INTRODUCTION
Acellular pertussis (aP) vaccination in Japan was introduced in 1981 after confirmation of antibody production in vaccinees and demonstration of the vaccine's clinical safety [Reference Sato, Kimura and Fukumi1] and prophylactic effect on secondary infection in family members [Reference Isomura, Suzuki and Sato2]. A number of randomized controlled trials (RCT) were conducted later, mainly in Europe, to evaluate aP vaccines. Since then, aP vaccines have been used in many countries [Reference Miller3, Reference Jefferson, Rudin and DiPietrantonj4].
Several observational studies have been conducted worldwide to evaluate the effectiveness of aP vaccine. However, most studies evaluated vaccine effectiveness (VE) during pertussis epidemics. Effectiveness of aP vaccine in a non-epidemic period has been evaluated in only a few studies [Reference Farrington5, Reference Ramsay, Farrington and Miller6]. By 1987, a Japanese observational study on secondary infection in family members reported the effectiveness of aP vaccine during an epidemic. The National Epidemiological Surveillance of Infectious Diseases showed that the number of reported cases of pertussis decreased dramatically as the vaccination rate increased, suggesting the effectiveness of the aP vaccine. No increases in the number of infected patients suggestive of apparent pertussis outbreaks have been reported since 1997 [7]. No observational study to directly evaluate the effectiveness of aP vaccine has been conducted since the number of reported cases of pertussis started to decrease in Japan.
In addition to the fact that pharmacoepidemiology in Japan is still an underdeveloped discipline, the lack of a database of personal vaccination histories may explain why so few observational studies of VE have been conducted in Japan. Accurate information about individual vaccination status is indispensable for observational studies of VE. In the United States, influenza vaccination information is recorded in the HealthPartners computerized influenza vaccination database and has been used for a cost-effectiveness study [Reference Hak8]. The General Practice Research Database (GPRD) in Great Britain contains vaccination information which has been used for a case-control study [Reference Smeeth9]. However, such an electronic database has not been developed in Japan. Instead, all parents living in Japan are required to keep their children's vaccination records in their Maternal and Child Health (MCH) Handbooks according to the Maternal and Child Health Law. Parents in Japan therefore have reliable information on their children's vaccination status [Reference Mamiya10]. Data from the MCH Handbooks could be used to check vaccination status and therefore be used in research studies.
Another limitation on observational studies to evaluate VE in Japan is the need for population sampling. In order to conduct an observational study in the general population, researchers need to check the personal vaccination statuses of individual residents. A telephone survey might be an effective way to collect such information; however, it requires tremendous time and effort to select appropriate subjects based on their age and sex. Many of the existing Japanese observational studies were therefore conducted in patients seeking treatment at hospitals without attempting to survey a representative and unbiased population sample. Fortunately, information such as individual residents' address, name, age, and sex can be obtained for each household from the Basic Resident Registration in Japan. Therefore, efficient random sampling of study subjects is possible if a copy of the resident card is available [11]. We therefore conducted a matched case-control study in Japanese children to evaluate the effectiveness of aP vaccine during a non-epidemic period based on data from MCH Handbooks and the Basic Resident Registration.
METHODS
Paediatricians at 57 medical institutions and paediatric general practitioners participated in the study. We recruited the paediatricians from the members of the Kitakyushu City Medical Association, via an invitation explaining the purpose and plan of our study. Of the paediatricians belonging to the Kitakyushu Medical Association, 66% (57/86) collaborated with our study. Cases and controls were selected from children who lived in Kitakyushu City (population 1·01 million in 1999). Based on a VE of 0·96, which was estimated in a previous small-scale pilot study (K. Okada et al., unpublished data), three controls were selected for each case to ensure a statistical power of 90%.
Vaccine studied
aP vaccines combined with diphtheria-tetanus (DT) toxoids (DTaP) were available in Japan from six manufacturers when the study was conducted. The DTaP vaccine produced by The Chemo-Sero-Therapeutic Research Institute (Kaketsuken; Kumamoto, Japan) was estimated to have been given to >95% of children in Kitakyushu City. The rate of use of the Kaketsuken vaccine was estimated from the total number of units supplied by the manufacturer in the city of Kitakyushu divided by the total number of DTaP vaccines that children received in the city of Kitakyushu. The Kaketsuken vaccine, a two-component aP vaccine, contains a modified formulation of the original aP vaccine; its pertussis toxin (PT) and filamentous haemagglutinin (FHA) were isolated by affinity chromatography to obtain a constant ratio of 1:4 PT to FHA [Reference Kimura12]. The standard immunization schedule for DTaP in Japan is an initial dose given three times from ages 3 to 12 months followed by a single booster injection given between 12 and 18 months.
