Introduction
Verminotic pneumonia caused by metastrongylids Parafilaroides spp. (Metastrongyloidea: Filaroididae) and Otostrongylus circumlitus (Metastrongyloidea: Crenosomatidae) is frequently reported in pinnipeds in different parts of the world (Onderka, Reference Onderka1989; Dailey, Reference Dailey, Dierauf and Gulland2001, Reference Dailey2009; Siebert et al., Reference Siebert, Wohlsein, Lehnert and Baumgartner2007; Reisfeld et al., Reference Reisfeld, Sacristán, Sánchez-Sarmiento, Costa-Silva, Díaz-Delgado, Groch, Marigo, Ewbank, Favero, Guerra, Réssio, Cremer, Esperón and Catão-Dias2019). While the nematode O. circumlitus affects different hosts, the genus Parafilaroides has several species that cause lung disease in pinnipeds from different regions (Dailey, Reference Dailey2006, Reference Dailey2009; Jacobus et al., Reference Jacobus, Marigo, Gastal, Taniwaki, Ruoppolo, Catão-Dias and Tseng2016; Reisfeld et al., Reference Reisfeld, Sacristán, Sánchez-Sarmiento, Costa-Silva, Díaz-Delgado, Groch, Marigo, Ewbank, Favero, Guerra, Réssio, Cremer, Esperón and Catão-Dias2019; Echenique et al., Reference Echenique, Pereira, Prado, Schild and Valente2020). The parasitic cycle of both genera is not well known, but experimental studies involving Parafilaroides decorus and O. circumlitus have concluded that fish preyed on by pinnipeds, respectively as opaleye (Giretta nigricans) (Dailey, Reference Dailey1970) and turbot (Psetta maxima) (Lehnert et al., Reference Lehnert, Von Samson-Himmelstjerna, Schaudien, Bleidorn, Wohlsein and Siebert2010), serve an important role as intermediate hosts. The great diversity of parasitic species from the same genus that affect pinnipeds is probably related to the affinity of each parasite to its respective intermediate and definitive hosts; thus, fur seals of the same species and from the same region, which feed on prey from similar habitats, usually have the same parasitic species (Wells and Clark, Reference Wells and Clark2019).
In fur seals, verminotic pneumonias are caused predominantly by Parafilaroides spp. and, to date, there is only evidence of an Antarctic fur seal with O. circumlitus pneumonia (Measures, Reference Measures, Samuel, Pybus and Kocan2001; Dailey, Reference Dailey2009; Jacobus et al., Reference Jacobus, Marigo, Gastal, Taniwaki, Ruoppolo, Catão-Dias and Tseng2016; Seguel et al., Reference Seguel, Nadler, Field and Duignan2018; Reisfeld et al., Reference Reisfeld, Sacristán, Sánchez-Sarmiento, Costa-Silva, Díaz-Delgado, Groch, Marigo, Ewbank, Favero, Guerra, Réssio, Cremer, Esperón and Catão-Dias2019; Echenique et al., Reference Echenique, Pereira, Prado, Schild and Valente2020). Parafilaroides spp. comprise 7 species of nematodes distributed around the world (Measures, Reference Measures, Samuel, Pybus and Kocan2001; Dailey, Reference Dailey2006, Reference Dailey2009; Jacobus et al., Reference Jacobus, Marigo, Gastal, Taniwaki, Ruoppolo, Catão-Dias and Tseng2016; Rhyan et al., Reference Rhyan, Garner, Spraker, Lambourn and Cheville2018; Reisfeld et al., Reference Reisfeld, Sacristán, Sánchez-Sarmiento, Costa-Silva, Díaz-Delgado, Groch, Marigo, Ewbank, Favero, Guerra, Réssio, Cremer, Esperón and Catão-Dias2019; Echenique et al., Reference Echenique, Pereira, Prado, Schild and Valente2020); of these, only Parafilaroides hydrurgae and Parafilaroides normani have been reported from the Southern Hemisphere in seals (Hydrurga leptonyx) and fur seals (Measures, Reference Measures, Samuel, Pybus and Kocan2001; Dailey, Reference Dailey2009; Jacobus et al., Reference Jacobus, Marigo, Gastal, Taniwaki, Ruoppolo, Catão-Dias and Tseng2016; Echenique et al., Reference Echenique, Pereira, Prado, Schild and Valente2020), respectively. The fur seal was infected by P. normani, which was a specimen of Arctocephalus australis and presented occurrence at Cassino beach, located on the Southern coast of Rio Grande do Sul in Brazil (Echenique et al., Reference Echenique, Pereira, Prado, Schild and Valente2020). This work aims to identify and quantify the occurrence of pneumonia concomitant with the presence of helminths detected by polymerase chain reaction (PCR) and histology, in South American fur seals found dead on the northern coast of Rio Grande do Sul, as well as to characterize the macroscopic and histological lesions caused by them.
