–2010); HPFS, Health Professionals Follow-up Study (1986–2010); TFA, trans-fatty acids.
* Multivariable model (for more details, see Division of Health, Labor and Welfare, Okinawa Prefectural Government(21)).
Table 3. Multivariable hazard ratios of CHD with isoenergetic (percentage of energy) substitutions of one dietary component for another in the Nurses’ Health
Study (NHS; 1984–2010) and the Health Professionals Follow-up Study (HPFS) (1986–2010) (adapted from Li et al. (Reference Li, Hruby and Bernstein42))
(Hazard ratios (HR) and 95 % confidence intervals)
In summary, during many years, especially since the Seven Countries Study(Reference Keys, Menotti and Karvonen3), it has been advocated that CHD was related to SFA intake and most if not all of international dietary recommendations counselled that their intake should be less than 10 % of TEI. Metabolic studies have shown that diets high in SFA and low in PUFA increased plasma cholesterol concentrations(Reference Hegsted, McGandy and Myers47). It was generally advocated that SFA increased CHD risk by elevating plasma cholesterol on the one hand, and that different types of SFA had a different effect on plasma lipoproteins, so that they could not be similarly atherogenic(Reference Mensink, Zock and Kester48) on the other hand. Meta-analysis failed to demonstrate a link between SFA intake and CHD events and/or CHD mortality at odds with individual cohorts (except the Prospective Urban Rural Epidemiology (PURE) cohort, which found no association). As very often in science and life, the truth is probably intermediate between these extremes. SFA are probably not as deleterious as previously thought, but the lack of association observed in most meta-analyses between their intake and CHD does not prove their total safety. Indeed, the substitution of a part of SFA for PUFA, MUFA or CHO from whole grains reduces CHD risk, which pleads against complete safety of SFA toward CHD. Thus, their consumption ad libitum (‘eat butter’) is certainly not a good way to prevent CHD. Altogether, this could explain controversies about adequacy of dietary SFA guidelines for CHD risk(Reference Nestle1,Reference Harcombe, Baker and DiNicolantonio2) . Among the reasons of controversy about the deleterious effect or not of SFA, the following can be cited: the CV effect of decreasing SFA depends on the nutrients and foods that are substituted, the effect of SFA on plasma LDL-cholesterol concentrations can vary significantly among individuals, individual SFA have differing biological effects, the food matrix can modify the CV risk in response to SFA, and overall nutrient distribution should be considered, which is also true for other types of FA, except industrially formed TFA which are clearly always deleterious.
MUFA intake and CHD
The most common MUFA of the human diet is oleic acid. The specific interest for their potential protective CV effect was born with the Seven Countries Study(Reference Keys, Menotti and Karvonen3), showing a lower CHD risk in populations consuming the MedDiet known to be rich in OO. We will separate studies aiming to assess the association of total MUFA whatever their dietary sources (animal and plant) from those studying the effect of OO, knowing that its content is not exclusively oleic acid but also polyphenols (especially in extra-virgin OO).
Cohorts
Hu et al. (Reference Hu, Stampfer and Manson49) prospectively studied 80 082 women aged 34–59 years who had no history of CHD events, followed up for 14 years. Dietary intake was assessed by a FFQ. The analysis of the association of FA intake with CHD risk was performed comparing quintiles of FA intake and by analysis of the effect of substitution of a dietary component by another one. There was no association between MUFA intake and CHD risk along the five quintiles of intake (from 11 to 19·3 % of energy) (RR 0·95; 95 % CI 0·64, 1·39). As compared with CHO, an increase in 5 % of energy from MUFA was associated with a 29 % decreased risk (RR 0·81; 95 % CI 0·65, 1·00; P=0·05). In an extension of follow-up to 20 years of the NHS, the authors(Reference Oh, Hu and Manson50) found no association across the quintiles of MUFA intake and CHD, while they found a significant inverse association for PUFA and a significant positive association for TFA. In the PREvencion con DIeta MEDiterranea (PREDIMED) study (7038 subjects at high CV risk followed up for 6 years)(Reference Guasch-Ferré, Babio and Martínez-González51), the highest quintile of MUFA intake (compared with the lowest) was inversely associated with CHD (RR 0·50; 95 % CI 0·31, 0·81). In substitution analysis, replacing 5 % of energy from SFA by MUFA decreased CHD by 37 % (RR 0·63; 95 % CI 0·43, 0·94)(Reference Marmot, Syme and Kagan24). In that study, MUFA were mainly from extra-virgin OO, which also contains polyphenols.
Meta-analyses
Jakobsen et al. (Reference Jakobsen, O’Reilly and Heitmann52), in 2009, performed a meta-analysis of eleven cohort studies (344 696 participants; 4–10 years of follow-up; 5249 CHD events; 2155 CHD deaths). There was tendency to a positive association between MUFA intake and CHD events (RR 1·19; 95 % CI 1·00, 1·42) when substituted for SFA. Chowdhury et al. (Reference Chowdhury, Warnakula and Kunutsor39), in 2014, included nine prospective cohorts (144 219 participants; 6031 events); they found no association of MUFA intake with CHD (RR 1·00; 95 % CI 0·91, 1·10). Schwingshackl & Hoffmann, in 2014, performed two meta-analyses(Reference Schwingshackl and Hoffmann53,Reference Schwingshackl and Hoffmann54) , one including thirty-two cohorts (841 211 participants; primary prevention)(Reference Schwingshackl and Hoffmann53), the other, twelve RCT (7150 participants; follow-up 1–6 years; secondary prevention)(Reference Schwingshackl and Hoffmann54). Regarding cohorts, MUFA intake was inversely associated with CHD mortality (RR 0·88; 95 % CI 0·80, 0·96; P=0·004) and CHD events (RR 0·91; 95 % CI 0·86, 0·96; P=0·001). The association was only significant with OO for CHD events. Regarding meta-analysis of RCT, comparing a reduced/modified fat intake (fat < 30 % of TEI, MUFA: 8–26 % TEI) with a control diet, MUFA intake was not associated with CHD mortality, CHD events and MI. Zhu et al. (Reference Zhu, Bo and Liu29), in 2019, performed a meta-analysis of forty-three cohort studies and found no association of MUFA with CHD events. Martínez-González et al. (Reference Martínez-González, Dominguez and Delgado-Rodríguez55) performed a meta-analysis of case–control, cohort and intervention studies to assess the association of OO consumption with CHD and/or stroke. Nine studies, all conducted in Mediterranean countries, were included (101 460 participants for CHD and 38 673 for stroke; follow-up 4·8 to 10·6 years). Combining all studies, with CHD and stroke as end points, showed an inverse association of OO consumption with CHD (RR 0·82; 95 % CI 0·70, 0·96; P=0·01). Secondary analysis showed no association of OO with CHD (ten studies) (for an increase in 25 g/d: RR 0·87; 95 % CI 0·78, 1·18; P=0·14).
In summary, discrepancies clearly exist; there is reasonable evidence of an inverse association of MUFA intake with CHD when substituted for SFA. For OO, there is no proof of a protective effect per se towards CHD, but possibly towards stroke. Some reasons for the controversy are that MUFA are tightly associated with SFA in many foodstuffs, such as red meat, and that OO is not only rich in oleic acid but also contains SFA and polyphenols (extra-virgin OO).
