INTRODUCTION
Toxoplasmosis, caused by the protozoan Toxoplasma gondii, is a worldwide zoonosis [1]. In general its seroprevalence is very high in South America and low in Asia. Fragmentary reports indicate a high prevalence of T. gondii infections in Africa [1]. Toxoplasmosis is usually asymptomatic in immunocompetent adults, but can cause mortality in the very young and the immunocompromised. Many patients infected with human immnuodeficiency virus (HIV) infection die of toxoplasmosis. This is of particular concern in many African countries because of the high prevalence of HIV and lack of resources to manage it. We summarize the current status of T. gondii infection in humans and other animals in Ethiopia.
Humans and other animals become infected with T. gondii mostly by ingesting undercooked meat of infected animals or by ingesting food or water contaminated with oocysts [1]. Cats are essential in the life-cycle of T. gondii because they are the only hosts that can excrete the environmentally resistant oocysts in nature. The prevalence of T. gondii antibodies varies with age, lifestyle of the cat (stray vs. pet), the serological test utilized, the screening dilution, and other undefined factors. In general, infection in cats increases with age and the prevalence is higher in stray cats. Infected cats can shed millions of oocysts in a matter of a few days, and after sporulation oocysts can survive in the environment for months or even years depending on the moisture and ambient temperature. Cats are thought to become infected by ingesting infected prey soon after they begin to hunt. Cats usually shed oocysts only for a short time and once in their life. However, poor nutrition, concurrent infections, and immunosuppression may affect the immune status of the cat and lead to increased oocyst shedding.
TOXOPLASMOSIS IN HUMANS IN ETHIOPIA
Limited data indicate a high seroprevalence of T. gondii antibodies in humans in Ethiopia (Table 1). Among these reports, the study by Guebre-Xabier et al. [7] is of note. They tested 1016 sera from different age groups from six geographical locations in Ethiopia. Seroprevalence varied from 47–96% with high rates in 97 children (aged 14–18 years) from leprosy families (85·5%) and from 427 blood donors (50–92%). This high prevalence in blood donors is important because toxoplasmosis can be transmitted by blood transfusion, especially in immunosuppressed persons or during acute infection. In an earlier report, unfortunately only published as an abstract, T. gondii antibodies were found in 42% of 614 persons sampled in 1981–1982 in 1:50 serum dilution tested with an in-house ELISA [4]. These authors reported that the prevalence was 41% in children aged 1–5 years but the number of children tested is not given [4].
Table 1. Summary of Toxoplasma gondii prevalence in humans in Ethiopia
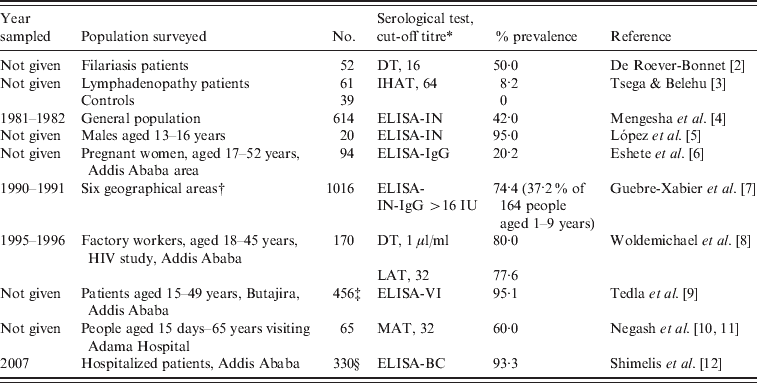
* DT, Dye test; IHAT, indirect haemagglutination test; LAT, latex agglutination test (Eiken Co., Japan); MAT, modified agglutination test; ELISA, enzyme-linked immunoabsorbent assay; ELISA-IN, ELISA in house; ELISA-BC, ELISA (BioCheck Inc., USA); ELISA-VI (Viro-immuno Diagnostica GmbH, Germany).
† Addis Ababa 427, Jima 186, Konso 164, Yirgalem 100, Asmara 97, Butajira 42.
‡ 97·7% of 214 schizophrenia patients, 95·3% of 171 bipolar disorder patients, 87·3% of 71 controls.
§ 93·3 of 154 HIV-positive patients, 86·7% of 143 HIV-negative patients.
Little is known of clinical toxoplasmosis in people or animals in Ethiopia. Tsega & Belehu [3] found T. gondii antibodies in five (8·2%) of 61 patients with lymphadenopathy but not in 39 control patients; however, these data are insufficient to imply that T. gondii was a cause of lymphadenopathy in the five patients. Shibre et al. [17] found no relationship between T. gondii infection and effectiveness of schizophrenia therapy in Addis Ababa based on treatment with the anti-T. gondii drug trimethoprim.