Material and methods
During the period between autumn and spring 2019–2020, weekly monitoring was carried out on the northern coast of Rio Grande do Sul between the beaches of Tramandaí (30°00′20.6″S 50°07′54.8″W) and the end of Palmares do Sul (30°28′40.4″S 50°19′18.9″W) in partnership with Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA; SISBIO: 77472-1; SISBIO: 61987). Twenty-six South American fur seals classified as codes 2 and 3, according to the decomposition classification, were identified and analysed as per the protocol of Geraci and Lounsbury (Reference Geraci and Lounsbury2005). The age of the animals was determined by the total length of the animals (Ponce de Léon, Reference Ponce de León1983; Katz et al., Reference Katz, Morgades and Castro-Ramos2012), while body condition was measured using the adipose tissue of the xiphoid process region and then classified as bad, moderate or good (Geraci and Lounsbury, Reference Geraci and Lounsbury2005; Katz et al., Reference Katz, Morgades and Castro-Ramos2012). Necropsy was performed in a conventional manner (Geraci and Lounsbury, Reference Geraci and Lounsbury2005) and fragments of different organs were collected for histological analysis, fixed in 10% buffered formalin and processed routinely. For each animal, 4 fragments were collected from different lung lobes, selected by macroscopic alterations or, if absent, randomly, for histological analysis, totalling 104 fragments.
Two lung fragments from each animal were collected, frozen at −20°C and pooled and subjected to DNA extraction using the commercial Pure Link® Genomic DNA Mini Kit (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer's recommendations. For the PCR, primers (5′GCAGACGCTTAGAGTGGTGAAA3′/R 5′ACTCGCCGTTACTAAGGGAATC3′) flanked in the adjacent genes 5.8S and 28S were used, targeting the ITS-2 gene according to Lenerth et al. (Reference Lehnert, Von Samson-Himmelstjerna, Schaudien, Bleidorn, Wohlsein and Siebert2010). The PCR products were subjected to electrophoresis in 1.5% agarose gel and later visualized in a light-emitting diode transilluminator.
Samples that were equally positive on PCR and histology were selected for purification and subsequent sequencing. Amplicons were purified with the InvitrogenTM PureLinkTM Quick PCR Purification Kit (Thermo Fisher Scientific), as per the manufacturer's recommendations and sequenced on an automated sequencer (Sanger, Cambridgeshire, UK). The generated sequences were submitted to BLAST® analysis (Altschul et al., Reference Altschul, Gish, Miller, Myers and Lipman1990) to determine similarity.
The partial sequences of the present study were aligned with another 12 corresponding sequences from Metastrongylids and a sequence from Dictyocaulus filaria (as an outgroup) available from GenBank® using Clustal/W v.1.8.1 (Thompson et al., Reference Thompson, Higgins, Gibson and Clustal1994). A maximum-likelihood phylogenetic tree with 260 informative sites was generated using the Hasegawa–Kishino–Yano + G substitution model. This was created with the aid of Mega 10 software, using 100 bootstrap replicas (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018), in accordance with the lowest Bayesian information criterion score. An identity matrix with 452 informational sites was calculated with the BioEdit software using partial sequences of ITS-2 from Parafilaroides species deposited in GenBank and those found in this study (Table 1).
