Atherosclerosis has become the leading cause of cardiovascular morbidity and mortality throughout the world, and 12·9 million people were killed by IHD and stroke in 2010, one in four deaths worldwide, compared with one in five deaths in 1990( Reference Lozano, Naghavi and Foreman 1 ). Observational studies have suggested that higher intakes of fruits and vegetables are associated with a low risk of CVD and stroke( Reference Martinez-Gonzalez, de la Fuente-Arrillaga and Lopez-Del-Burgo 2 , Reference Dauchet, Amouyel and Hercberg 3 ), and carotenoids, abundant in many fruits and vegetables, have been reported to play an important role in the protection of the cardiovascular system( Reference Agarwal, Parameswari and Vasanthi 4 , Reference Dwyer, Paul-Labrador and Fan 5 ). Lutein and lycopene have been reported to be the most powerful carotenoids in vivo to inhibit the oxidative modification of LDL in the vascular endothelium( Reference Karppi, Nurmi and Kurl 6 ), and the synergistic effects of lutein and lycopene have been reported to be most pronounced during the protection of multilamellar liposomes against oxidative damage( Reference Stahl, Junghans and de Boer 7 ).
In most trials, numerous compounds labelled as antioxidant vitamins are grouped together and often the results are extrapolated from one compound to another, implying that they all would yield similar results. Although large randomised controlled trials have reported that antioxidant vitamins such as single β-carotene and vitamin E show limited benefits in CVD events( Reference Hennekens, Buring and Manson 8 , Reference Omenn, Goodman and Thornquist 9 ), it is important to find those that may have beneficial effects and those that do not. Grouping together various agents under a broad category can be misleading. The Los Angeles Atherosclerosis Study has reported that subjects with the highest serum lutein concentrations (0·42 μmol/l) have 80 % less arterial wall thickening relative to those at the lowest quintile of serum lutein concentrations (0·15 μmol/l)( Reference Dwyer, Navab and Dwyer 10 ). Such a relationship has not been reported for serum β-carotene. Buttressing these findings are the animal experiments reported in the same study, where lutein supplementation was found to reduce the size of atherosclerotic lesions in apoE-null mice by 44 % and inhibit LDL oxidation in a dose-dependent manner up to almost 80 %( Reference Dwyer, Navab and Dwyer 10 ). Moreover, it is well established that the bioavailability of lutein from the diet is higher than that of β-carotene( Reference van het Hof, Brouwer and West 11 ). The Rotterdam Study also has reported that lycopene is inversely associated with the calcified plaques of the abdominal aorta( Reference Klipstein-Grobusch, Launer and Geleijnse 12 ). However, the effects of lutein and lycopene on atherosclerosis have not been examined in randomised controlled trials, and there is an agreement that not high concentrations of a single antioxidant but a high intake of diverse phytochemicals present in fruits and vegetables can lead to health benefits( Reference Milde, Elstner and Grassmann 13 ).
In our previous studies, it was found that high serum lutein concentrations were inversely associated with carotid artery intima–media thickness (CAIMT)( Reference Zou, Xu and Huang 14 ) and that an increase in serum lutein concentrations after 20 mg lutein supplementation for 3 months could reduce the levels of inflammatory cytokines and regulate the levels of serum lipids, which may play important roles in early atherosclerosis( Reference Xu, Zou and Xiao 15 ). Therefore, in the present study, we evaluated the effects of lutein and lycopene supplementation on the development of CAIMT in Chinese subjects with subclinical atherosclerosis and investigated whether the combination of lutein and lycopene supplementation was more effective than lutein alone in human subjects.
Methods
Study population
Participants aged 45–68 years were recruited from three communities in Haidian District, an urban district of Beijing, China (Table 1). They were eligible for the study if they had a clinical diagnosis of subclinical atherosclerosis (defined as CAIMT >0·75 mm for those aged below 59 years or CAIMT >0·85 mm for those aged above 60 years), according to the Chinese guideline for early vascular disease detection( 16 ). Subjects were excluded if they had carotid calcified plaques (CAIMT >1·30 mm) or moderate-to-severe fatty liver, cirrhosis, chronic renal failure or a history of myocardial infarction, stroke, revascularisation, or coronary bypass operation or were using statin medications or consuming dietary supplements containing vitamins or carotenoids 3 months before enrolment. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the medical ethics committee of Peking University. Written informed consent was obtained from each participant before participation in the study.