α-Linolenic acid and CHD
Cohorts
The Multiple Risk Factor Intervention Trial(Reference Dolecek56) was a US primary prevention study in men at high CHD risk (usual-care group 6250 participants; 10·5 years of follow-up); the highest quintile of α-linolenic acid (ALA) intake expressed as a percentage of TEI was inversely associated with CHD deaths (RR 0·58; P<0·05). When expressed as quintiles of g/d of intake, the association was not significant (RR 0·66; NS). It is noteworthy that mean LC n-3 PUFA intake was low (175 mg/d). The HPFS(Reference Ascherio, Rimm and Giovannucci57) included 43 757 participants, followed for 16 years. Intake of ALA (as % TEI) was inversely associated with MI (RR 0·41 for a 1 % increase in energy; P trend < 0·01), but not with fatal CHD. The association was not significant for a range of ALA intake of 0·9–1·5 g/d. In an update of the HPFS, Mozzafarian et al. (Reference Mozaffarian, Ascherio and Hu58) reported that after 14 years of follow-up, for each 1 g/d intake of ALA, there was lower risk of non-fatal MI (RR 0·42; 95 % CI 0·23, 0·75; P = 0·003) and total CHD (RR 0·53; 95 % CI 0·34, 0·83; P = 0·006), but only when LC n-3 PUFA intake was < 100 mg/d. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study was a Finnish study in male smokers (n 21 930; 6·1 years of follow-up)(Reference Pietinen, Ascherio and Korhonen59). No association was found between ALA intake and CHD death over a range of intake of 0·9 to 2·5 g/d. The Singapore Chinese Health Study(Reference Koh, Pan and Wang60) included 63 257 participants, aged 45–74 years, with a follow-up of 13 to 18 years. ALA intake was inversely associated with CHD mortality when comparing the highest (0·80 g/d) with lowest (0·40 g/d) quartiles (RR 0·82; 95 % CI 0·71, 0·93; P trend = 0·001). The association was significant only in individuals without prior history of CHD. The NHS(Reference Hu, Stampfer and Manson61) recruited 76 283 US women without prior CHD, followed for 10 years. In multivariate analysis, fatal CHD was inversely associated with the highest quintile of intake of ALA (1·36 g/d) as compared with the lowest (0·71 g/d) (RR 0·55; 95 % CI 0·32, 0·94; P trend = 0·01). Non-fatal CHD was not significantly associated (RR 0·85; 95 % CI 0·61, 1·19; P = 0·50). An updated analysis of the NHS (76 763 women; follow-up 18 years)(Reference Albert, Oh and Whang62) showed an inverse association with the fourth (0·60 % TEI) and fifth (0·75 % TEI) quintiles of ALA intake as compared with the lowest (0·37 % TEI) for sudden cardiac death (RR 0·62 (95 % CI 0·39, 0·98) and RR 0·60 (95 % CI 0·37, 0·96), respectively; P trend = 0·02) but not for other fatal CHD or non-fatal CHD. A Netherlands cohort(Reference de Goede, Verschuren and Boer63) included 20 069 healthy men and women, aged 20–65 years with a follow-up of 8–13 years. No association was observed between the highest quintile of ALA intake (> 1·9 g/d) v. the lowest (< 0·9 g/d) (RR 1·12; 95 % CI 0·72, 1·75). The Pooling Project of Cohort Studies on Diet and Coronary Disease(Reference Vedtofte, Jakobsen and Lauritzen64) gathered eight cohorts (four from the USA, two from Finland and one from Sweden) including 148 675 women and 80 368 men with a follow-up of 4–10 years. For each additional 1 g of ALA consumed, a 15 % lower risk of CHD events (hazard ratio 0·85; 95 % CI 0·72, 1·01; NS) and a 23 % lower risk of CHD deaths (hazard ratio 0·77, 95 % CI 0·58, 1·01; NS) were observed. No consistent association was observed among women. No effect of the modification by the intake of LC n-3 PUFA was observed. Other studies found no association(Reference Oomen, Ocké and Feskens65–Reference Sala-Vila, Guasch-Ferré and Hu68).
Adipose tissue α-linolenic acid and CHD
Three studies assessed the association between adipose tissue content of ALA with CHD. The Diet, Cancer and Health Study(Reference Bork, Jakobsen and Lundbye-Christensen69) included 3500 Danish participants, aged 50–64 years, with a 17-year follow-up. Multivariate analysis showed that ALA dietary intake (range < 1·67 g/d to ≥ 2·54 g/d) was not associated with MI (RR 0·90 (95 % CI 0·76, 1·07) for men; RR 1·10 (95 % CI 0·83, 1·45) for women). ALA concentrations in adipose tissue were also not associated with MI (RR 1·27 (95 % CI 0·94, 1·72) for men, and RR 1·13 (95 % CI 0·75, 1·71) for women). In a case–control study from Costa-Rica(Reference Campos, Baylin and Willett70) including 1819 cases with a first MI and 1819 controls, the levels of ALA intake ranged from 0·42 to 0·86 % of TEI. Greater ALA (in adipose tissue or as dietary intake) was associated with a lower risk of MI (RR 0·41 (95 % CI, 0·25, 0·67) for adipose tissue ALA, and 0·61 (95 % CI 0·42, 0·88) for dietary ALA). The relationship between ALA and MI was non-linear; risk did not decrease with intakes > 0·65 % of energy (1·79 g/d). The EURopean multicenter case–control study on Antioxidants, Myocardial Infarction and breast Cancer study (EURAMIC)(Reference Guallar, Aro and Jiménez71) is a case–control study (639 cases and 700 controls) conducted in eight European countries and Israel. After adjustment for classical CHD risk factors, comparing the highest quintile with the lowest concentrations of ALA in adipose tissue, there was no significant association with MI (RR 0·68; 95 % CI 0·31, 1·49).
Meta-analyses
Pan et al. (Reference Pan, Chen and Chowdhury72), in 2012, included nineteen prospective cohorts and eight retrospective case–control studies (251 049 individuals, 15 327 CVD events, follow-up 5–30·7 years). The overall pooled RR was 0·86 (95 % CI 0·77, 0·97). In subgroups, the association was significant only for fatal CHD (RR 0·80; 95 % CI 0·65, 0·98). Chowdhury et al. (Reference Chowdhury, Warnakula and Kunutsor39), in 2014, included seven prospective cohorts (157 258 participants) and four RCT (9444 participants). No association was found. Del Gobbo et al. (Reference Del Gobbo, Imamura and Aslibekyan73), in 2016, pooled nineteen cohort studies. Biomarkers of ALA were inversely associated with fatal CHD (RR 0·91; 95 % CI 0·84, 0·98) but not total CHD (RR 1·00; 95 % CI 0·95, 1·05). Balk & Lichtenstein(Reference Balk and Lichtenstein74) have summarised the 2016 update of the 2004 Agency of Healthcare Research and Quality evidence review of n-3 FA and CVD. By meta-analysis, no association was found. Wei et al. (Reference Wei, Hou and Xi75), in 2018, carried out a meta-analysis of fourteen cohorts. Higher ALA intake was associated with modest reduced CHD (RR 0·91; 95 % CI 0·85, 0·97) and fatal CHD (RR 0·85; 95 % CI 0·75, 0·96). Abdelhamid et al. (Reference Abdelhamid, Brown and Brainard76), in 2020, performed a meta-analysis of RCT and found no effect of ALA on CHD mortality or events.
In summary, results are contradictory. Most cohorts do not find association of ALA intake or its biomarkers with CHD. Meta-analyses generally conclude that there is a lack of or a weak significant association. Whether ALA has a protective effect towards CHD, it might exist in individuals with a very low intake of LC n-3 PUFA (< 100 or < 175 mg/d) as suggested by two studies(Reference Dolecek56,Reference Mozaffarian, Ascherio and Hu58) . Among reasons explaining the controversial effect of ALA, one should consider that it may not be protective per se, but only when LC n-3 PUFA intake is low so that it could participate in restoring by elongation and desaturation to a higher, even if modest, increase in LC n-3 PUFA concentrations in membrane phospholipids, or when given as part of a MedDiet.
n-6 PUFA and CHD
The main n-6 PUFA in the diet is linoleic acid (LA). Because maize or safflower oil is used as the main source of PUFA in RCT, the LA effect per se is included in this section.