Table 1. Identity matrix of the Parafilaroides sp. sequence for the present study (1 at 6) and isolates of Parafilaroides deposited in GenBank®

One slide for each case that was identified as positive both during histology and following sequencing for Parafilaroides sp. was selected for immunohistochemical analysis for the detection of Brucella spp. associated with parasites. The detection system used was the universal polymer bound to horseradish (MACH-A Universal HRP-Polymer, Biocare, Pacheco, USA) and the polyclonal anti-Brucella abortus primary antibody (non-commercial) produced in rabbits. Antigen retrieval was achieved with Proteinase K (Dakocytomation, Carpinteria, CA, USA) for 10 min and the reactions were revealed with chromogen 3-amino-9-ethylcarbazole (AEC Romulin; Biocare, Pacheco, USA). As a positive control, lung slides from a bovine fetus infected with B. abortus (Antoniassi et al., Reference Antoniassi, Juffo, Pescador, Corbellini, Sonne, Gomes, Nakazato and Driemeier2016) were used and negative controls were incubated with commercial universal serum (Universal Negative Control Serum; Biocare, Pacheco, USA).
Results
Of all 26 fur seals of the species A. australis collected, 14 animals (53.8%) were classified as sub-adults, 8 as juveniles (30.8%) and 4 as adults (15.4%). Fifteen animals were males (57.7%) and 11 were females (42.3%). At necropsy, poor body condition was observed in 11 (42.3%) South American fur seals, good in 8 (30.7%) and moderate in 7 animals (26.9%). Gross and histological changes were analysed and the cause of death was determined for each fur seal when possible. Of these, 9 (34.6%) were diagnosed with traumas of different natures according to the macroscopic characteristics of the lesions – blunt trauma, puncture-cutting and blunt-blunt trauma (most frequent); 8 animals with poor body condition score and an empty stomach, sometimes containing liquid, were diagnosed with starvation (30.7%); and 9 were inconclusive (34.6%) due to unspecific morphological alterations (Table 2).
Table 2. Causes of death of South American fur seals infected by Parafilaroides sp., stranded in Southern Brazil

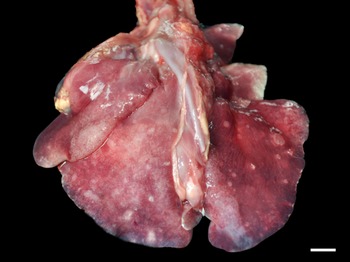
Grossly, only 1 fur seal infected by Parafilaroides sp. (1/26; 4%) presented pulmonary alterations in all lung lobes, characterized by a moderate amount of circumscribed, white and sub-pleural nodular areas, with slight elevation, ranging from 0.5 to 2.5 cm in diameter, in addition to moderate diffuse oedema and multifocal to coalescing emphysema (Fig. 1). In another 14 animals analysed, which were later diagnosed with parafilaroidiasis by histology or PCR, lesions were interpreted as non-specific, such as pulmonary oedema, multifocal emphysema and small foci of atelectasis.

Fig. 1. Verminotic pneumonia by Parafilaroides sp. in the South American fur seal. Multiple, whitish nodules measuring 0.5–2.5 cm in the lung lobes (3 cm bar).
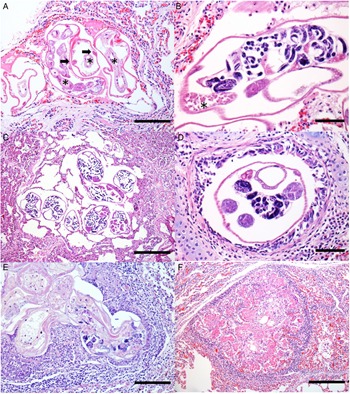
With respect to the histology results, the pulmonary alterations found in the 12 animals, related to verminotic pneumonia, were classified as mild or moderate according to their extension and severity. No animal was diagnosed with severe verminotic pneumonia, and the individuals identified with the most severe conditions were associated with the most necrotic, inflammatory lesions, predominantly formed by granulomas randomly distributed in the lung parenchyma. In 46.1% (12/26) of the South American fur seals, transverse and longitudinal sections of nematodes were observed, ranging from 30 to 130 μm. Groups of 2–8 parasites were observed that exhibited a thin eosinophilic cuticle, pseudocoelomatic cavity, polymyar/coelomian musculature, thin hypodermis, lateral cords and an evident digestive system (Fig. 2A). They were dioecious, with the male being smaller and less observed than females. The females had ovaries, eggs (Fig. 2B) and a uterus with or without larval eggs. Sperm deposits were sometimes observed in females (Fig. 2B).