Table 1 Characteristics of the subjects in the Beijing atherosclerosis intervention study* (Mean values and standard deviations; number of subjects and percentages)
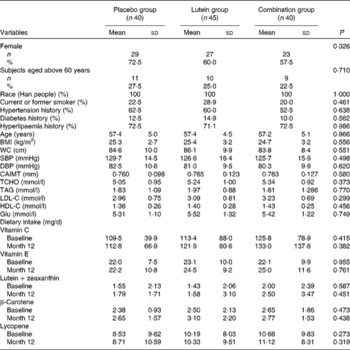
Placebo group, receiving capsules containing starch; lutein group, receiving capsules containing 20 mg lutein/d; combination group, receiving capsules containing 20 mg lutein/d+20 mg lycopene/d; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; CAIMT, carotid artery intima–media thickness; TCHO, total cholesterol; LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol; Glu, glucose.
* There was no difference in dietary intake between baseline and month 12 in the three groups.
Study design
A total of 144 participants who met all the protocol criteria were randomly assigned to one of the following three treatment groups: placebo (receiving capsules containing starch); lutein group (receiving capsules containing 20 mg lutein/d); combination group (receiving capsules containing 20 mg lutein/d+20 mg lycopene/d) for 12 months. All participants, investigators and clinical centre personnel were unaware of treatment allocation throughout the study. Supplements or placebo for the three groups were prepared as capsules identical in size, weight or colour by the Beijing Yuguang Bioscience Research Center, China. The pharmacy technician, who prepared the capsules on the basis of the randomisation list, was not involved in the collection of outcome data. The participants were instructed to take their assigned capsules daily, and adherence to treatment was assessed using capsule counts and a diary of the medication taken at monthly visits. At each visit, they were asked about adverse events and were counselled to follow their usual diets. Diet stability was validated using 24 h dietary recalls conducted at baseline and after 12 months.
Assessment of subclinical atherosclerosis
Common CAIMT was measured using high-resolution B-mode ultrasonography with a 5 MHz liner scanner (Probe 5412, Aloka prosound α-10; Aloka Company Limited). Carotid sonography was carried out by three medical technicians who were blinded to the information of the present study. The subjects were examined in the supine position, and for each participant, CAIMT was measured three times for each side of the carotid artery and the mean value of CAIMT was recorded. To assess inter-reader variation, five participants were examined separately by the three medical technicians, and a CV < 3·0 % was obtained. Blood pressure was measured at least two times before the subjects were subjected to ultrasound for CAIMT measurements, and the mean value of blood pressure was recorded.
Serum biochemistry and HPLC analysis of carotenoids
Overnight fasting venous blood samples were collected from all the subjects at months 0, 4, 7 and 12. The samples were protected from light and centrifuged for 10 min (3000 g , 4°C). Serum total cholesterol, TAG, HDL-cholesterol, LDL-cholesterol and glucose concentrations were measured within 2 d of sample collection using an autoanalyser at the Beijing Lawke Health Laboratory. The aliquots of the specimens were frozen at − 70°C until HPLC analysis.