Patients, controls, and diagnosis
From April 1999 to March 2001, the participating paediatricians registered 116 children with clinically suspected pertussis who had attended their hospital or paediatric clinic, and definitive diagnoses were made by another paediatrician responsible for case diagnosis. Clinically suspected pertussis was determined by the participating paediatricians according to the reporting standards used for Japan's pertussis surveillance, i.e. coughing lasting >1 week with either: (a) coughing episodes with staccato, whooping, or paroxysmal cough at night and/or (b) neonates or children with otherwise unexplained vomiting or apnoea after cough. For these cases, we examined bacterial isolates from nasopharyngeal swab samples, PT paired serum, and levels of antibody to the fimbriae antigen which is not included in the two-component vaccine, in addition to WBC count and lymphocyte count. After these tests, the paediatrician responsible for case diagnosis reviewed the diagnosis of pertussis on the basis of patient files and test results supplied by the participating paediatricians, and definitively diagnosed pertussis in 15 children. Definitive diagnosis was based on the following: (1) characteristic coughing attacks (repeated staccato, whoop or paroxysmal cough that lasted ⩾7 days and (2) either isolation of Bordetella pertussis, serodiagnosis (at least fourfold increase of PT-IgG or agglutinin titre) or contact with a family member with confirmed pertussis.
Controls were randomly selected from the Basic Resident Registration of Kitakyushu City. In Japan, all citizens are registered on the Basic Resident Registration. Individuals can view details including names, dates of birth, sex and address. Controls [matched by age (±6 months of the date of birth) and sex] were selected who were living in the same residential area as the cases during the study period.
The number of vaccinations was defined as the number of vaccinations received at least 28 days before definitive case diagnosis. A period of 28 days was chosen as the valid period to allow for the development of antibodies (around 14 days) and the normal incubation period (14 days). The age of cases at the time of the valid period was used in the data analysis.
Study method and questionnaires
The study description and a questionnaire with a manual were sent to parents of potential controls between October and December 2005. In a previous small-scale pilot study (using the same method), a 43% valid response rate from the parents of 30 children was achieved. Based on this response rate, we randomly selected nine candidates, which included ‘reserves’ if additional mailing of questionnaire forms was needed, for each case from the Basic Resident Registration. Questionnaire forms were sent according to the selection order to the first six consecutive candidates per case. If valid responses from three or more controls for each case were not received within 2 weeks, questionnaires were sent to the three ‘reserve’ candidates. The study description and general information on pertussis were posted on the Kitakyushu Medical Association website to provide information for parents.
The questionnaire sent to the parents of candidate controls included questions on: (1) the history of DTaP vaccination (date, vaccine manufacturer and lot number), (2) whether the control child was born in Kitakyushu City or had moved from another city (in which case the date of arrival was requested), and (3) any history of pertussis, including the date of diagnosis and name of diagnosing institution if available. Parents who consented to taking part in the study also copied the history of DTaP vaccination from their MCH Handbooks. Questionnaires could be returned to the Kitakyushu Medical Association by fax or mail.
The same definitions of number of vaccination and age used for the cases were applied to the controls. The exclusion criteria for controls were as follows: (1) onset of pertussis before the definitive diagnosis of pertussis in the matched case, (2) moving to Kitakyushu City after the onset of pertussis in the matched case, and (3) use of vaccines other than Kaketsuken DTaP vaccine.
The reason for this 4-year delay in surveying controls after the identification of the cases is that there was a change in the method of identifying control subjects. Initially, we planned to collect hospital-based controls, but we received advice from our statistician that the collection of community-based controls would be more appropriate. However, the method of collecting community-based controls in Japan was not established at that point, and it took some time to change the research protocol. Moreover, due to the enactment of the Protection of Personal Information Act in Japan, obtaining permission to view the Basic Resident Registration from the local government also takes a long time. Therefore, there was a delay between collecting the cases and starting to identify suitable controls.