Fig. 2. Histology of verminotic pneumonia caused by Parafilaroides sp. in the South American fur seal. (A) Metastrongylids composed of a thin eosinophilic cuticle; a thin hypodermis where the lateral cords are inserted (arrows) is observed. In the pseudocoelomic cavity, there are several sections of intestine composed of a single cell layer (asterisks) (50 μm bar), haematoxylin and eosin (H&E) stain. (B) Female nematode containing larvae eggs inside the uterus and sperm deposit (asterisk) (20 μm bar), H&E stain. (C) Cross-sections of female nematode parasites with intrauterine larval eggs are observed, organized in groups and located in the alveolar spaces, generating marked alveolar emphysema with the absence of an inflammatory infiltrate. Adjacent to the emphysematous areas, the parenchyma is moderately atelectatic (100 μm bar), H&E stain. (D) Bronchiole lumen with nematode cross-section. Female, with a female reproductive system and eggs hatched inside the uterus (20 μm bar), H&E stain. (E) Degenerated parasitic sections, with and without larval eggs, and surrounded by mononuclear inflammatory cells and fibrin (50 μm bar), H&E stain. (F) Nodular lesion with a granulomatous appearance, characterized by eosinophilic fibrillar material, associated with a mononuclear inflammatory infiltrate and cellular debris, and surrounded by fibrous connective tissue (100 μm bar), H&E stain.
The parasites were predominately found in the alveoli, comprising 75% (9/12) of the fur seals that were positive on microscopy. They were mostly seen in the periphery of the lung lobes, close to the visceral pleura (sub-pleural) or, less commonly, with a random multifocal distribution. Of the 9 cases in which parasites were seen in the alveoli, 5 were found exclusively in the alveolar lumen, while in 4 cases, there were also parasitic cuts in the bronchi or bronchioles. In 16.7% (2/12) of cases, parasites were seen in the bronchial space, and in 41.7% (5/12) they were identified in the bronchiolar space. Numerous emphysematous areas of variable extent, generally related to the number of nematodes (Fig. 2C), were associated with the luminal location of the parasites. Obstructive pulmonary atelectasis was frequently observed in these cases, and they were mainly adjacent to the areas of emphysema. The presence of the parasite inside the bronchi and bronchioles also caused alveolar pulmonary emphysema, and also led to the accumulation of a discrete amount of mucinous material in the lumen. In 33.3% of the animals (4/12), multifocal circumscribed areas composed of epithelioid and foamy macrophage infiltrates, and a smaller amount of eosinophils and fibrin, were observed, which involved parasites in different stages of degeneration (Fig. 2E). Externally to these structures, there was a moderate infiltrate of lymphocytes and plasma cells, surrounded by bundles of collagenous connective tissue (Fig. 2F). In the other 8 cases (66.7%), there was a slight interstitial infiltrate of lymphocytes around the adult nematodes. Of these animals with mild-to-moderate inflammation associated with these nematodes, different areas in the lung parenchyma with groups of parasites without an associated inflammatory reaction were frequent, comprising 66.6% of cases (8/12). Multifocal and moderate pulmonary arterial thrombosis was observed in 2 parasitized animals (2/12; 16.7%). Circulatory changes, such as congestion and diffuse alveolar oedema, were frequently noted in the lungs of these infected animals.
Of the 26 lung samples tested during PCR with ITS-2, 9 had bands of the expected size. The 6 sequenced samples (selected because they were also positive during histology) showed sequences compatible with Parafilaroides sp. registered in GenBank with accession numbers KP402084 and KP402085, by Jacobus et al. (Reference Jacobus, Marigo, Gastal, Taniwaki, Ruoppolo, Catão-Dias and Tseng2016), with a percentage similarity ranging from 99.82 to 100%. The sequences were deposited in GenBank under the following accession numbers: ON059340, ON059341, ON059342, ON059343, ON059344 and ON059345. The respective amplified base pairs are specified in Table 3.
Table 3. GenBank BLAST and results for the ITS-2 region in Parafilaroides sp.

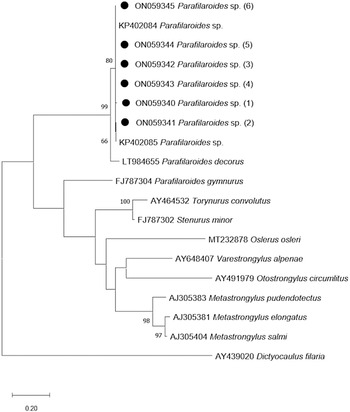
The phylogenetic tree that was developed in this study demonstrates a close genetic relationship with the species of Parafilaroides spp. (Fig. 3), forming a clade with the sequences KP402084 and KP402085 (Jacobus et al., Reference Jacobus, Marigo, Gastal, Taniwaki, Ruoppolo, Catão-Dias and Tseng2016) with 80% bootstrap. In addition, with 99% bootstrap, a macroclade including the species P. decorus that also parasitizes on pinnipeds was formed.