Lutein, zeaxanthin, all-trans-β-carotene, trans-lycopene and β-apo-8′-carotenal were purchased from Sigma Chemical Company. Methanol and acetonitrile were obtained from Honeywell B&J, and methyl tert-butyl ether (MTBE) was obtained from J. T. Baker. Serum lutein, zeaxanthin, β-carotene and lycopene concentrations were determined using HPLC based on the method described previously( Reference Zou, Xu and Huang 14 , Reference Yeum, Booth and Sadowski 17 ). Into a 5 ml disposable test-tube, 500 μl of serum and 500 μl of internal standard (β-apo-8′-carotenal) were pipetted and then 500 μl ethanol were added and vortexed for 1 min. To the sample, 700 μl hexane was added and vortexed for 2 min, followed by centrifugation for 5 min (10 000 g and 4°C). The upper layer was collected, and the hexane process was repeated three times. The hexane extract was evaporated under N2 at 4°C. The residue was dissolved in solvent A (methanol–acetonitrile 40:60, v/v), and 20 μl were injected into the HPLC column.
Lutein, zeaxanthin, β-carotene and lycopene were adequately separated, and carotenoid concentrations were quantified by determining peak areas in HPLC chromatograms calibrated against known amounts of the standard. The concentrations were corrected for extraction and handling losses by monitoring the recovery of the internal standard. The lower limit of detection was 0·1 μg/ml for carotenoids.
Statistical analyses
All continuous data are presented as means and standard deviations or standard errors, and categorical data are reported as percentage. The comparison of continuous variables among the three groups was made using one-way ANOVA, and the difference in categorical data was evaluated using the χ2 test. Differences between baseline and follow-up measurements within the groups were determined using a paired-samples t test and a general linear regression analysis. Independent-samples t test was used to compare differences between variables between two groups. A linear correlation was used to assess the association between the change in CAIMT from baseline and the change in serum lutein or lycopene concentrations adjusted for sex and age. All the analyses were carried out with SPSS 11.0 for Windows (SPSS, Inc.).
Results
Participant characteristics
Of the 360 participants screened for the present study, 144 met the study criteria and were assigned randomly to receive treatment (Fig. 1). Among these subjects, three discontinued the treatment and seven were lost to follow-up. Furthermore, nine participants who were using statin medications during the intervention were also excluded. Therefore, all analyses for the present study were based on a sample of 125 participants undergoing the treatment. Of the 125 participants, seventy-nine (63·2 %) were women and forty-six (36·8 %) were men. The mean age of the participants was 57·3 (sd 4·8) years, and demographic variables and baseline characteristics were well balanced across all the groups, including the percentage of participants aged above 60 years in each group (Table 1). The participants reported no bloating, diarrhoea, allergies or other adverse events during the 12 months. Capsule compliance, defined as taking at least twenty-seven capsules per month, was 100 % in all the groups, and no significant change in the patterns of dietary intake was observed among the groups for the treatment.
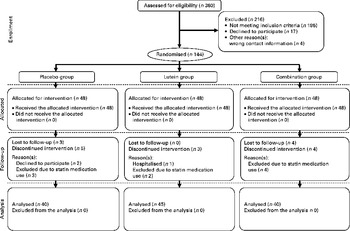
Fig. 1 Flow diagram showing the allocation of the participants of the present study. Placebo group, receiving capsules containing starch; lutein group, receiving capsules containing 20 mg lutein/d; combination group, receiving capsules containing 20 mg lutein/d+20 mg lycopene/d.
Changes in serum lutein and lycopene concentrations
A significantly positive change in serum lutein and lycopene concentrations in response to the supplementation was observed, whereas no significant change was observed in the placebo group (Table 2). Serum lutein concentrations increased significantly in both the lutein and combination groups from baseline to month 4 (both P< 0·01), with no significant difference being observed between the two groups. Serum lutein and lycopene concentrations increased from 0·34 to 1·96 μmol/l (5·8-fold) in the lutein group and from 0·35 to 1·66 μmol/l (4·7-fold) in the combination group, respectively (P< 0·01). No significant difference in serum lutein concentrations in the same period was observed between the lutein and combination groups. Similarly, serum lycopene concentrations increased significantly from 0·18 to 0·71 μmol/l (3·9-fold) at month 12 (P< 0·01), whereas no significant changes were observed in the lutein and placebo groups.