Randomised clinical trials
Several RCT have been designed over the years. Rose et al. (Reference Rose, Thomson and Williams77), in 1965, performed a secondary prevention RCT (eighty London patients included but analysis from forty-three after 2 years). Patients were divided into three groups: no advice (controls), reduction of animal fats plus OO (80 g/d), and reduction of animal fats plus maize oil (80 g/d). After 2 years of follow-up, 75 % of controls remained free of new CHD, whereas only 57 and 52 % in the OO and maize oil groups, respectively (0·1 > P>0·05). The conclusion of the authors was that under the circumstances of their study, maize oil could not be recommended in the treatment of CHD. The main limitation of the study is the small number of patients remaining included at 2 years. The Oslo Diet-Heart Study(Reference Leren78), initiated in the 1950s, is a secondary prevention RCT (412 men; aged 30–64 years; followed for 11 years). Patients were randomly allocated to the experimental diet group (39 % of TEI from fat including 8·5 % SFA, 20·7 % PUFA and 10·1 % MUFA) or to the control diet group. The detailed clinical follow-up including dietary advice was undertaken only during the first 5 years of the 11-year follow-up. We only report the results of the 11-year follow-up. The experimental diet analysed in seventeen participants contained 39 % of TEI from fat including 8·5 % SFA, 20·7 % PUFA and 10·1 % MUFA. The composition of the control diet is not reported but probably was the usual Norwegian diet at that time. After 5 years of follow-up the mean cholesterol reduction in the experimental diet group was 17·6 % v. 3·7 % in the control group, and combined CHD major relapses (MI and new angina pectoris) were reduced by 24·7 % (P=0·05), but not MI or CV deaths. After 11 years of follow-up fatal MI was 43·9 % lower in the experimental diet group (P=0·004). The Finnish Mental Hospital Study(Reference Turpeinen79), initiated in the 1960s, aimed to study the association of an experimental diet rich in PUFA with CHD mortality in patients from two mental hospitals regardless of pre-existing CHD, with a 12-year follow-up. A cross-over design was used (6 years × 6 years). The PUFA:SFA ratio of the usual diet was 0·22–0·29 v. 1·42–1·78 for the experimental one. Mean plasma cholesterol was 12–18 % lower with the experimental diet. The experimental diet decreased age-adjusted CHD deaths: 6·61 v.14·8 per 1000 person-years for males (P<0·002) and 5·21 v. 7·90 for females (NS). The Minnesota Coronary Survey(Reference Frantz, Dawson and Ashman80,Reference Ramsden, Zamora and Majchrzak-Hong81) was a secondary prevention RCT conducted from 1968 in six Minnesota mental state hospitals and one nursing home (4393 men and 4664 women; follow-up 4·5 years). The trial compared a 39 % fat control diet (18 % SFA; 5 % PUFA; 16 % MUFA; 446 mg cholesterol per d) with a 38 % fat treatment diet (9 % SFA; 15 % PUFA; 14 % MUFA; 166 mg cholesterol per d) on plasma cholesterol levels and the incidence of MI and sudden deaths. No difference was observed between the two groups for those end points. The Sydney Diet Heart Study(Reference Ramsden, Zamora and Leelarthaepin82) is a secondary prevention RCT (men aged 30–59 years followed from 1966 to 1973). The intervention group (n 221) increased PUFA intake (from safflower) to about 15 % of TEI, and reduced SFA to less than 10 % of TEI and dietary cholesterol to 300 mg/d. The control group (n 237) received no instructions. CHD mortality was higher in the intervention group (16·3 v. 10·1 %; RR 1·74; 95 % CI 1·04, 2·92; P=0·036). It is noteworthy that the n-6 PUFA intervention was delivered in a margarine that probably had a high content of TFA, which may have contributed to the higher CHD mortality. The other studies modelling, in cohorts such as the NHS, HPHS and others, the replacement of percentage of energy (generally 5 %) from SFA with PUFA have been discussed earlier (Substitution/reduction studies of SFA and CHD section). They generally showed a decrease in CHD events and/or mortality.
In summary, all these studies increased the PUFA:SFA ratio to decrease plasma cholesterol, so that the specific effect of n-6 PUFA towards CHD is not proved.
Meta-analyses
Ramsden et al. (Reference Ramsden, Hibbeln and Majchrzak83), in 2010, performed a meta-analysis including seven RCT responding to the following criteria: dietary PUFA were increased in replacement of SFA and/or TFA, and end points were non-fatal MI and CHD deaths. A separate analysis of whole datasets, of RCT with specific n-6 PUFA diet, and of RCT with mixed n-6/n-3 PUFA diets was made. For non-fatal MI + CHD death, the pooled risk reduction for mixed n-3/n-6 PUFA diets was 22 % (RR 0·78; 95 % CI 0·65, 0·93) compared with an increased risk of 13 % for n-6 specific PUFA diets (RR 1·13; 95 % CI 0·84, 1·53). Risk of non-fatal MI + CHD death was higher in n-6 specific PUFA diets compared with mixed n-3/n-6 PUFA diets (P=0·02). It was concluded that substitution of SFA with n-6 PUFA was not useful and even deleterious, whereas substituting with mixed n-6/n-3 PUFA was protective. Ramsden et al. (Reference Ramsden, Zamora and Majchrzak-Hong81), in 2016, have included the results of their recovered data from the Minnesota Coronary Experiment in an updated meta-analysis of LA intervention trials. There was a tendency towards an increased risk of death from CHD (RR 1·33; 95 % CI 0·99, 1·79; P=0·06). Farvid et al. (Reference Farvid, Ding and Pan84), in 2014, included thirteen cohort studies (310 602 individuals; 12 470 CHD events including 5882 CHD deaths). Comparing the highest v. the lowest category of intake, LA was associated with a lower total CHD risk (RR 0·85; 95 % CI 0·78, 0·92) and CHD deaths (RR 0·79; 95 % CI 0·71, 0·89). A 5 % energy increment in LA intake replacing the equivalent energy from SFA was associated with a 9 % lower risk of events (RR 0·91; 95 % CI 0·86, 0·96) and a 13 % lower risk of CHD deaths (RR 0·85; 95 % CI 0·82, 0·94). Hamley(Reference Hamley85), in 2017, performed a meta-analysis of nineteen RCT aiming to analyse the replacement of SFA with mostly n-6 PUFA. Five of them were adequately controlled. No association of replacement of SFA with n-6 PUFA was found with major CHD events (RR 1·06; 95 % CI 0·86, 1·31), total CHD events (RR 1·02; 95 % CI 0·84, 1·23) or CHD mortality (RR 1·13; 95 % CI 0·91, 1·40). The pooled results from all nineteen trials suggested that replacing SFA with mostly n-6 PUFA significantly reduced total CHD events (RR 0·80; 95 % CI 0·65, 0·98, P=0·03), but neither major CHD events nor CHD mortality. Hooper et al. (Reference Hooper, Al-Khudairy and Abdelhamid86), in 2018, performed a meta-analysis of twelve trials. n-6 PUFA generally replaced SFA or MUFA. They found no association with CHD events (RR 0·88; 95 % CI 0·66, 1·17; seven trials) or MI (RR 0·88; 95 % CI 0·76, 1·02; seven trials). Marklund et al. (Reference Marklund, Wu and Imamura87), in 2019, performed an individual-level pooled analysis of thirty cohort studies aiming to assess the association between biomarkers of dietary n-6 FA intake (in plasma and erythrocyte phospholipids, total plasma or cholesteryl esters, adipose tissue) and incident CVD and mortality. Mean age was 49–77 years, median follow-up was 2·5–31·9 years. Higher levels of LA were significantly associated with lower risks of total CVD and CV mortality, with RR per interquintile range of 0·93 (95 % CI 0·88, 0·99) and 0·78 (95 % CI 0·70, 0·85), respectively. By comparing extreme quintiles, higher levels of LA were associated with a significantly lower risk of CVD mortality (RR 0·77; 95 % CI 0·69, 0·86) but not of total CVD or CHD. Li et al. (Reference Li, Guasch-Ferré and Li88), in 2020, performed a meta-analysis of the association of dietary intake and biomarkers of LA with mortality including CVD mortality. They included forty-four prospective cohorts (eighteen reporting association of LA intake and twenty-two of LA biomarkers with mortality; 811 069 participants with dietary intake assessment (50 786 CVD deaths) and 65 411 participants with biomarker measurements (6492 CVD deaths)). The pooled RR comparing extreme categories of dietary LA intake was 0·87 (95 % CI 0·82, 0·92) for CVD mortality. The pooled RR for each 1 sd increment in LA concentrations in adipose tissue/blood compartments was 0·89 (95 % CI 0·85, 0·94) for CVD mortality. The different causes of CVD mortality are not reported.
In summary, n-6 PUFA are not deleterious for CV risk as suggested before, but are probably not cardioprotective per se. They contribute, in association with ALA, to decrease the CHD risk when replacing SFA. There is not much controversy about the fact that n-6 PUFA have been studied in substitution of SFA to decrease plasma cholesterol or associated with ALA as a component of plant PUFA, which can explain that their protective effect per se cannot be studied, on difference with ALA or LC n-3 PUFA (EPA/DHA) which can be added as a dietary supplementation and not as substitutes of other FA.
Long-chain n-3 PUFA and CHD
The CV protective effect of LC n-3 PUFA has become over time, as well as for SFA, a subject of hard controversy due mainly to an extreme heterogeneity of designs of RCT, further complicated by the multiplication of contradictory meta-analyses. They sometimes include studies of a very different nature, with very different populations, different doses given, different forms (fish oils, TAG, ethyl esters, others), EPA + DHA or EPA alone, sometimes associated with CV treatments (statin, aspirin), and different placebos.