Fig. 3. Maximum-likelihood phylogenetic tree 2. Performed with the Hasegawa–Kishino–Yano model with a gamma distribution (+G) of partial sequences of the ITS-2 gene. The numbers in the branches indicate bootstrap values. Only bootstrap values >50 are counters. The numbers displayed next to the species name are the GenBank® accession numbers of the sequences used. The generated sequences for the Parafilaroides sp. are in bold, indicated by a black circle.
The 6 individuals with confirmed parafilaroidiasis at PCR and histology had their lungs submitted for an immunohistochemical test to detect anti-B. abortus, and the results were negative (absence of immunostaining).
Discussion
The diagnosis of Parafilaroides sp. in the South American fur seals from the present study was established by histological analysis, PCR and sequencing. At necropsy, Parafilaroides spp. are described as small and delicate parasites (Jacobus et al., Reference Jacobus, Marigo, Gastal, Taniwaki, Ruoppolo, Catão-Dias and Tseng2016) and are responsible for the formation of parasitic granulomas commonly described in pinnipeds (Measures, Reference Measures, Samuel, Pybus and Kocan2001; Reisfeld et al., Reference Reisfeld, Sacristán, Sánchez-Sarmiento, Costa-Silva, Díaz-Delgado, Groch, Marigo, Ewbank, Favero, Guerra, Réssio, Cremer, Esperón and Catão-Dias2019). However, such lung injury was observed in only 1 animal in the present study. Another macroscopic presentation characterized by the formation of small, yellowish pustules, which contained parasites inside them, was described in pinnipeds parasitized by Parafilaroides spp. (Dailey, Reference Dailey1970; Echenique et al., Reference Echenique, Pereira, Prado, Schild and Valente2020). According to Dailey (Reference Dailey1970), such a lesion was visualized when the infection was massive, and these small, firm nodules were associated with the formation of parasitic granulomas in degenerated specimens. This type of inflammatory reaction would occur in animals that are more susceptible to infections, such as in debilitated individuals and in stressful situations (Measures, Reference Measures, Samuel, Pybus and Kocan2001). As in the present study, most cases of pulmonary verminosis in fur seals were not diagnosed macroscopically (Onderka, Reference Onderka1989; Measures, Reference Measures, Samuel, Pybus and Kocan2001).
The localization and histological characterization of the identified pulmonary parasites was compatible with the Metastrongyloidea Superfamily (Bowman, Reference Bowman2014). The 2 pulmonary nematodes described in otarids, O. circumlitus and Parafilaroides spp., are mainly distinguished based on the characteristics inherent to their respective families, in which Crematosotidae has a bursa, while in Filaroididae, it is absent or indistinct, observed by the parasitic morphology that unfortunately we were unable to perform due to the absence of adult worms for analysis. Otostrongylus circumlitus is larger and therefore easily identified on macroscopy, in addition to being located in the bronchi, bronchioles and occasionally in the arteries. On the other hand, in Parafilaroides spp., it is smaller and predominantly alveolar in location (Onderka, Reference Onderka1989; Kelly et al., Reference Kelly, Greig, Colegrove, Lowenstine, Dailey, Gulland and Haulena2005). Microscopically, parasitic size may help suggest metastrongylid genus; however, in this study only the molecular tool concluded the diagnosis. Otostrongylus circumlitus presents with an approximately 900 μm, with thick cuticle and hypodermis (Barnett et al., Reference Barnett, Bexton, Fraija-Fernández, Chooneea and Wessels2019), while in Parafilaroides spp., as seen in the present work, they have a diameter ranging from 30 to 130 μm, and a smooth and thin cuticle (Garner et al., Reference Garner, Lambourn, Jeffries, Hall, Rhyan, Ewalt, Polzin and Cheville1997).
