The active form of vitamin D is known to be involved in the regulation of the bone mineral metabolism, and the role of vitamin D deficiency in the risk of rickets in children and osteomalacia in adults is well established(Reference Holick1). However, recent epidemiological evidence has linked low vitamin D status to several other conditions such as insulin resistance(Reference Chiu, Chu and Go2), metabolic syndrome(Reference Ford, Ajani and McGuire3), obesity(Reference Wortsman, Matsuoka and Chen4), multiple sclerosis(Reference MacLean and Freedman5), CVD(Reference Wallis, Penckofer and Sizemore6) and certain cancers(Reference Holick7). Therefore, the high prevalence of vitamin D insufficiency in certain population groups, in particular among the elderly who have a decreased capacity for cutaneous vitamin D formation, is a matter of concern(Reference MacLaughlin and Holick8).
Low vitamin D status is caused by several factors, including reduced skin synthesis caused by low UVB exposure and/or ageing and low dietary intake(Reference Holick7). In countries at high latitudes, e.g. the Nordic countries, very little, if any, pre-vitamin D is formed from October through March(Reference Holick9), and adequate vitamin D status is therefore highly dependent on sufficient dietary vitamin D intake. Furthermore, during spring and summer time, the use of sunscreen – as recommended for other reasons – may substantially decrease the production of pre-vitamin D(Reference Sayre and Dowdy10), making the dietary intake important for most population groups throughout the year.
However, few studies have been conducted in countries where UV exposure is low due to latitude, but where the population is expected to have an increased intake of dietary vitamin D due to high intake of marine food. The Faroe Islands is such a community at a geographical location at about 62°N. It is therefore of importance to consider whether dietary intake is sufficient to compensate for the low UV exposure, especially during the months October–March.
Given the limited available data on the prevalence of vitamin D insufficiency among elderly with high intake of marine food in the Nordic countries(Reference Vieth11, Reference Pearce and Cheetham12), we examined the vitamin D status in a population of 70–74-year-old Faroese subjects to determine the proportion with adequate vitamin D levels. Furthermore, we wanted to estimate the relative contribution of various predictors of vitamin D status, including obesity.
Materials and methods
Study population and design
The present study is part of a larger study of cardiovascular and neurobehavioural effects of lifetime methylmercury exposure. The Faroe Islands constitute a unique setting in which the residents have an increased exposure to methylmercury and persistent organic pollutants due to their intake of traditional marine food, including pilot whale meat and blubber. To examine the subjects aged 70–74 years, a cohort of Faroese men and women born in mid-1930s was established.
All 1131 Faroese citizens born between 2 January 1934 and 31 August 1937 received a letter of invitation, and 713 gave consent corresponding to a participation rate of 63 %. Serum was available for 669 subjects (59 % of the eligible population). The participants underwent a thorough clinical examination, including measurement of weight, height, waist circumference and blood pressure. We calculated BMI by weight (in kg)/height2 (in m2). All the participants travelled to the same examination location, and were thus examined by the same research nurses and a single physician. The participants completed health and lifestyle questionnaires, including FFQ on the local food items, i.e. fish, seabirds, pilot whale meat and blubber, and a question whether they avoid fat food. The FFQ addressed the consumption during childhood, adulthood and the preceding year. We used the information regarding intake during the preceding year as the half-life of 25-hydroxyvitamin D3 (25(OH)D3) in blood is reported to be approximately 4 weeks(Reference Vieth, Bischoff-Ferrari and Boucher13). Fish intake was assessed as total fish intake in the questionnaires, thus it was not possible to separate intake into lean and oily fish.
The study was approved by the Faroese Ethical Review Committee, and all the participants provided written informed consent.
Vitamin D status
Blood samples were obtained at the clinical examination under fasting conditions. Serum was stored at − 80°C until analysis. Vitamin D status was assessed using LC-MS/MS for the determination of serum 25(OH)D3 (S-25(OH)D3) concentrations at the ISO 15 189 accredited clinical biochemical laboratory of Lillebaelt Hospital, Vejle. Serum proteins were precipitated with an internal standard (d 6-25(OH)D3; Synthetica, Oslo, Norway) and dissolved in acetonitrile, and solid-phase extraction of the supernatant was performed followed by ultra performance liquid chromatography. The lower detection limit was 10 nmol/l, and the CV was 9·6 % at 20 nmol/l and 6·7 % at 60 nmol/l. 25-OH-Vitamin D3/D2 Serum Calibration Standard obtained from Chromsystems, Munich, Germany, was used as a calibrator. According to the Vitamin D External Quality Assessment Scheme, there was a slight negative bias of 3·4 nmol/l.