Table 2 Change in serum lutein and lycopene concentrations in subjects with subclinical atherosclerosis (Mean values and standard deviations)
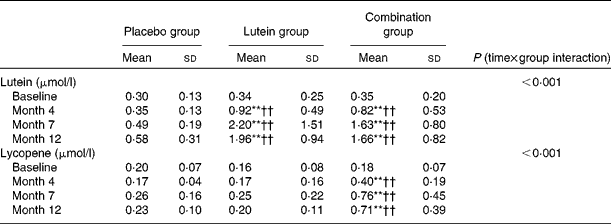
Placebo group, receiving capsules containing starch; lutein group, receiving capsules containing 20 mg lutein/d; combination group, receiving capsules containing 20 mg lutein/d+20 mg lycopene/d.
Mean values were significantly different from the baseline values: ** P< 0·01 (paired t test).
Mean values were significantly different from those of the placebo group: †† P< 0·01 (independent-samples t test).
Changes in common carotid artery intima–media thickness
The changes in CAIMT among the three groups are shown in Fig. 2. Compared with baseline values, the mean values of CAIMT decreased significantly by 0·035 mm (P= 0·042) and 0·073 mm (P< 0·001) in the lutein and combination groups at month 12, respectively. The mean change in CAIMT in the combination group was also significantly higher than that in the lutein and placebo groups. A significant decrease in CAIMT from baseline was observed in the lutein (P< 0·05) and combination (P< 0·01) groups at month 12, and no significant change in CAIMT was detected in the placebo group.
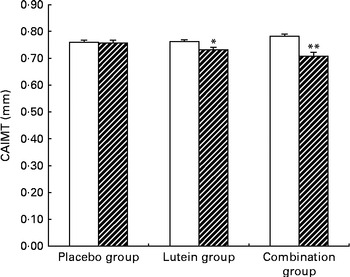
Fig. 2 Average carotid artery intima–media thickness (CAIMT) among the groups at baseline (□) and month 12 (![]() ). Placebo group, receiving capsules containing starch; lutein group, receiving capsules containing 20 mg lutein/d; combination group, receiving capsules containing 20 mg lutein/d+20 mg lycopene/d. Values are means, with their standard errors represented by vertical bars. Mean values were significantly different from the baseline values of CAIMT: * P< 0·05, ** P< 0·01.
). Placebo group, receiving capsules containing starch; lutein group, receiving capsules containing 20 mg lutein/d; combination group, receiving capsules containing 20 mg lutein/d+20 mg lycopene/d. Values are means, with their standard errors represented by vertical bars. Mean values were significantly different from the baseline values of CAIMT: * P< 0·05, ** P< 0·01.
An association between the change in CAIMT and the change in serum lutein concentrations (Fig. 3(a)) or the change in serum lycopene concentrations (Fig. 3(b)) was observed. The change in CAIMT was inversely associated with the increase in serum lutein concentrations in the lutein (β = − 0·340, P= 0·030) and combination (β = − 0·365, P= 0·021) groups and also inversely associated with the increase in serum lycopene concentrations (β = − 0·342, P= 0·031) in the combination group.
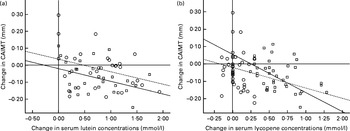
Fig. 3 Scatter plots showing the relationship between the change in carotid artery intima–media thickness (CAIMT) and the change in (a) serum lutein and (b) serum lycopene concentrations in the treatment groups after 12 months. Placebo group, receiving capsules containing starch; lutein group, receiving capsules containing 20 mg lutein/d (![]() ); combination group, receiving capsules containing 20 mg lutein/d+20 mg lycopene/d (
); combination group, receiving capsules containing 20 mg lutein/d+20 mg lycopene/d (![]() ). The curves in both plots indicate the linear regression functions for each group. Linear regression coefficients β and nominal P values were calculated. (a) Lutein group: β = − 0·340, P= 0·030; combination group: β = − 0·365, P= 0·021. (b) Lutein group β = − 0·251, P= 0·114; combination group: β = − 0·342, P= 0·031.