Parafilaroides spp. cause respiratory disease in pinnipeds and can result in high mortality rates (Dailey, Reference Dailey1970; Onderka, Reference Onderka1989; Garner et al., Reference Garner, Lambourn, Jeffries, Hall, Rhyan, Ewalt, Polzin and Cheville1997; Siebert et al., Reference Siebert, Wohlsein, Lehnert and Baumgartner2007). However, in South American fur seals, infection by these agents usually causes mild-to-moderate lung lesions, possibly suggesting a strategic co-evolution process to avoid host loss (Onderka, Reference Onderka1989; Sukhdeo, Reference Sukhdeo1994). Although areas of atelectasis and emphysema in the lungs were observed in the present study, they were associated with a slight reduction in the respiratory parenchyma. Furthermore, the inflammatory process was more severe and granulomatous when there were degenerated parasitic structures, similarly seen by Onderka (Reference Onderka1989). Although such pulmonary worm infections have not been considered serious enough to lead to death by themselves, they are associated with some degree of debilitation in these individuals, interfering with their feeding behaviour, their ability to escape from predators and threats, and to rest, even favouring the emergence of secondary infections (Measures, Reference Measures, Samuel, Pybus and Kocan2001). Two animals infected by Parafilaroides sp. died from traumatic causes, and 1 from starvation. These frequent causes of death in South American fur seals were also reported by other authors (Seguel et al., Reference Seguel, Paredes, Pavés, Molina, Henríquez, De Groote and Schlatter2011, Reference Seguel, Paves, Paredes and Schlatter2013; Katz et al., Reference Katz, Morgades and Castro-Ramos2012; Baldassin et al., Reference Baldassin, Amorim, Werneck, Mariani and Alava2017). In Brazil and other South American countries (outside the breeding colonies), traumatic causes are related to the interaction of these animals with dogs, and to human interactions, either when fishing or due to aggression (Katz et al., Reference Katz, Morgades and Castro-Ramos2012; Baldassin et al., Reference Baldassin, Amorim, Werneck, Mariani and Alava2017). Of the 9 cases diagnosed as trauma in this study, 7 were characterized in juvenile or sub-adult animals as resulting from blunt force trauma with frequent perforations in the skin and lacerations of muscle and adjacent tissues, which resembled a dog bite. The animals in this study, whose final diagnosis was determined to be starvation, and who also had verminotic pneumonia, did not present with any lung lesions that were severe enough to justify this as a cause of death. This is unlike what was found in the sub-Antarctic fur seal, as reported in SC state by Reisfeld et al. (Reference Reisfeld, Sacristán, Sánchez-Sarmiento, Costa-Silva, Díaz-Delgado, Groch, Marigo, Ewbank, Favero, Guerra, Réssio, Cremer, Esperón and Catão-Dias2019) which, despite being cachectic and carrying infections by Sarcocystis sp. and gammaherpesviruses, had pneumonia caused by Parafilaroides sp. and was suggested as the cause of death, determined by the most severe lesion.
Studies often describe pinnipeds parasitized by Parafilaroides spp. and these are affected by other aetiological agents. It is further suggested that this nematode is a host for Brucella pinnipedialis (Rhyan et al., Reference Rhyan, Garner, Spraker, Lambourn and Cheville2018; Reisfeld et al., Reference Reisfeld, Sacristán, Sánchez-Sarmiento, Costa-Silva, Díaz-Delgado, Groch, Marigo, Ewbank, Favero, Guerra, Réssio, Cremer, Esperón and Catão-Dias2019). However, the South American fur seals studied by our team did not show immunohistochemical staining with anti-B. abortus polyclonal antibody (Rhyan et al., Reference Rhyan, Garner, Spraker, Lambourn and Cheville2018), suggesting that lung nematodes in this region are not related to this bacterial species or to other species of the same genus, possibly due to the distance between the reproductive colonies and the places in which these animals were found.