Statistical analyses
S-25(OH)D3 was not normally distributed, hence data are presented as median and the 50 % range (25–75th percentiles). Serum concentrations of 25(OH)D3 were compared between groups using non-parametric tests.
To test the differences in vitamin D status between these groups, we dichotomised the following specific variables: BMI in obese (BMI ≥ 30 kg/m2) v. non-obese; smoking status in daily smokers v. never smokers; whale blubber or whale meat intake more than once per month v. less during the most recent year; and fish intake more than twice per week v. less during the most recent year. This was performed using the Mann–Whitney U test.
The association between vitamin D status and BMI, sex, age, season of blood sampling, dietary intake of fish, whale meat and whale blubber, and avoidance of eating fat food were analysed by simple and multiple logistic regression models using a binary outcome of sufficient vitamin D status defined as 25(OH)D3 >80 nmol/l. A value of P < 0·05 (two-tailed) was taken to indicate statistical significance. Stata version 10.0 (Stata Corporation, College Station, TX, USA) was used for statistical analyses.
Results
The population had a mean age of 72·4 (sd 1·2) years, and the proportion of sexes was equal. Of the population, 19 % had a BMI lower than 25 kg/m2, and 37 % were obese (BMI ≥ 30 kg/m2). The median 25(OH)D3 concentration was 47·6 (29·8–64·8) nmol/l.
A total of 44 and 26 % of the subjects, respectively, reported that they consumed pilot whale blubber and meat more than once per month during the preceding year, and 64 % reported that they had two or more fish dinners per week in the same period. Less than 20 % reported smoking daily or at least sometimes.
Vitamin D status
Vitamin D status among the 669 subjects is shown in Table 1. For 54 % of the population, vitamin D status was < 50 nmol/l, and only 10·3 % had S-25(OH)D3 levels above 80 nmol/l.
Table 1 Vitamin D status in 669 Faroese men and women aged 70–74 years
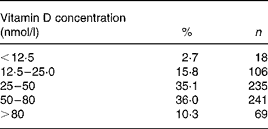
Less than 41 % of the men and 52 % of the women had S-25(OH)D3 concentrations >50 nmol/l. However, twice as many of the women (14 %) had a vitamin D status >80 nmol/l compared with the men (7 %). Vitamin D deficiency (S-25(OH)D3 < 25 nmol/l(Reference Dawson-Hughes, Heaney and Holick14)) was present in 20 and 17% of the men and women, respectively. The differences between sexes were statistically significant (χ2 = 9·3651, P = 0·025; Table 2).
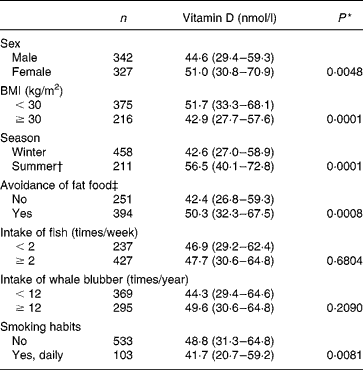
Table 2 Median serum 25-hydroxyvitamin D3 concentrations (50 % range given in the parentheses) in relation to the selected characteristics in Faroese subjects aged 70–74 years
* Tested using the Mann–Whitney or Wilcoxon rank-sum test.
† Includes the months July, August and September.
‡ Participants were asked ‘Do you avoid eating fat food?’.
S-25(OH)D3 concentration varied with the month in which blood sampling was done (Fig. 1). Only for the months July and September were S-25(OH)D3 concentrations above 80 nmol/l for about 22–24 % of the population. The overall difference between the sampling months is shown in Table 2. Obesity, as measured by BMI, was negatively correlated to the S-25(OH)D3 concentration, such that the subjects with a higher BMI had a lower vitamin D status (r s − 0·1034, P = 0·008). The difference in vitamin D status between obese and non-obese is shown in Table 2.

Fig. 1 Fluctuation of serum 25-hydroxyvitamin D3 (25(OH)D3) concentrations according to the month in which blood sampling was done. Boxes represent lower and upper quartiles, and the centre line represents the median value. The grey circle represents an outlier observation. The median values for July, August and September (summer months) are significantly different from those for the other months (P < 0·0001; see Table 2).