Primary prevention
In the NHS, the highest quintile of LC n-3 PUFA intake was inversely associated with CHD events (RR 0·69; 95 % CI 0·57, 0·84; P<0·001)(Reference Hu, Bronner and Willett89). As to non-fatal MI, RR were 0·23 (95 % CI 0·09, 0·55; P=0·001) for plasma EPA and 0·46 (95 % CI 0·18, 1·16; P trend = 0·07) for DHA(Reference Sun, Ma and Campos90). Still in the NHS (30-year follow-up)(Reference Chiuve, Rimm and Sandhu91), sudden cardiac death was reduced by 37 % for the highest quintile of intake (RR 0·63; 95 % CI 0·44, 0·90; P trend = 0·03). In the Physicians’ Health Study, Albert et al. (Reference Albert, Campos and Stampfer92) (22 071 men; aged 40–80 years; 17 years of follow-up) found an inverse association of plasma LC n-3 PUFA with sudden cardiac death for the highest quartile of intake (RR 0·19; 95 % CI 0·05, 0·71; P trend = 0·001). Lemaitre et al. (Reference Lemaitre, King and Mozaffarian93) carried out a case–control study from the Cardiovascular Health Study. In those who died from MI, plasma phospholipid concentrations of EPA + DHA were lower than those of controls (RR 0·23; 95 % CI 0·12, 0·76; P=0·01), but no association was observed in those victims of a non-fatal MI. In the Ludwigshafen Risk and Cardiovascular Health Study(Reference Kleber, Delgado and Lorkowski94) (3316 patients hospitalised for coronary angiography; follow-up 9·9 years), the highest tertile of omega-3 index (sum of EPA + DHA/total FA in phospholipids of erythrocyte membranes)(Reference von Schacky95) was associated with a lower CHD mortality (RR 0·78; 95 % CI 0·64, 0·95; P=0·015). The Framingham Heart Study Offspring cohort(Reference Harris, Tintle and Etherton96) (2500 participants; mean age 66 years; follow-up of 7·3 years) found that an omega-3 index > 6·8 % was inversely associated with total CVD events (RR 0·61; 95 % CI 0·37, 0·99; P=0·008). The NIH-AARP-HS (617 119 participants aged 50–71 years; 16 years of follow-up)(Reference Zhuang, Zhang and He36) found that the highest quintile of intake was associated with a modest lower CHD mortality (RR 0·98; 95 % CI 0·97, 0·99). The Japan Public Health Centre-based study(Reference Hamazaki, Iso and Eshak97) (116 896 participants, aged 40–69 years, 14–17 years of follow-up) showed that the highest quartile of plasma concentrations of LC n-3 PUFA (divided into quartiles) was inversely associated with sudden cardiac death (RR 0·08; 95 % CI 0·01, 0·88; P trend = 0·04) and CHD mortality (RR 0·12; 95 % CI 0·02, 0·75; P trend = 0·03). ASCEND (A Study of Cardiovascular Events in Diabetes)(98) is a primary prevention study involving 15 480 diabetics followed for 7·4 years. Four groups were compared. Two groups received 460 mg EPA plus 380 mg DHA/d with (n 3870) or without (n 3870) concomitant 100 mg/d aspirin. There was a significant decrease in CHD mortality (RR 0·81; 95 % CI 0·67, 0·99). VITAL (Vitamin D and Omega-3 Trial)(Reference Manson, Cook and Lee99,Reference Manson, Bassuk and Cook100) is a primary prevention study aiming to evaluate the effect of daily vitamin D3 (2000 IU) or fish oil (460 mg EPA + 380 mg DHA) on primary prevention of cancer and CHD (in patients at high CHD risk; 25 871 US men aged ≥ 50 years and women aged ≥ 55 years, including 5106 African Americans). After a 5·3-year follow-up, LC n-3 PUFA supplementation had no effect on the composite end point (MI, stroke, CHD mortality), but was associated with significant reductions in total MI (RR 0·72; 95 % CI 0·59, 0·90), percutaneous coronary intervention (RR 0·78; 95 % CI 0·63, 0·95) and fatal MI (RR 0·50; 95 % CI 0·26, 0·97). In African Americans, MI was reduced by 77 % (RR 0·23; 95 % CI 0·11, 0·47). The UK Biobank study(Reference Li, Zhong and Liu101) (427 648 men and women aged 40–69 years from the general population living in England, Scotland and Wales; follow-up 8–12 years) assessed the association of habitual fish oil supplementation with CVD and mortality. RR associated with fish oil use was 0·84 (95 % CI 0·78, 0·91) for CVD mortality, 0·80 (95 % CI 0·70, 0·91) for MI mortality, 0·93 (95 % CI 0·90, 0·96) for CVD, and 0·92 (95 % CI 0·88, 0·96) for MI (all P<0·05). The association was stronger among participants with hypertension (P=0·005).
Secondary CHD prevention
The Diet and Reinfarction Trial(Reference Burr, Gilbert and Holliday102) (2033 patients; 2-year follow-up) showed that the consumption of 200–400 g/week of fatty fishes was inversely associated with CHD mortality (RR 0·71; 95 % CI 0·54, 0·93; P<0·01), but not new CHD events. The GISSI-Prevenzione trial(103) (11 324 patients with MI within the previous 3 months before inclusion, followed for 3·5 years) showed that 850 mg/d of EPA+DHA ethyl esters reduced the composite end point (CV death, non-fatal MI, and non-fatal stroke) by 15 % (RR 0·85; 95 % CI 0·68, 0·95; P=0·008), and CHD deaths by 35 % (RR 0·65; 95 % CI 0·51, 0·84). The Japan EPA Lipid Intervention Study (JELIS)(Reference Yokoyama, Origasa and Matsuzaki104) was both a primary (n 14 981) and secondary CHD prevention Japanese study (n 3664 patients, plasma cholesterol ≥ 2·52 g/l, follow-up: mean 4·6 years). EPA (1·8 g/d) plus statin, as compared with statin alone, reduced CHD events by 19 % in the whole group (RR 0·81; 95 % CI 0·69, 0·95; P=0·011). The decrease was significant for the ‘secondary prevention’ group (RR 0·81; 95 % CI 0·66, 1·00; P=0·048), but not for the ‘primary prevention group’ (RR 0·82; 95 % CI 0·63, 1·06; P=0·132). In a subsequent analysis, it was shown that EPA decreased risk of CHD by 38 % in subjects who did not achieve the objective of non-HDL and/or of LDL-cholesterol plasma concentrations with statin(Reference Sasaki, Yokoyama and Matsuzaki105). OMEGA(Reference Rauch, Schiele and Schneider106) (4837 patients; aged 60–80 years with a history of MI; follow-up 40 months) found no effect of a small dose of EPA+DHA (400 mg/d). The SUpplementation with FOLate, vitamins B6 and B12 and/or OMega-3 fatty acids (SU.FOL.OM3) trial(Reference Galan, Kesse-Guyot and Czernichow107) assessed the effects of vitamins B and EPA+DHA (600 mg/d) (2501 patients with a recent history of ischaemic stroke; follow-up 4·7 years). No effect of EPA+DHA was observed. The Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial(108) studied the effects of EPA+DHA supplementation (840 mg/d) on CV deaths and CHD events in 12 536 pre-diabetic or diabetic patients, followed for 6·2 years. No effect was observed. The Risk and Prevention Study(109) (12 513 patients, 5 years of follow-up) assessed the efficacy of a 1 g/d intake of EPA+DHA (ethyl esters). No benefit was observed for whole patients but the main composite end point (mortality, MI and non-fatal stroke) was decreased in women (RR 0·82; 95 % CI 0·67, 0·99; P=0·04). The Cardiovascular Outcome Study, an ancillary study of the Age-Related Eye Disease Study 2 study(Reference Bonds, Harrington and Worrall110) (4203 patients; aged 50–85 years; 4·8 years of follow-up), found no benefit of 1 g/d EPA+DHA. The following studies used pharmaceutical forms of EPA, beyond the scope of our paper, but we decided to include them because of their novelty. The Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT)(Reference Bhatt, Steg and Miller111) included 8179 patients with a prior CHD history (70 % of patients) or not (secondary + primary prevention subgroups), all with plasma TAG ≥ 2 g/l and receiving a statin; 58 % had diabetes. Patients received either 4 g/d of EPA icosapentethyl or placebo. After a median follow-up of 4·9 years, the primary composite end point (non-fatal CHD, MI and stroke mortality, coronary revascularisation or unstable angina) was reduced by 25 % with EPA icosapentethyl (P<0·001). The effect was significant only in the secondary prevention cohort. The key secondary composite end point was reduced by 26 % (P<0·001). All pre-specified end points were also reduced with EPA: CV death or non-fatal MI by 25 % (P<0·001), fatal or non-fatal MI by 31 % (P<0·001), urgent or emergency revascularisation by 35 % (P<0·001), CV death by 20 % (P=0·03), hospitalisation for unstable angina by 32 % (P=0·002), fatal or non-fatal stroke by 38 % (P=0·01). REDUCE-IT USA(Reference Bhatt, Miller and Brinton112) analysed the results of the REDUCE-IT trial in the group of the 3146 US patients only (38·5 % of the trial). The primary composite end point was reduced by 31 % (P=0·000001) as well as the key secondary composite end point (P=0·00008). CHD death was reduced by 34 % (P=0·007), and MI by 28 % (P=0·01). The STRENGTH (STatin Residual Risk Reduction With EpaNova in HiGh CV Risk PatienTs With Hypertriglyceridemia) trial(Reference Nicholls, Lincoff and Garcia113) aimed to evaluate the effects of 4 g daily of carboxylic acids of EPA+DHA compared with maize oil as a placebo, on major adverse cardiovascular events (MACE) in patients on optimal statin therapy with mixed dyslipidaemia and at high risk for CVD. It has been prematurely halted based on an interim analysis indicating a low probability of clinical benefits of EPA+DHA carboxylic acids. A total of 13 078 patients at high CV risk (aged 62·5 ± 9 years, 70 % with diabetes and at least 50 % with criteria of secondary CV prevention), with mixed dyslipidaemia (hypertriacylglycerolaemia: 180 mg/dl (2·03 mmol/l) to 500 mg/dl (5·65 mmol/l); HDL-cholesterol < 42 mg/dl (< 1·08 mmol/l) in men and < 47 mg/dl (< 1·22 mmol/l) in women), and all treated with statins (LDL-cholesterol < 100 mg/dl (< 2·59 mmol/l)) were enrolled at 675 sites in twenty-two countries. After 54 months, RR for MACE was 0·99 (95 % CI 0·90, 1·09). Several explanations have been proposed to explain the discrepant results with those of the REDUCE-IT trial: lower duration of STRENGTH, maize oil v. mineral oil as placebo, carboxylic acids of EPA + DHA v. EPA icosapentethyl, 70 % of patients with diabetes v. 58 %. Even if the design of the STRENGTH and REDUCE-IT trials look very similar, the absence of an effect observed in the STRENGTH trial, for whatever reason, does not invalidate the protective effect observed in the REDUCE-IT study. The United States Food & Drug Administration(114) approved, in December 2019, the use of icosapentethyl of EPA as an adjunctive therapy to reduce the risk of CV events in patients meeting the inclusion criteria of REDUCE-IT.