Apparently, the species of Parafilaroides have higher occurrences in certain regions, according to the literature already described (Measures, Reference Measures, Samuel, Pybus and Kocan2001; Dailey, Reference Dailey2009; Echenique et al., Reference Echenique, Pereira, Prado, Schild and Valente2020). In this way, although in this present study the pulmonary nematodes were concluded only at the gender level, it is noted that the descriptions in fur seals from the Southern Hemisphere were more frequently related to the species P. normani (Dailey, Reference Dailey2009; Jacobus et al., Reference Jacobus, Marigo, Gastal, Taniwaki, Ruoppolo, Catão-Dias and Tseng2016; Echenique et al., Reference Echenique, Pereira, Prado, Schild and Valente2020). In Brazil, previous reports suggest (Jacobus et al., Reference Jacobus, Marigo, Gastal, Taniwaki, Ruoppolo, Catão-Dias and Tseng2016) or affirm (Echenique et al., Reference Echenique, Pereira, Prado, Schild and Valente2020) that the species of lung worms that affected fur seals was P. normani.
In addition to the fact that the morphology observed in the histological analyses was consistent with the genus Parafilaroides, the phylogenetic analysis results showed high similarity with the sequences KP402084 and KP402085 with respect to ITS-2, which was deposited as Parafilaroides sp. by Jacobus et al. (Reference Jacobus, Marigo, Gastal, Taniwaki, Ruoppolo, Catão-Dias and Tseng2016). Although the authors deposited the ITS-2 sequences as Parafilaroides sp. when they sequenced a fragment of the cytochrome c oxidase subunit I mitochondrial DNA gene from one of their samples, it showed 100% similarity with P. normani (Jacobus et al., Reference Jacobus, Marigo, Gastal, Taniwaki, Ruoppolo, Catão-Dias and Tseng2016). Since the sequences of the present study present high similarity with those of the study by Jacobus et al. (Reference Jacobus, Marigo, Gastal, Taniwaki, Ruoppolo, Catão-Dias and Tseng2016), as demonstrated in the identity matrix, it is highly likely that the species involved in the pulmonary verminosis of the specimens studied here is also P. normani. Furthermore, the monophyletic relationship of the sequences from the present study with the sequences produced by Jacobus et al. (Reference Jacobus, Marigo, Gastal, Taniwaki, Ruoppolo, Catão-Dias and Tseng2016), as well as the high bootstrap value (80%) of the branch in question and the percentage of similarity shown in the matrix, reaffirms the possibility that they are the same species. When compared to the molecular test, the morphological evaluation proved to be superior for the detection of pulmonary parasites. Since the fragments were randomly removed from different areas, the difference in analysis results was possibly due to the multifocal nature of the lesion, which was associated with the greater volume of the lung tissue analysed during histology. The 25 mg scanty fragments used for DNA extraction may not contain the parasite. In the same way, we obtained 3 positive results during the molecular analysis of animals that histologically did not present parasitic structures, possibly also due to the random selection of tissues for the different techniques. However, the molecular examination proved to be an excellent complementary tool, not only because of its high specificity, but also because it constitutes another form of diagnostic screening. Furthermore, the results of the PCR products for the ITS-2 gene were satisfactory for sequencing and phylogenetic analysis.
Metastrongylids verminotic pneumonia was morphologically observed in the studied South American fur seals, and the lesions featured a mild-to-moderate morphological pattern. The characteristic macroscopy results of the lesion by Parafilaroides spp. were evidenced in 1 animal, which presented with a nodular, multifocal and moderate lesion due to the formation of granulomas associated with the degenerated parasite. The histology results established the diagnosis, characterized the lung lesion and determined its intensity. Furthermore, PCR, with the amplification of a fragment of the ITS-2 gene, confirmed that the genus was involved in verminotic pneumonia. Combined, these approaches made it possible to suggest that the species involved in this infection is P. normani.
Data
The data that support the findings of this study are available from the authors upon reasonable request.
Acknowledgment
The authors are grateful to Journal Prep Services for the English-language editing of this manuscript.
Author's contributions
Y. D. conceived all stages of the study. R. F. M. conceived the PCR analyses. M. B. B. conceived the immunohistochemical analyses. J. V. Z. E. participated in the histopathological analyses. P. G. C. W. conducted monitoring of beaches where South American fur seals were collected. J. F. S. and S. P. P. conceived and designed the study. All authors participated in the writing of the study.
Financial support
This project was supported by Comissão de Aperfeiçoamento de Pessoal do Nível Superior (CAPES) for the Master scholarship to Y. Daoualibi (Finance Code 001); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the productivity scholarship (S. P. Pavarini 303008/2018-0).
Conflict of interest
None.
Ethical standards
This project was supported by the Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) that granted the Biodiversity Authorization and Information (SISBIO: 77472-1; SISBIO: 61987).