Correlates of vitamin D status
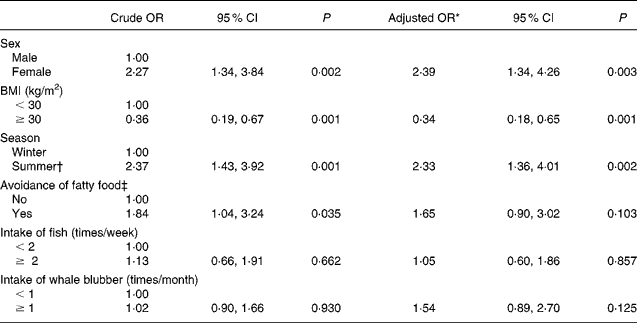
In the bivariate logistic regression analysis, female sex, BMI and summer season significantly predicted sufficient vitamin D status, while age, smoking, avoidance of fat food, and intake of whale blubber and meat or fish were NS cofactors. Having a BMI = 25 kg/m2 (cut-off for normal weight) compared to having a BMI = 30 kg/m2 (cut-off for obesity) decreased the odds of having a vitamin D concentration higher than 80 nmol/l by 56 %. Blood sampling done in the summer season, July–September, increased the odds of having vitamin D status above 80 nmol/l by more than twofold (Table 3).
Table 3 Unadjusted and adjusted odds ratios for vitamin D status >80 nmol/l
(Odds ratios and 95 % confidence intervals)
* The adjusted model includes age, sex, BMI, season, avoidance of fat food, intake of fish and intake of whale blubber.
† Includes the months July, August and September.
‡ Participants were asked ‘Do you avoid eating fatty food?’.
Intake of marine food did not correlate with vitamin D status in the bivariate logistic regression analysis. However, in the full regression model with adjustment for sex, BMI, age, season and avoidance of fat food, the subjects with at least a monthly intake of whale blubber had 56 % increased odds of having a vitamin D status >80 nmol/l, although this did not reach significance (P = 0·11). In the adjusted model, neither intakes of fish and whale meat nor smoking habits predicted vitamin D status. We have performed more detailed analysis regarding grouping the intake of fish and whale blubber into more categories. Although the results were in the same directions as when dichotomised, they were less clear due to the smaller group size and the imprecise exposure assessment. Table 3 shows the crude and adjusted OR for vitamin D concentration above 80 nmol/l.
Discussion
Fish and marine foods are believed to be crucial sources of dietary vitamin D, and of importance for vitamin D status, especially for populations at northern latitudes. However, even a high intake of fish and whale blubber as in this population of elderly Faroese subjects, at a latitude of 62°N, does not prevent low vitamin D status, with an average of 54 % having a vitamin D status lower than 50 nmol/l and approximately 10 % having a vitamin D concentration above 80 nmol/l, as recently suggested to be optimal(Reference Hollis15). Only during summer season (July through September) did the median vitamin D concentration rise above 50 nmol/l, and median values never rise above 80 nmol/l. During November through February, the median levels were 15 % lower than the levels during summer season.
The optimal serum vitamin D concentration is a matter of debate, mostly ranging between 70 and 80 nmol/l(Reference Brock, Graubard and Fraser16), although levels above 80 nmol/l are required to obtain maximal bone mineral density(Reference Choi, Weihe and Budtz-Jorgensen17). However, the most recent Danish report from 2004 on vitamin D suggests that levels above 50 nmol/l might be considered sufficient. These differences in the classification of vitamin D sufficiency are important, as the use of higher cut-off values results in considerably higher proportions of subjects with insufficient vitamin D status (Table 1).
In this fishing population, the average intake of fish and other marine foods is high and includes pilot whale blubber. Nonetheless, the vitamin D status is low in the majority of the elderly subjects. One factor of importance may be that the most frequent fish eaten are cod and haddock with vitamin D content lower than 1 μg/100 g, which may explain why we did not observe a correlation with overall fish intake and vitamin D status, as other studies have demonstrated(Reference Vieth11). Unfortunately, the design of the questionnaire prevented a separation of fish intake into oily v. lean fish. In contrast, the intake of whale blubber did increase the odds of having a serum vitamin D concentration above 80 nmol/l by a factor of 1·5. As blubber ingestion may reflect a general intake of the Faroese traditional food, this traditional marine food may to some degree counteract the limited UVB exposure during winter with regard to the vitamin D status. However, at the same time, the traditional diet imposes an increased exposure to environmental contaminants, such as methylmercury and organochlorines, which may involve independent risks of CVD and neurobehavioural disturbances(Reference Orton, Morris and Herrera18). Still, even during the summer months and among frequent blubber consumers, i.e. eating blubber more than three times per month, the prevalence of vitamin D levels above 80 nmol/l was just about 20 %.