The controls were surveyed in accordance with the ‘Ethical Guidelines for Epidemiological Studies’ issued by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare in July 2002. The study protocol was approved by the ethical committee of the Public Health Research Foundation.
Statistical analysis
We used SAS software (SAS Institute Inc., Cary, NC) and a conditional logistic regression model to calculate the matched odds ratio (OR) and two-tailed 95% confidence interval (CI) for pertussis events by number of vaccination [1–2 vaccination(s) or 3–4 vaccinations]. The formula (1−OR)×100 was used to calculate VE. This was stratified according to the duration of coughing attacks: ⩾7 days, ⩾14 days, or ⩾21 days.
RESULTS
Questionnaires for cases
During the study period, 15 children, aged 4 months to 6 years, received a definitive diagnosis of pertussis. Eight of the children were aged <1 year [their ages were 4 and 10 months (one case each) and 5, 6 and 7 months (each in two cases)], four were aged 1 year, and the remaining three were aged 2, 3, and 6 years (Table 1). Six were boys and nine were girls. Twelve had never been vaccinated, while one child had been vaccinated once and two children had been vaccinated three times.
Table 1. Age of children and number of DTaP vaccinations
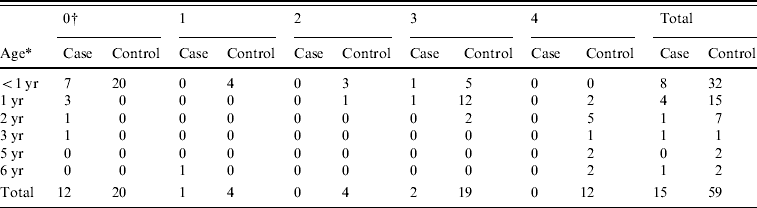
* Age of children.
† Number of DTaP vaccinations.
Ten children had persistent staccato cough, six whooped, 14 had nocturnal paroxysmal cough, two had cyanosis (lasting 1 day and 3 days respectively) and one patient presented with apnoea. Coughing attacks lasted 10–90 days, with one child having coughing attacks that lasted 7–13 days, two children having attacks that lasted 14–20 days, and 12 children having attacks that lasted >21 days. One patient was admitted to hospital. The WBC count was ⩾15 000/μl in 11 children, and the percentage of lymphocyte was ⩾70% in three.
The definitive diagnosis of pertussis was based on Bordetella pertussis isolation in two children, on positive PT-IgG antibody titre or significant increase in antibody level in 10 children, and on positive agglutinin titre or its significant increase in three children. No child had contact with a family member with confirmed pertussis. However, of the 15 confirmed cases, 10 had siblings, and among these, three had a sibling who showed signs of pertussis (i.e. consistent coughing lasting ⩾14 days), and could have been an infection source.
Questionnaires for controls
For four cases, valid responses were not received within 2 weeks of sending questionnaires from three or more matched controls, so questionnaires were also sent to the parents of the ‘reserve’ candidates until valid responses were received from three or more controls for each case.
The questionnaire return rate was 69·6% (71/102). We received valid responses from the parents of 59 children; this amounted to three controls for six cases, four controls for four cases, and five controls for five cases. The age of the control children ranged between 4 months and 6 years: 32 were aged <1 year; 15 were aged 1 year; seven were aged 2 years; one was aged 3 years; two were 5 years and two were 6 years (Table 1). Of the controls, 25 were boys, and 34 were girls. Twenty had never been vaccinated, four had been vaccinated once, four twice, 19 three times, and 12 four times.
Twelve children were excluded from the efficacy analysis: one had an onset of pertussis before the onset in the matched case, four had moved to Kitakyushu City after the onset of pertussis in the matched cases, and seven had been vaccinated with vaccines other than the Kaketsuken DTaP vaccine.
Age of children and number of vaccinations
The rate of vaccination with Kaketsuken DTaP was 87% (comprising a total of 136 injections) in the 67 children (obtained from the 71 completed questionnaires excluding the four children who had moved to Kitakyushu City after the study period).