). The curves in both plots indicate the linear regression functions for each group. Linear regression coefficients β and nominal P values were calculated. (a) Lutein group: β = − 0·340, P= 0·030; combination group: β = − 0·365, P= 0·021. (b) Lutein group β = − 0·251, P= 0·114; combination group: β = − 0·342, P= 0·031.
Discussion
The primary findings of the present study were the significantly positive changes in serum lutein and lycopene concentrations in response to the lutein and lycopene treatment and the significant decrease in CAIMT from baseline in the lutein (P< 0·05) and combination (P< 0·01) groups after 12 months of intervention. Furthermore, the change in CAIMT was inversely associated with the increase in serum lutein concentrations (P< 0·05) in both the active treatment groups and with that in serum lycopene concentrations (P< 0·05) in the combination group, and no significant change in CAIMT was detected in the placebo group.
CAIMT can be used to detect early atherosclerosis as a marker to predict cardiac events including myocardial infarction and stroke( Reference Tu, Wang and Lin 18 ). Though atherosclerosis is an inevitable degenerative consequence of ageing, the progress of atherosclerosis can be slowed down through some approaches( Reference Lusis 19 ). In the present study, the mean baseline value of lutein concentrations in all the participants was 0·33 (sd 0·20) μmol/l, which was similar to that in the participants free of symptomatic CVD in the Los Angeles Study (0·28 (sd 0·12) μmol/l)( Reference Dwyer, Navab and Dwyer 10 ). The mean baseline value of lycopene concentrations was 0·18 (sd 0·07) μmol/l, which was slightly higher than that reported in the Rotterdam Study (0·13 (sd 0·09) μmol/l)( Reference Klipstein-Grobusch, Launer and Geleijnse 12 ), but far lower than that reported in the Los Angeles Study (0·76 (sd 0·72) μmol/l)( Reference Dwyer, Navab and Dwyer 10 ). The Los Angeles Atherosclerosis Study also has reported that subjects with the highest serum lutein concentrations (0·42 μmol/l) have 80 % less arterial wall thickening relative to those at the lowest quintile of serum lutein concentrations (0·15 μmol/l)( Reference Dwyer, Navab and Dwyer 10 ), which is consistent with the findings of our previous study( Reference Zou, Xu and Huang 14 ). The Atherosclerosis Risk in Communities (ARIC) Study also has found that there is a possible beneficial effect of consuming a lutein-rich diet( Reference Kritchevsky, Shimakawa and Tell 20 ), which is acknowledged by the CUDAS (Perth Carotid Ultrasound Disease Assessment Study) investigators( Reference McQuillan, Hung and Beilby 21 ). In both the lutein and combination groups, serum lutein concentrations increased after lutein intervention and the mean value of CAIMT also decreased significantly after the 12-month trial, whereas no significant change in CAIMT was detected in the placebo group. Furthermore, the linear correlation showed that the decrease in CAIMT was inversely associated with the increase in serum lutein concentrations in the lutein (β = − 0·340, P= 0·030) and combination (β = − 0·365, P= 0·021) groups. These results indicate that high serum lutein concentrations might be protective against the development of CAIMT in Chinese subjects with subclinical atherosclerosis, which is consistent with the findings of previous studies( Reference Dwyer, Navab and Dwyer 10 , Reference Zou, Xu and Huang 14 ).