The logistic regression models showed that season, whale blubber intake, sex, BMI and avoidance of fat food predicted approximately 10 % of the variation in 25(OH)D3 concentrations. A poor precision of the proxy variables relating to sun exposures and dietary vitamin D may contribute to the poor correlations observed. However, other factors may also influence the concentration of 25(OH)D3 in the blood. Among these factors are the use of vitamin D supplements, sun-exposure habits and recent travels or vacations at lower latitudes. Of note is that food available at the Faroe Islands is not vitamin D enriched, and the major dietary source is therefore the marine food. An additional factor of possible importance is genetic variation in the enzymes involved in vitamin D metabolism(Reference Uitterlinden, Fang and van Meurs19–Reference Webb, Pilbeam and Hanafin22). Furthermore, we observed that the behaviour of avoiding fat food was associated with higher vitamin D levels. This may seem counterintuitive as blubber intake (which is fat) is also associated with higher vitamin D status. But in Faroese food habits, whale blubber is not considered as a ‘fat food’, but it is considered more as a kind of complement eaten mainly at traditional meals together with whale meat, or fermented or dried fish. Responding ‘yes’ to the question ‘Do you avoid high-fat food?’ may be interpreted as an indication of healthy behaviours that may include taking supplemental vitamins or being more physically active outdoor. This question was not addressed in the questionnaire available, but it may possibly impact on vitamin D status.
The age of the cohort members in the present study was 72 years. It is well known that ageing causes a decrease in the subcutaneous production of vitamin D3 (cholecalciferol) partly due to an age-related decline in the content of 7-dehydrocholesterol in the epidermis and dermis(Reference MacLaughlin and Holick8), and partly due to a change in the sun-exposure behaviour(Reference Pekkarinen, Turpeinen and Hamalainen23). However, due to the frequent intake of marine food in this Faroese population, we did expect higher vitamin D levels and thus lower prevalence rates of insufficient vitamin D status. Still, our median vitamin D concentration is in accordance with the majority of the reported observations in middle-aged and older populations in the northern countries. For instance, in most recent studies of elderly Finnish(Reference Vieth11, Reference Pearce and Cheetham12, Reference Kilkkinen, Knekt and Aro24), Norwegian(Reference Holvik, Meyer and Sogaard25) and elderly Danish women(Reference Andersen, Mølgaard and Skovgaard26) showed comparable levels. Although a Swedish study in elderly women showed much higher values, also during winter(Reference Burgaz, Akesson and Michaelsson27). Differences in food fortification programmes between countries may explain these differences as well as different habits regarding dietary and supplementary vitamin D intake. In fact, it is a general tendency that among elderly people also in England(Reference Hirani, Tull and Ali28) and the US(Reference Yetley29), a substantial proportion is at risk of vitamin D insufficiency.
In addition, among the obese subjects, who constituted more than one-third of the elderly Faroese population, the prevalence of low vitamin D concentrations was even higher than that in the group as a whole, thus supporting recent observations that obese subjects in general have lower vitamin D levels compared with the normal-weight subjects(Reference Burgaz, Akesson and Michaelsson27, Reference Konradsen, Ag and Lindberg30, Reference Lagunova, Porojnicu and Lindberg31). Recent studies suggest that vitamin D is better correlated to fat mass than to fat-free mass both in adolescents(Reference Lenders, Feldman and Von Scheven32) and in adults(Reference Snijder, van Dam and Visser33), so the inverse association between vitamin D and BMI observed in several studies does not seem to be explained entirely by the distribution volume, and also that subjects with a high fat mass, perhaps especially subcutaneous fat, may sequester vitamin D in the fat to a higher extent than in the muscle.
The clinical importance of the observed low vitamin D levels in an elderly population is reflected by randomised studies that have shown that vitamin D intake reduces fall risk and risk of hip fracture in the most compliant women. Additionally, epidemiological evidence demonstrates advantageous associations between cancer risk, CVD, autoimmune diseases such as multiple sclerosis and diabetes(Reference Holick and Chen34). Thus, even though the Faroese subjects eat fish and other marine foods regularly, they do not obtain enough vitamin D to reach desirable levels, and the low levels observed may therefore increase their susceptibility to several chronic diseases.
Vitamin D concentrations below 80 nmol/l are very common among the elderly of the Faroe Islands in spite of a high consumption of marine food, with deficiency being particularly common among obese subjects during the winter. Hence, efforts to improve vitamin D status are warranted, and it appears clinically appropriate to recommend supplementary vitamin D intake.
Acknowledgements
The present study was supported by grants from the National Institute of Health (ES013692) and the European Commission FP6 integrated project PHIME (contract no. FOOD-CT-2006-016253). The authors are solely responsible for all the results and conclusions, which do not necessarily reflect the position of any of the funding agencies. All the authors declare no conflicts of interest. C. D. and P. G. designed the present study, with the cohort being planned and conducted by P. W. and P. G. P. W. and M. S. P. carried out the clinical examinations. A. V. S. and I. B. performed the analysis of 25(OH)D3 concentrations in the serum. C. D. drafted the paper and performed the statistical analysis. C. D., P. G. and P. W. have the primary responsibility for the final content. All the authors contributed to critical comments on the paper and approved the final version.