Meta-analyses
We will only pick up the recent largest ones. For others, see Innes & Calder(Reference Innes and Calder115). The very large meta-analysis by Alexander et al. (Reference Alexander, Miller and Van Elswyk116), in 2017, included eighteen RCT and sixteen prospective cohort studies. The overall meta-analysis of RCT found no association of LC n-3 PUFA with CHD events (RR 0·94; 95 % CI 0·85, 1·05). In contrast, LC n-3 PUFA reduced significantly the risk of CHD events in subgroups of patients at high CV risk with plasma TAG ≥ 1·5 g/l (RR 0·84; 95 % CI 0·72, 0·98) or LDL-cholesterol ≥ 1·30 g/l (RR 0·86; 95 % CI 0·76, 0·98). An EPA + DHA dose > 1 g/d lowered the risk in those with plasma TAG ≥ 1·50 g/l (RR 0·75; 95 % CI 0·64, 0·89). CHD deaths were reduced by 19 % (RR 0·81; 95 % CI 0·65, 1·00) only in secondary prevention (RR 0·80; 95 % CI 0·64, 0·99). As to cohorts, CHD events were reduced by 18 % (RR 0·82; 95 % CI 0·74, 0·92) for the highest intakes, by 23 % for CHD mortality (RR 0·77; 95 % CI 0·66, 0·90), and by 47 % for sudden cardiac death (RR 0·53; 95 % CI 0·41, 0·67). The meta-analysis by Maki et al. (Reference Maki, Palacios and Bell117), in 2017, assessed CV mortality alone and included fourteen RCT (71 899 subjects; follow-up ≥ 6 months). The overall analysis concluded a decrease in 8 % of CHD mortality (RR 0·920; P=0·015). The risk reduction was more pronounced (–13 to – 29 %; RR 0·709–0·871) for a dose > 1 g/d (28 % of all subjects included), in patient groups with plasma TAG ≥ 1·50 g/l, LDL-cholesterol ≥ 1·30 g/l, and in RCT where the proportion of patients treated with statins at inclusion was < 40 % (all significant). These results are consistent with those of Alexander et al. (Reference Alexander, Miller and Van Elswyk116). The meta-analysis by Hu et al. (Reference Hu, Hu and Manson118), in 2019, included thirteen RCT (127 477 participants; mean follow-up 5 years). When excluding REDUCE-IT, LC n-3 PUFA were inversely associated with MI (RR 0·92; 95 % CI 0·86, 0·99; P=0·020), CHD deaths (RR 0·92; 95 % CI 0·86, 0·98; P=0·014) and total CHD (RR 0·95; 95 % CI 0·91, 0·99; P=0·008). Including REDUCE-IT reinforced the inverse associations. CHD and CHD death were linearly related to LC n-3 PUFA dose. The meta-analysis by Popoff et al. (Reference Popoff, Balaciano and Bardach119), in 2019, included eleven RCT, carried out from 1966 to 2016, in patients who had suffered a MI. They had been randomised to receive within 6 weeks after MI to receive n-3 FA from very different sources (fish oils, soyabean oils, seeds, refined EPA, DHA, ALA) or, oil or capsule form or as foodstuffs (MedDiet). The authors conclude that there were no benefits to patient important outcome. However, CV mortality was significantly reduced (RR 0·77; 95 % CI 0·65, 0·91), as well as risk of new MI (RR 0·77; 95 % CI 0·6, 0·90). Aung et al. (Reference Aung, Halsey and Kromhout120), in 2018, performed a meta-analysis including ten trials involving 77 917 individuals at high CV risk, followed for a mean of 4·4 years. The range of EPA dose supplementation was 226–1800 mg/d. They found no significant associations with CHD death rate (RR 0·93; 99 % CI 0·83, 1·03; P = 0·05), non-fatal MI (RR 0·97; 99 % CI 0·87, 1·08; P = 0·43) or any CHD events (RR 0·96; 95 % CI 0·90, 1·01; P = 0·12).