Other studies have reported that lycopene is also inversely associated with CAIMT and the calcified plaques of the abdominal aorta( Reference Klipstein-Grobusch, Launer and Geleijnse 12 , Reference McQuillan, Hung and Beilby 21 ) and that the synergistic effect of lutein and lycopene is most pronounced during the protection of multilamellar liposomes against oxidative damage( Reference Stahl, Junghans and de Boer 7 ). In the present study, serum lycopene concentrations increased from 0·18 μmol/l at baseline to 0·71 μmol/l (3·9-fold) at month 12 in the combination group, whereas no significant changes were observed in the lutein and placebo groups. A pooled analysis of thirteen lipid-lowering trials has reported an annual rate of change in mean common CAIMT of 0·0147 mm (95 % CI 0·0122, 0·0173 mm) and estimates of 0·0170 mm/year for subjects with previous CHD (95 % CI 0·0114, 0·0227 mm)( Reference Bots, Evans and Riley 22 ). In the present study, the mean value of CAIMT decreased significantly by 0·035 mm (P= 0·042) and 0·073 mm (P< 0·001) in the lutein and combination groups at month 12, respectively. The improvements observed in the present study were greater than the change reported in the thirteen lipid-lowering trials of CAIMT of 0·0147 or 0·0170 mm. In the present study, the decrease in CAIMT was inversely associated with the increase in serum lycopene concentrations (β = − 0·342, P= 0·031). Therefore, these results suggest that the combination of lutein and lycopene supplementation was more effective than lutein alone, and the present study reports a new strategy for preventing the development of atherosclerosis.
To our knowledge, this is the first randomised controlled trial to study the effects of lutein and lycopene in subjects with subclinical atherosclerosis, and the present study provides evidence for their subclinical application. Both the Beta-Carotene and Retinol Efficacy Trial (CARET) and the Alpha-Tocopherol Beta-Carotene Cancer Prevention (ATBC) Trial have reported that supplements containing β-carotene are harmful to cigarette smokers, causing increased incidences of lung cancer and overall mortality( Reference Omenn, Goodman and Thornquist 9 , Reference Albanes, Heinonen and Taylor 23 ). However, the supplements used in the present study did not contain β-carotene, and no adverse events were reported by the participants of the present study and other lutein trials on age-related macular degeneration patients( Reference Olmedilla, Granado and Blanco 24 , Reference Aleman, Duncan and Bieber 25 ). The present study has limitations that need to be considered. First, no completely blank control population was studied to assess the dietary intake of lutein and lycopene. As we know, people eat lots of fruits and vegetables in China and carotenoids including lutein and lycopene are abundant in most fruits and vegetables. A food survey carried out in 2009–10 by the United States Department of Agriculture has reported that people had daily dietary intakes of 1·29 (sd 0·08) mg lutein+zeaxanthin and 5·14 (sd 2·04) mg lycopene in the USA( 26 ). Therefore, even subjects in the placebo group ingested a certain amount of lutein and lycopene with their diet everyday. Second, the trial duration of 12 months may not be long enough to assess the potential long-term effects on the progression of atherosclerosis, and further research is needed to evaluate the effects of the combination of diverse carotenoids on the incidence of myocardial infarction and stroke.
Conclusions
In conclusion, the present study indicates that lutein and lycopene supplementation could lead to high serum concentrations of lutein and lycopene, with a decrease in CAIMT being associated with both concentrations. In addition, the combination of lutein and lycopene supplementation was more effective than lutein alone for protection against the development of CAIMT in Chinese subjects with subclinical atherosclerosis, which can be used as a new strategy for preventing the development of atherosclerosis. Further studies are needed to confirm whether synergistic effects of lutein and lycopene exist.
Acknowledgements
The authors thank Jiawei Zuo, Aiyan Zhu and Yuanyuan Li (Department of Ultrasound Diagnosis, Peking University Third Hospital) for their technical help in the assessment of CAIMT and also thank Bodi Hui (Beijing Union University) and Jingyu Wang, Ninghua Huang, Yan Xie, Jing Zeng and Lailai Yan (Central Laboratory, Peking University) for their instructions on HPLC analysis of carotenoids.
The present study was supported by grants from the National Natural Science Foundation of China (NSFC-30972472). The National Natural Science Foundation of China had no role in the design and analysis of the present study or in the writing of this article.
X.-M. L. was the principal investigator and the leader of the experimenters; Z.-Y. Z. was the chief experimenter of the study and responsible for manuscript preparation; X.-R. X. was an experimenter and carried out the statistical analyses; H.-B. Z., X. X., L. O., Y.-M. H., X. W. and Y.-Q. L. were the experimenters.
None of the authors has any conflicts of interest.