Vaccination status by age (defined as having received at least one vaccination) was 1/8 in children aged <1 year, 1/4 in children aged 1 year, 0/1 in the children aged 3 or 5 years, and 1/1 in the child aged 6 years (Table 1). Vaccination status in control children was 12/32 in those aged <1 year, 15/15 in those aged 1 year, 7/7 in those aged 2 years, 1/1 in the child aged 3 years, and 2/2 in children aged 5 or 6 years. Based on the number of vaccinations by age, the vaccination rate was lower in the cases than in the controls.
Effectiveness of pertussis vaccine
The cases were divided into three groups based on the duration of coughing attacks characteristic of pertussis: ⩾7 days, ⩾14 days, and ⩾21 days. The cases were further divided into two groups, depending on whether they had received one or two vaccination(s), or three or four vaccinations, to estimate VE by using a conditional logistic regression model (Table 2). The VE of three or four vaccinations compared with non-aP vaccination (baseline) was 96·9% (95% CI 62·2–99·7) for coughing attacks that lasted ⩾7 days, 96·4% (95% CI 53·5–99·7) for ⩾14 days, and 95·9% (95% CI 46·1–99·7) for ⩾21 days. The point estimate of VE for one or two vaccination(s) was 86·8–83·9%. However, the number of subjects who received only one or two vaccination(s) was small, and therefore the confidence interval was large. Therefore, comparing the effectiveness of one or two with three or four vaccinations was difficult.
Table 2. Effectiveness of aP vaccine
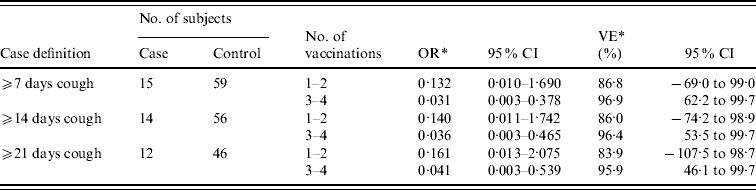
* Effectiveness of acellular pertussis vaccinations compared with non-vaccination.
DISCUSSION
The effectiveness of DTaP vaccine has never previously been evaluated using appropriate epidemiological methods in Japan. We surveyed the DTaP vaccination history of children with pertussis who had coughing attacks that lasted >7 days and of controls who were matched by age and sex in Kitakyushu City. The effectiveness of the DTaP vaccine used in more than 95% of children living in the region for prevention of pertussis was 95·9–96·9% in children who had received three or four vaccinations, equivalent to VE of 96·0% (95% CI 67·4–99·5) in the practice-based controls (children who, for reasons other than pertussis, visited the hospital where the cases received treatment for pertussis around the same time and had the same background as the cases) in the previous study.
A case-control study on DTaP vaccine was conducted in Munich, Germany, from 1993 to 1995. In the cases who were defined as having continual cough for at least 21 days, the VE of three DTaP vaccinations was 93% (95% CI 63–99) [Reference Beloradsky13], which is comparable to the VE of three or more DTaP vaccinations in patients who had continual cough for ⩾21 days estimated in our study (95·9%, 95% CI 46·1–99·7). The Centers for Disease Control and Prevention (CDC) in the United States conducted a case-control study in children aged 6–59 months in seven states and territories by using two types of whole-cell pertussis vaccines combined with DT toxoids (DTwP) and three types of DTaP vaccines from 1999 to 2000 [Reference Bisgard14]. The VE estimated in the US study was also comparable to that in our study: 95·4% (95% CI 88·7–98·2) with three DTaP vaccinations and 96·7% (95% CI 90·8–98·8) with four vaccinations.
Since 1991, large-scale field RCTs and cohort studies on DTaP vaccines including aP vaccines developed in Japan have been conducted in children in Europe and Africa. These studies used different designs and case definitions. When evaluated based on the case definition most similar to that used by the World Health Organization (WHO), most DTaP vaccines (with two or more components) showed >80% effectiveness [Reference Edwards, Decker, Mortimer, Plotkin and Orenstein15].
Thus, the VE reported in the case-control studies are higher than that reported in the RCTs or cohort studies. Pertussis symptoms are often milder in vaccinated children than in their unvaccinated counterparts. VE may therefore be overestimated if the definition ‘moderate-to-severe pertussis’ is used in the study [Reference Orenstein, Bernier and Hinman16]. Liese et al. estimated VE separately based on two sets of definitions, ‘pertussis with paroxysmal cough’ and ‘pertussis with cough’, and reported a higher VE among patients who met the stricter criteria [Reference Beloradsky13]. The high VE estimated in our study may be due to the fact that the majority of patients included in the analysis had more severe forms of pertussis (Table 2).