The meta-analysis of RCT by Abdelhamid et al. (Reference Abdelhamid, Brown and Brainard76), in 2020, focused on the effect of LC n-3 PUFA in primary and secondary CHD prevention. LC n-3 PUFA reduced CHD events by 9 % (134 116 participants; 8791 events; RR 0·91; 95 % CI 0·85, 0·97; thirty-two RCT), and CHD mortality by 10 % (127 378 participants; 3598 deaths; RR 0·90; 95 % CI 0·81, 1·00; twenty-four RCT). The meta-analysis by Doshi et al. (Reference Doshi, Kumar and Thakkar121), in 2020, aimed to compare the effects of combined use of EPA + statin with statin alone. Five RCT were included (27 415 patients; dose of EPA ≥ 1800 mg/d) associated or not with a usual dose of statin. EPA + statin resulted in an 18 % reduction in the incidence of MACE (RR 0·78; 95 % CI 0·65, 0·93; P<0·01) and a 30 % reduction in MI (RR 0·71; 95 % CI 0·61, 0·82; P<0·01) as compared with statin alone. Hoang & Kim(Reference Hoang and Kim122), in 2020, performed a meta-analysis of eighteen RCT using LC n-3 supplements for at least 1 year. They decreased CHD by 10 % (RR 0·90; 95 % CI 0·84, 0·96) and MI by 12 % (RR 0·88; 95 % CI 0·83, 0·94). Lombardi et al.(Reference Lombardi, Chiabrando and Vescovo123) included fourteen RCT (125 763 patients). They compared a high dose (> 1 g/d of LC n-3 PUFA supplementation v. control or v. a low dose ≤ 1 g/d). The high dose was associated with a 21 % decrease in cardiac mortality (RR 0·79; 95 % CI 0·65, 0·96; P=0·03 v. control), a 29 % decrease in MI (RR 0·71 (95 % CI 0·62, 0·82); P<0·0001 v. control; and 0·79 (95 % CI 0·67, 0·92); P=0·003 v. low dose), a 26 % decrease in coronary revascularisation (RR 0·74 (95 % CI 0·66, 0·83); P < 0·0001 v. control; and 0·74 (95 % CI 0·66, 0·84); P < 0·0001 v. low dose) and a 27 % decrease in unstable angina (RR 0·73 (95 % CI 0·62, 0·86); P = 0·0001 v. control; and 0·74 (95 % CI 0·62, 0·89); P=0·002 v. low dose). Casula et al. (Reference Casula, Olmastroni and Gazzotti124), in 2020, included sixteen RCT (81 073 patients with previous CV events, dose of LC n-3 PUFA ≥ 1 g/d for ≥ 1 year). LC n-3 PUFA supplementation was associated with a 9 % decrease in cardiac mortality (RR 0·91; 95 % CI 0·85, 0·98), a 9 % decrease in MACE (RR 0·90; 95 % CI 0·82, 0·99) and a 17 % decrease in MI (RR 0·83; 95 % CI 0·71, 0·98). Cabiddu et al.(Reference Cabiddu, Russi and Appolloni125), in 2020, performed a trial-sequential analysis of eleven RCT in primary/secondary prevention (100 609 patients; EPA + DHA dose: 376–2000 mg/d). Cardiac mortality was reduced by 6·3 % (RR 0·937; 95 % CI 0·88, 0·98; P=0·018). They also concluded from their analysis that no further trials were needed to better evaluate the efficacy of LC n-3 PUFA in preventing cardiac mortality. Bernasconi et al. (Reference Bernasconi, Wiest and Lavie126), in 2020, performed a meta-analysis and meta-regression of all forty RCT (135 267 participants) published before August 2019 evaluating the effects of an intervention consisting exclusively in dietary supplementation with EPA/DHA (dosage: 400 mg/d to 5500 mg/d, weighted average dosage: 1221 mg/d) on CV outcomes. The authors extracted the data from a meta-analysis by Abdelhamid et al. (Reference Abdelhamid, Brown and Brainard76), except for four studies with EPA/DHA dietary advice only. Supplementation was associated with a 13 % decrease in MI (RR 0·87; 95 % CI 0·80, 0·96), a 10 % decrease in CHD events (RR 0·90; 95 % CI 0·84, 0·97), a 35 % decrease in fatal MI (RR 0·65; 95 % CI 0·46, 0·91) and a 9 % decrease in CHD mortality (RR 0·91; 95 % CI 0·85, 0·98). Meta-regression showed that the effect was dose dependent for MI (each additional 1 g/d was associated with a significant risk reduction of 9·0 %) and for CHD mortality and fatal MI (protective effect achieved with less than 800–1200 mg/d, then quickly plateauing). The authors were unable to conclude that EPA alone had a superior effect to EPA+DHA. They found no evidence that the effect magnitude for any of the CV outcomes studied depended on the year of publication of the RCT. Regarding the effectiveness of EPA/DHA in primary or secondary prevention, most trials have been performed in patients at high CV risk, so that it can be concluded that EPA/DHA supplementation was protective in secondary prevention. However, the fact that the effect of supplementation did not increase with baseline risk in that meta-analysis provides, according to the authors, some confidence that findings about effectiveness can be generalised to prevention in lower-risk populations.
In summary, prospective cohort studies in primary prevention are all consistent in concluding that LC n-3 PUFA are protective in primary prevention for the highest or intermediate intakes, confirmed by meta-analysis by Alexander et al. (Reference Alexander, Miller and Van Elswyk116). Most large RCT v. placebo also support a CHD protective effect in secondary prevention with about a 20 % decrease in CHD death and 30 % decrease in CHD events with doses of EPA + DHA ≥ 900 mg/d in secondary CHD prevention. This is confirmed by several recent meta-analyses(118,120–126). Negative studies for some of them can be criticised for their lack of power and/or by the use of OO as a placebo. In addition, it should be noted that CV drugs (statins, aspirin and post-infarction medications) have probably masked partially the effects of LC n-3 PUFA. The populations likely to benefit the most in secondary prevention are those at high CV risk with in particular hypertriacylglycerolaemia (≥ 1·50 g/l) and/or high LDL (≥ 1·30 g/l)(Reference Alexander, Miller and Van Elswyk116). The EPA + DHA dose that appears to be effective is the one at least for cardiac mortality that makes it possible to achieve an omega-3 index of ≥ 8 %(Reference von Schacky127), corresponding to an intake of approximately ≥ 900 mg/d of EPA + DHA, which can be achieved by a multiple-week intake of marine products at high concentrations of LC n-3 PUFA. The recommendations of the American Heart Association expert panel that revised the previous recommendations in 2017 are in agreement with this(Reference Siscovick, Barringer and Fretts128). The reasons for the controversy on the CV protective effect of EPA/DHA have been cited above, but it is now, in 2020, in view of the consistent results of the seven most recent meta-analyses (the last one including forty RCT), very difficult to assert that LC n-3 PUFA do not have a protective effect against CHD, especially in patients at high CV risk. It is very unlikely that the negative result of the recent STRENGTH trial will significantly change this conclusion when included in a further meta-analysis, although it is useful to better understand why no effect was observed. In all cases, LC n-3 PUFA should be associated with lifestyle modifications and specific management/treatment of other risk factors.
Are the recommendations concerning fatty acid intakes for cardiovascular prevention useful today?
The answer is both yes and no. Yes, because it is always useful to assess on the basis of basic, epidemiological and nutritional sciences what specific nutrients can be beneficial or deleterious. Typical examples are the proved deleterious effect of TFA. No, because assessing and/or proposing recommendations for specific nutrient intakes have some pitfalls, especially the discrepant results of CHD effect of SFA, MUFA, n-6 PUFA, ALA and even LC n-3 PUFA per se although for these last controversy is less marked in 2020 than in the past years. From this observation, the whole food pattern may be more important to consider(Reference Hu129). Indeed, as underlined by Martínez-González et al. (Reference Martínez-González, Gea and Ruiz-Canela130), this approach limits confounding by individual dietary factors and captures the synergistic effects of individual foods and nutrients. It may also provide a more powerful tool to assess the effect of dietary habits on CV health because the effect of a single dietary element is likely to be too small as to be detected in epidemiological studies or RCT. Furthermore, specific nutrient recommendations such as an intake of FA as percentage of energy is not very pertinent for counselling a healthy population and individuals at high CHD risk or with a prior history of CHD, because almost nobody is able to calculate a percentage of energy from specific FA in her/his diet. So, if the response for that reason to our question is no, what dietary pattern(s) should we propose to them? Certainly not the Western diet, which increases CHD risk as compared with other types of dietary pattern(Reference Iqbal, Anand and Ounpuu131–Reference Brunner, Mosdøl and Witte134).
If we come back to the Seven Countries Study, cohorts with the lowest CHD incidence during follow-up were those from Tanushimaru and Ushibuka (Japan) and those from Mediterranean areas (Dalmatia, Montegiorgio, Crevalcore, Crete and Corfu). It is of special interest to note that both the MedDiet and Japanese Dietary Culture were inscribed the same year (2013) in the List of the Intangible Cultural Heritage of Humanity(135,136) . Thus, can the MedDiet or the JapDiet be considered as the best one(s) if not panacea(s)?
Mediterranean diet and CHD
Randomised clinical trials
Three RCT compared the effects of a MedDiet with another diet on CVD, but two of them for different reasons have been criticised. The Lyon Diet Heart study was a secondary prevention French single-blind trial, first reported in 1994 after a mean of 27 months of follow-up(Reference de Lorgeril, Renaud and Mamelle137). Patients with previous MI were separated into two groups. The experimental group (n 302) received a Mediterranean-type diet: more bread, more root vegetables and green vegetables, more fish, less meat (beef, lamb and pork to be replaced with poultry, no day without fruit. Butter and cream had to be replaced with a rapeseed (canola) oil-based margarine whose composition was comparable with OO with 15 % SFA, 48 % oleic acid but 5–4 % 18 : 1 TFA, 16·4 % LA and 4·8 % ALA). Seasoning oils were rapeseed oil and OO exclusively. Moderate wine consumption was allowed at meals. The control group (n 303) received a low-fat step-1 diet of the National Cholesterol Education Program for secondary prevention. The National Cholesterol Education Program diet recommends less than 30 % of energy from total fat, less than 10 % from saturated fat, and less than 300 mg/d of cholesterol. As compared with the control group, the RR of the ‘MedDiet’ group for CV deaths and CV deaths + non-fatal MI were 0·24 (95 % CI 0·07, 0·85) and 0·27 (95 % CI 0·12, 0·59), respectively. In a further paper, de Lorgeril et al. (Reference de Lorgeril, Salen and Caillat-Vallet138) excluded bias possibly due to the single-blind randomisation. The extension to 4 years of follow-up of the trial was published in 1999(Reference de Lorgeril, Salen and Martin139) and its results confirm the previous ones. RR for major primary end points (cardiac deaths and cardiac deaths + non-fatal MI) were 0·35 (95 % CI 0·15, 0·83; P=0·01) and 0·28 (95 % CI 0·15, 0·53; P=0·0001), respectively. It is to note that at the end of the study plasma total cholesterol, LDL-cholesterol, HDL-cholesterol and TAG plasma concentrations were not different between the two groups. However, criticisms have been made. The diet was not strictly a MedDiet because of the addition of the margarine rich in ALA on one hand, and because no sufficient consideration was given to OO with an amount of MUFA of 12·9 %, below the usual 15–20 % of the traditional MedDiet on the other hand. Other criticisms formulated were: wide CI and relatively small number of events. In a statement of three American Heart Association committees(Reference Kris-Etherton, Eckel and Howard140), these others criticisms have been made: the baseline diet was only assessed in the experimental group at the beginning of the study, and the diet of the control group at baseline was presumed to be comparable. Nutrient intake in the control group was only assessed at the conclusion of the study so that it is not clear whether the control group made any dietary changes. In addition, dietary data were reported for only 27 % of the control group and 48 % of the experimental group. It cannot be excluded that the results of the Lyon Diet Heart Study appeared too good to be true (70 % decreased risk!), but, in spite of criticisms made, it would be excessive to consider this trial as discredited.