Much medical attention is being focused on the acquired immune deficiency syndrome (AIDS) epidemic in Africa. Ethiopia is the second-most populous nation in the horn of Africa, with over 82 million inhabitants, and a high rate of AIDS. The finding of 93·3% seroprevalence of T. gondii antibodies in HIV patients by Shimelis et al. [12] is notable. Although clinical toxoplasmosis has been suspected in many HIV-infected patients treated with highly active antiviral therapy (HAART), and immune reconstitution [18–20], there are no histologically verified cases of toxoplasmosis in HIV-infected or immunocompetent persons in Ethiopia because histological diagnosis has not been pursued. Of 566 HIV-related deaths in a teaching hospital in Addis Ababa, a central mass lesion (with suspicion of toxoplasmosis) was diagnosed in brains of 74 (13·1%) by Bane et al. [21], but the diagnosis was not pursued further. The strongest positive evidence of clinical toxoplasmosis in AIDS is that reported by Amogne et al. [22]. They diagnosed neurological toxoplasmosis in 323 AIDS patients solely on the basis of clinical signs and favourable response to treatment with the anti-T. gondii medicine sulfadoxine-pyrimethamine (Fansidar; Hoffmann-La Roche, Switzerland). Serology for HIV was positive in all patients, and T. gondii IgG antibodies were found in 19 (83%) of 23 cases; no T. gondii serological data were given for the remaining 300 patients. The symptoms reported in these 323 patients were headache (91%), fever (83%), altered sensorium (62%), and seizures (48%). Radiographically, 79% of patients had enhancing lesions. In total, 248 (77%) patients responded to treatment with clinical improvement, 64 (20%) died in hospital, and 11 (3%) did not show clinical improvement. There is no mention of post-mortem examination. In summary, all the evidence presented is presumptive, and there are no definitive data with respect to clinical toxoplasmosis in humans in Ethiopia.
TOXOPLASMOSIS IN OTHER ANIMALS IN ETHIOPIA
There are no reports of clinical toxoplasmosis in other animals. Serological surveys indicate a high prevalence of T. gondii antibodies in sheep and goats, although these surveys are more than a decade old (Table 2). Recently, Teshale et al. [16] reported 74·9% seroprevalence in 641 goats from central and southern regions of Ethiopia. Seroprevalence in cattle was low [13]. To our knowledge, there is no report of isolation of viable T. gondii from animals (or humans) in Ethiopia.
Table 2. Summary of Toxoplasma gondii prevalence in animals in Ethiopia
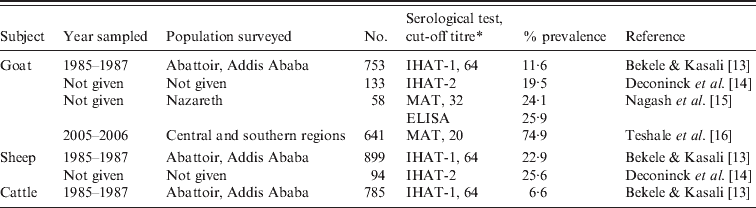
* IHAT-1, Indirect hemagglutination test (Wellcome Diagnostics, UK); IHAT-2, indirect haemagglutination test (Toxoplasmose Fumouze, France); ELISA, enzyme-linked immunoabsorbent assay (Enzygnost, bioMérieux, France); MAT, modified agglutination test.
PROSPECTIVE
Limited data in Table 1 indicate that the prevalence of T. gondii in humans in Ethiopia is very high. As noted above, a study conducted in 1981–1982, mentioned a seroprevalence of 41% in children aged 1–5 years, which is quite high for this age group, but the number of children tested out of a total of 614 people tested was not given [4]. About 1 million adults in Ethiopia are considered to be infected with HIV, with less than one-third of them likely receive HAART [23]. Based on a conservative T. gondii seroprevalence of 50%, thousands might die of concurrent opportunistic infections, including toxoplasmosis. However, exact figures are not available, and most serological surveys are not current. To start, a planned survey is needed for T. gondii prevalence in different age groups, especially pregnant women. Attempts should be made to isolate viable T. gondii from food animals, cats, and humans because nothing is known of the genetic diversity of T. gondii strains prevalent in humans and other animals in Ethiopia.
ACKNOWLEDGEMENTS
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Department of Health and Human Services or the Centers for Disease Control and Prevention or the U.S. Department of Agriculture.
DECLARATION OF INTEREST
None.