We consider the wide confidence intervals and relatively high point estimates for the effectiveness of one or two doses of vaccine are due to the small number of cases included. However, a similar case-control study conducted in the United States reported the effectiveness of a single vaccination to be 70%, and two doses to be 89%; and they therefore reported similar effectiveness for one or two doses as the present study, i.e. 86% [Reference Bisgard14].
The effectiveness of aP vaccines in the Japanese population was evaluated based on secondary infection within families in the studies conducted during the pertussis epidemic in the 1980s [Reference Noble17, Reference Kato18]. However, no placebo-controlled RCT or cohort study was done. In overseas studies, effectiveness of aP vaccines has been evaluated by individual brands. However, other than the study conducted by Kato et al. [Reference Kato18], previous Japanese studies on secondary infection within families did not distinguish vaccines by brand.
An RCT or cohort study is ideal for evaluating aP VE. However, because the number of pertussis cases in Japan has decreased substantially due to the increased vaccination rate [Reference Kuno-Sakai and Kimura19], conducting a cohort study is difficult. Additionally because infant pertussis vaccination is generally recommended in Japan, a randomized study would be unethical. Fine pointed out that the study design for evaluation of secondary infection within families included potential biases that might affect study results [Reference Fine20]. Thus, in the present study, we believe that our use of a case-control design using aP vaccine was the most appropriate to evaluate the effectiveness of DTaP vaccine during a non-epidemic period.
We inspected a copy of the Basic Resident Registration to identify a random sample of control candidates from the community. Sampling efficiency with this method was considered far superior to a telephone survey since control candidates could be matched with cases based on their date of birth and sex provided in the Basic Resident Registration. Unlike authors of studies conducted in Europe and the United States where databases of personal vaccination statuses are available, we used MCH Handbooks kept by parents of study participants as information sources. In Japan, all pregnant women receive an MCH Handbook, and these are retained by most parents or guardians at least until the child reaches the sixth grade of elementary school, which is the end of last required periodical vaccination in Japan. Several reports have been published on epidemiological studies in which MCH Handbooks were used [Reference Kawai21, Reference Tsuchiya22]. Documentation of health-care records of mothers and their children including infant vaccination status (including a record of the type of vaccine, manufacturer, lot number, date of vaccination, and administering physician) in MCH Handbooks by physicians is required under Japanese law. Since we asked the parents to copy the details such as manufacturer, lot number and date of vaccination from their MCH Handbooks onto questionnaire forms, the information obtained in the survey was thought to be more reliable than that which could have been obtained from telephone interviews. The study method based on the Basic Resident Registration and MCH Handbooks used in this case-control study on paediatric aP vaccine could therefore be used in Japan as an alternative to the database-oriented study method used in Europe and the United States. The Basic Resident Registration and MCH Handbooks could also be used in other epidemiological studies in Japanese children.
Our study suggests that the Kaketsuken DTaP vaccine effectively prevented pertussis in Japanese children during a non-epidemic period. However, because this study was conducted in a specific region and had a small number of cases, further research is needed to reach a definitive conclusion.
ACKNOWLEDGEMENTS
We express our gratitude to the following individuals for their cooperation in this study, including patient registration activities: Dr Hideko Morita, Dr Hiroshi Kurashige, Dr Hiroyoshi Hashizume, Dr Katsuko Sato, Dr Kotaro Ichikawa, Dr Kunihiro Katayama, Dr Kunio Kishida, Dr Mitsuko Koga, Dr Osamu Okita, Dr Takahisa Sakuma, Dr Tomohiko Uozumi, Dr Yasufumi Hidaka, Dr. Yasuhiko Takahashi, Dr. Yusuke Amamoto, and Dr. Yutaka Ebisu. Moreover, we thank Dr Tsugumichi Sato, Department of Pharmacoepidemiology, Graduate School of Medicine, University of Tokyo, for his instruction on epidemiological procedures. We are indebted to Toshiya Tanaka and Mika Watanabe and the staff at the Kitakyushu Medical Association, and Ryoji Kido, The Chemo-Sero-Therapeutic Research Institute.
DECLARATION OF INTEREST
None.