The Indo-Mediterranean Diet Heart Study(Reference Singh, Dubnov and Niaz141) was both a primary (patients with surrogate markers of CV risk) and secondary prevention trial (patients with a history of CHD) with a 2-year follow-up. All patients were randomly allocated to two groups: one receiving an Indo-Mediterranean Diet (IMedDiet) (n 499) and the other as control a low-fat step 1 National Cholesterol Education Program diet (n 501). The IMedDiet was rich in whole grains, fruits, vegetables, walnuts and almonds (250–300 g/d of fruit, 125–150 g/d of vegetables and 25–50 g/d of walnuts or almonds). The experimental group was also encouraged to eat 400–500 g/d of whole grains, legumes, rice, maize and wheat, as well as mustard seed or soyabean oil, in 3–4 servings per d). The RR for non-fatal MI, fatal MI, sudden cardiac death, and total cardiac end points were 0·47 (95 % CI 0·28, 0·79), 0·67 (95 % CI 0·31, 1·42), 0·33 (95 % CI 0·13, 0·86) and 0·48 (95 % CI 0·33, 0·71), respectively. However, The Lancet published in 2015 an expression of concern(Reference Horton142) because of lack of clarity about original data; other concerns have been addressed by Martínez-González et al. (Reference Martínez-González, Gea and Ruiz-Canela130) considering it was reasonable to consider the IMedDiet study as discredited, even though it has been included in many reviews and meta-analyses. Such an inclusion questions the validity of the conclusions of these meta-analyses.
The PREvencion con DIeta MEDiterranea (PREDIMED) Study(Reference Estruch, Ros and Salas-Salvadó143) is the more recent RCT assessing the effects of a MedDiet for primary CV prevention in 7447 participants with a high CV risk (median follow-up: 4·8 years). They were assigned to a MedDiet supplemented with extra-virgin OO (increase in extra-virgin OO intake to 50 g/d), or a MedDiet supplemented with mixed nuts (increase in extra-virgin OO intake to 32 g/d plus in nuts from 0·9 to > 6 servings/week), or a control diet (advice to reduce dietary fat) (Table 4). The primary end point was a major CV event (MI, stroke, or CV death). RR for the primary end point was 0·70 (95 % CI 0·55, 0·89). RR for the secondary end points were for stroke: 0·58 (95 % CI 0·42, 0·82), for MI: 0·80 (95 % CI 0·53, 1·21), and for CV deaths: 0·80 (95 % CI 0·51, 1·24). Thus, the RR for the composite end point was reduced by 30 %, but when separating the secondary end points, it was significant for stroke only, with a reduction of 42 % of risk.
Table 4. Experimental (Mediterranean) diet used during the PREvencion con DIeta MEDiterranea (PREDIMED) study (adapted from Estruch et al. (Reference Estruch, Ros and Salas-Salvadó143))

* In the group assigned to the Mediterranean diet with extra-virgin olive oil, the goal was to consume 50 g (approximately 4 tablespoons) or more per d of the polyphenol-rich olive oil supplied.
† For participants assigned to the Mediterranean diet with nuts, the recommended consumption was one daily serving (30 g, composed of 15 g of walnuts, 7·5 g of almonds and 7·5 g of hazelnuts).
Meta-analyses
Sofi et al. (Reference Sofi, Macchi and Abbate144), in 2013, performed a meta-analysis of prospective cohorts studies (4 172 412 subjects) assessing the association of adherence to a MedDiet with CVD incidence and/or mortality risk. A two-point increase in adherence score to the MedDiet was associated with a 10 % reduction of CV risk (RR 0·90; 95 % CI 0·87, 0·92). In 2017, Grosso et al. (Reference Grosso, Marventano and Yang145) performed a meta-analysis of prospective studies and cohorts comparing adherence to a MedDiet with CHD events and CHD deaths. It is noteworthy that the criticised Indo-Mediterranean Diet Heart Study was included(Reference Brunner, Mosdøl and Witte134), CHD incidence was reported in four prospective studies (RR 0·72; 95 % CI 0·60, 0·86) and MI incidence in three (RR 0·67; 95 % CI 0·54, 0·83). After pooling four RCT, MI incidence, stroke incidence, CVD mortality and composite end point were all significantly decreased by 40, 36, 41 and 45 %, respectively. Liyanage et al. (Reference Liyanage, Ninomiya and Wang146), in 2016, included six RCT comparing the MedDiet with a control diet (10 950 participants). Three RCT, including 9052 participants and 477 events, reported major CV events (RR 0·63; 95 % CI 0·53, 0·75; P<0·001). Exclusion of the Indo-Mediterranean Diet Heart Study did not alter the direction of the effect (RR 0·69; 95 % CI 0·55, 0·86; P<0·001). No effect on CV mortality was observed (four studies; 10 623 participants and 315 deaths; RR 0·90; 95 % CI 0·72, 1·11; P=0·32). Three RCT reported 221 CHD events. The MedDiet was associated with a 35 % decrease in CHD events (RR 0·65; 95 % CI 0·50, 0·85) but the RR was no more significant when the Indo-Mediterranean Diet Heart Study was excluded from analysis. Rosato et al. (Reference Rosato, Temple and La Vecchia147), in 2017 (published in 2019), included twenty-nine observational studies. Comparing the highest with the lowest MedDiet score, they found for total CVD (eleven studies) a RR of 0·81 (95 % CI 0·74, 0·88), and for CHD/MI (eleven studies) a RR of 0·70 (95 % CI 0·62, 0·80). Galbete et al. (Reference Galbete, Schwingshackl and Schwedhelm148), in 2018, performed an umbrella review of meta-analyses of cohort studies evaluating the association of the MedDiet (by using adherence to a Mediterranean Diet Score) with type 2 diabetes, CVD, cancer and cognitive-related diseases. They included twenty-seven meta-analyses based on seventy primary studies. Regarding CVD end points, they found twelve meta-analyses including a total of thirty-one primary studies. A two-point increase in adherence to the MedDiet score was associated with a 28 % lower risk of CHD (RR 0·72; 95 % CI 0·60, 0·86), MI (RR 0·67; 95 % CI 0·54, 0·83) and a 26 % lower risk of acute MI (RR 0·74; 95 % CI 0·66, 0·83). The authors indicate that the MedDiet adherence score utilised was heterogeneous; the two more used scores in primary studies were that proposed by Trichopoulou et al. (Reference Trichopoulou, Costacou and Bamia149) and that proposed by Fung et al. (Reference Fung, Rexrode and Mantzoros150), but this did not alter significantly the results. Rees et al. (Reference Rees, Takeda and Martin151), in 2019, performed a Cochrane meta-analysis of RCT aiming to study the effects of a Mediterranean-style diet in primary prevention in subjects at high CV risk and in secondary prevention. The intervention could be dietary advice, provision of relevant foods, or both. The comparison group received no intervention, minimal intervention, usual care or another dietary intervention. Outcomes included clinical events and CVD risk factors. The follow-up periods were of 3 months or more defined as the intervention period plus post-intervention follow-up. The meta-analysis included for primary prevention nine trials (1337 participants) comparing a Mediterranean dietary intervention v. no intervention or minimal intervention for primary prevention and thirteen trials comparing the MedDiet with another diet (8687 participants), and for secondary prevention two trials (706 participants) comparing the MedDiet with usual care and six trials (1731 participants). The authors observed a great heterogeneity and high risk of bias. The authors’ conclusion is that the quality of evidence for the modest benefits on CVD risk factors in primary prevention is low or moderate, with a small number of studies reporting minimal harms and that there is a paucity of evidence for secondary prevention. Recently, O’Keefe et al. (Reference O’Keefe, Torres-Acosta and O’Keefe152) proposed that a pesco-Mediterranean diet could be more cardioprotective because of associating the benefits of seafood rich in LC n-3 PUFA with that of the traditional MedDiet.
In summary, the MedDiet has clearly a protective effect towards CHD in primary prevention (decreased risk by 10 to 30 %). In secondary prevention, the paucity of trials necessitates further ones.
Japanese diet and CHD
In 1958, at the initiation of the Seven Countries Study, the incidence of CHD and plasma cholesterol levels of the two Japanese cohorts were the lowest among the eleven included(Reference Keys, Aravanis and Blackburn153). Adachi et al. (Reference Adachi, Enomoto and Fukami154) have recently studied over 60 years the trends in nutritional intake and CHD risk factors among Japanese men in Tanushimaru, one of the two Japanese cities included in the Seven Countries Study. Tanushimaru is a farming city located on Kyushu island, in the south-western island of Japan. For that purpose, they compared the data of seven independent successive cohorts from 1958 to 2018 (1958, 1977, 1982, 1989, 1999, 2009 and 2018). In parallel, total plasma cholesterol increased from 1·68 g/l to 2·09 g/l, and BMI from 21·7 to 24·4 kg/m2. By comparing, in three cohorts (1958, 1977 and 1999), 15-year mortality of stroke and MI, the authors found a decrease in stroke mortality from 4·6/1000 participants in cohort 1958 to 0·8/1000 participants and 0·7/1000 participants in cohorts 1977 and 1999, respectively. MI mortality and sudden deaths remained relatively stable. Thus, stroke and MI deaths drastically decreased or remained stable, respectively, in regard of an increase in fat intake, plasma cholesterol and BMI. Other Japanese studies showed no significant increase in CHD incidence over 20 years since the mid-1960s(Reference Toshima155,Reference Tanizaki, Kiyohara and Kato156) except for the metropolitan cities of Tokyo and Osaka where westernisation is more marked than in the rest of Japan(Reference Iso157). Furthermore, while the incidence of CHD decreased over the same period in the USA(Reference Weir, Anderson and Coleman King158,Reference Benjamin, Muntner and Alonso159) , CHD in Japan remains approximately one-fourth of that of USA(Reference Finegold, Asaria and Francis160), in regard of a higher prevalence of hypertension, smoking, stress and lower physical activity in Japanese than in their American counterparts(Reference Adachi, Enomoto and Fukami154); Japanese have also almost the same level of plasma cholesterol as Americans(Reference Adachi, Enomoto and Fukami154). Altogether, these observations suggest that the whole dietary pattern of Japanese could be protective. The WHO-Coordinated Cardiovascular Diseases and Alimentary Comparison (CARDIAC) Study(Reference Yamori, Miura and Taira161) has been carried out in sixty-one communities from twenty-five countries around the world (1983–2005). It aimed to confirm the association of diets rich in seafood and soyabeans with the healthy longevity of Japanese. It showed a positive association between plasma cholesterol and CHD on one hand, and an inverse association with 24 h urinary excretion of taurine (an amino acid contained in fish proteins) (Fig. 2), plasma LC n-3 PUFA, and 24 h urinary excretion of isoflavones (an index of soya product intake) (Fig. 3) on the other hand. It is noteworthy that a high seafood/fish consumption (≥ 86 g/d) abolished the positive relationship between heavy smoking and CHD risk in a population of Japanese without history of CHD followed for 5 years (878 163 person-years)(Reference Yamori, Miura and Taira161–Reference Yamori, Liu and Mizushima163).

Fig. 2. Relationship between age-adjusted IHD mortality rate and 24 h urinary taurine excretion (from Yamori et al. (Reference Yamori, Liu and Mizushima163)). Au, Perth, Australia; Bu, Sofia, Bulgaria; Ca, St Johns, Canada; Cs, Shijiazhuang, China; Ch, Shanghai, China; Ct, Taiwan, China; Eq, Quito, Ecuador; Fk, Kuopio, Finland, rural; Fu, Kuopio, Finland, urban; Fr, Orleans, France; Ge, Tbilisi, Georgia; Is, Tel Aviv, Israel; Ja, Amino, Japan; Jb, Beppu, Japan; Jo, Ohda, Japan; Jk, Okinawa, Japan; Jt, Toyama, Japan; Nz, Dunedin, New Zealand; Po, Lisbon, Portugal; Ru, Moscow, Russia; Sp, Madrid, Spain; Sw, Göteborg, Sweden; Us, Stornoway, UK; Ub, Belfast, UK; Uw, Western Isles, UK.

Fig. 3. Relationship between 24 h urinary isoflavone excretion and age-adjusted mortality rates of CHD (from Yamori et al. (Reference Yamori, Miura and Taira161)). GBR, Great Britain; NZ, New Zealand; SW, Sweden; CIS, Commonwealth of Independent States (Russia); BLG, Belgium; ECD, Ecuador; PO, Portugal; SPN, Spain; PRC, People’s Republic of China; JPN, Japan.
Characteristic components of the traditional JapDiet (Washoku) include rice, miso soup, soyabean products, vegetables, fruits, fish, Japanese pickles, seaweed, mushrooms and green tea(Reference Adachi, Enomoto and Fukami154) customarily eaten using chopsticks and wooden bowls. Gabriel et al. (Reference Gabriel, Ninomiya and Uneyama164) detailed the arguments in favour of the beneficial health benefits of the JapDiet. ‘The style of eating in Washoku – a large variety of foods, small portions, the inclusion of soups, abundant vegetables, the cooking method, the large content of water, and the effective usage of umami taste – promotes not only the pleasant experience of eating, combined with the large incorporation of bioactive compounds from vegetables, but also ensures an adequate signal for satiety that prevents overeating.’ The other components of diet are various types of seafood/fish that are a rich source of high-quality proteins (rich in taurine) as well as LC n-3 PUFA and soyabean-based foods (fermented miso and tofu). Miso soup and salted vegetables account for a persistent high intake of Na of Japanese even if it has decreased markedly since the 1950s. Conversely, the high intake of K is protective. ‘In summary, the main elements of Washoku that promote positive health outcomes are: (1) the great variety of seasonal foodstuffs, including vegetables and fishes; (2) the way of cooking dishes based on large amounts of high quality water; (3) the well-balanced nutrition; and finally (4) the value of its connection with health and family ties.’(Reference Gabriel, Ninomiya and Uneyama164)
When compared with the MedDiet, we lack interventional studies or RCT demonstrating the protective effect of the traditional JapDiet towards CHD. Beyond that, the two diets have common characteristics but also different ones. The JapDiet has a lower amount of fat and a higher amount of seafood/fish, soya and salt than the MedDiet(Reference Yamori, Liu and Mizushima163). The MedDiet has been proved to be sustainable(Reference Dernini, Berry and Serra-Majem165) while the JapDiet has not yet demonstrated it is, even if Gabriel et al.(Reference Gabriel, Ninomiya and Uneyama164) suggested that because of its composition it could be. The MedDiet is more easily transposable to Westerners because it is more easily acceptable owing to their usual habits and foodstuffs available than the traditional JapDiet. On the other hand, the JapDiet itself should be revitalised for Asians because several epidemiological data plead for its contribution to the low incidence of CHD disease especially in Japanese provided that salt intake reach the current recommendations (about 6 g/d).
Conclusion
With the exception of TFA, whose harmfulness has been unanimously recognised, discrepancies remain as to the effect of other types of FA on CHD. These discrepancies explain some of the criticisms that have been made for many years against specific recommendations for the use of FA in primary/secondary CHD prevention. They also prevent us from proposing consensual recommendations adapted to all populations or to populations as a whole; personalised recommendations could be an alternative but are currently not so easy to propose in a pragmatic way. We propose that, instead of continuing to issue specific recommendations on FA intake, new updated recommendations should better promote/revitalise dietary habits that are proved or reasonably suspected to be protective, taking into account regional specificities to facilitate their acceptance by individuals. The traditional MedDiet and traditional JapDiet meet the criteria for a protective effect against CHD. These diets are low in SFA (both diets), high in MUFA (MedDiet), high in plant PUFA (both diets) and LC n-3 PUFA (both diets) and also contain polyphenols (both diets) and isoflavones (JapDiet) consistent with epidemiological data on the protective effects of these nutrients against CHD. Of course, this should not prevent further research on the effects of specific FA on CHD and, where possible, promote intervention studies on these FA or on dietary patterns.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
J. D. is the sole author who planned and wrote this article.
There are no conflicts of interest.