The beneficial effects of the Mediterranean diet are well recognised(Reference Hu1–Reference Estruch3). Olive oil is an integral ingredient of this diet, and it has been suggested that its regular consumption, as the main source of fat, exerts protective effects against the development of CVD(Reference Fung, Rexrode and Mantzoros2–Reference López-Miranda, Pérez-Jiménez and Ros5). Converging evidence suggests that the cardioprotective effects of extra-virgin olive oil are related not only to its high content of oleic acid, which exerts anti-atherosclerotic and anti-inflammatory effects(Reference Harwood and Yaqoob6, Reference Massaro, Carluccio and De Caterina7), but also to the presence of antioxidants, including phenols, in the non-saponifiable fraction(Reference Bendini, Cerretani and Carrasco-Pancorbo8, Reference Cicerale, Lucas and Keast9).
Recently, Bianco et al. (Reference Bianco, Coccioli and Guiso10) have identified in extra-virgin olive oil a new class of ortho-diphenols, 6,7-dihydroxy-isochromans: 1-phenyl-6,7-dihydroxy-isochroman (encoded L137) and 1-(3-methoxy-4-hydroxy-phenyl)-6,7-dihydroxy-isochroman. It has also been demonstrated that these compounds are not present in fresh olive fruits(Reference Guiso, Marra and Arcos11), but their synthesis may begin during the malaxation process and continue during the oil storage, in competition with the ortho-diphenol oxidative degradation, through the reaction between hydroxytyrosol and aldehydes and ketones, concurrently present in the olive oil(Reference Montedoro, Servili and Baldioli12, Reference Cartoni, Coccioli and Jasionowska13). The antioxidant power of the olive oil dihydroxy-isochromans and their ability to inhibit the human platelet response to agonists that induce reactive oxygen species-mediated platelet activation has been demonstrated in our previous study(Reference Togna, Togna and Franconi14).
On the basis of previous reports suggesting anti-atherogenic and anti-inflammatory properties of extra-virgin olive oil(Reference Carluccio, Siculella and Ancora15–Reference Perona, Cabello-Moruno and Ruiz-Gutierrez17), the present study aims to investigate the potential of L137 to modulate the production of key inflammatory mediators by human monocytes, by evaluating its in vitro effects on prostanoid (thromboxane A2 and PGE2) and TNF-α production induced by lipopolysaccharide (LPS).
The effect of L137 on NF-κB-mediated expression of the inducible form of cyclo-oxygenase (COX-2), a pro-inflammatory enzyme responsible for elevated levels of prostanoids(Reference Raso, Meli and Di Carlo18), was also verified.
Methods
Preparation of human peripheral blood mononuclear cell cultures
Fresh EDTA-treated buffy coats from the blood of healthy volunteers were provided by the blood transfusion centre (‘Sapienza’ University of Rome). Peripheral blood mononuclear cells were isolated by centrifugation on a Ficoll–Metrizoate density gradient. The mononuclear cells at the interface were washed twice with Mg2+/Ca2+-free phosphate-buffered solution by re-suspension and centrifugation at 300 g at room temperature. Cells (>90 % monocytes, as determined by non-specific esterase staining) were subsequently tested for their viability by trypan blue exclusion and resuspended in Roswell Park Memorial Institute (RPMI)-1640 medium, supplemented with 10 % heat-inactivated fetal bovine serum, 4 mm-glutamine, penicillin (100 U/ml) and streptomycin (100 μg/ml). Approximately 107 mononuclear cells were plated in twelve-well tissue culture plates, and monocytes were obtained by selective adherence (120 min, 37°C, 5 % CO2). Non-adherent cells were removed and discarded, while the adherent cells were washed carefully twice with pre-warmed medium. Complete medium was then added to the plates, and the cells were cultured for 24 h before treatments.
Experimental procedure
L137 synthesised in our laboratory by the reaction between hydroxytyrosol and benzaldehyde under very mild conditions(Reference Guiso, Marra and Cavarischia19) was dissolved in Tris–HCl buffer solution (pH 7·8) and assayed at concentrations ranging from 0·5 to 100 μm. To investigate the effect of the tested compound on LPS-induced activation, L137 was added to monocyte cultures 30 min before the stimulus (LPS 50 ng/ml) for 24 h incubation. Then, the supernatants were collected for the measurement of prostanoid and cytokine contents and lactate dehydrogenase release, and the cells were used for subsequent protein measurement(Reference Bradford20), electrophoresis separation and the methylthiazoletrazolium test.
In addition, some experiments were conducted in cultured monocytes pre-treated with aspirin or NS-398 to block constitutive COX-1 or inducible COX-2, respectively. According to Demasi et al. (Reference Demasi, Caughey and James21), to block constitutive COX-1, monocytes were pre-treated with 0·05 mm-aspirin for 30 min, and then washed twice with pre-warmed medium. NS-398, a selective COX-2 inhibitor, was added to the culture medium at a concentration of 10 μm, 30 min before the treatment. Human monocytes were then incubated for 24 h in a complete medium and primed with LPS (50 ng/ml), in the presence or absence of L137. TXB2 (the stable breakdown product of TXA2) and PGE2 concentrations were determined by RIA. The least detectable concentration was 2 pg/ml for both assays.
In order to rule out the possible effect of L137 on the peroxidase activity of COX-2 (conversion of PGG2 to PGE2), the cells were pre-incubated with 5 mm-aspirin to block COX, but not peroxidase, activity of COX-2, and then treated with L137 (10 and 50 μm). PGG2 (5 μm) was used as the substrate to generate PGE2 bypassing the COX step(Reference Wang, Bai and Zhu22).
TNF-α immunoreactivity was measured using a specific human ELISA kit (sensitivity < 9 pg/ml). Cell viability was determined by the lactate dehydrogenase and methylthiazoletrazolium tests.
Western blotting analysis of cyclo-oxygenase-2 and NF-κB expression
Proteins (30 μg) from each sample were denatured in boiling Laemmli buffer for 5 min and resolved by SDS-PAGE on a polyacrylamide gel consisting of a 4 % stacking and a 10 % resolving layer using a Mini-PROTEAN II apparatus (BioRad, Hercules, CA, USA). After electrophoresis, proteins were transferred to nitrocellulose membranes; the membranes were blocked for 1 h at room temperature in Tris-buffered saline–0·1 % Tween 20 (supplemented with 1 % fat-free dried milk and 1 % bovine serum albumin) incubated with primary antibodies, with horseradish peroxidase-conjugated secondary antibodies, and then detected by the enhanced chemiluminescence detection system.
Statistical analysis
Data are presented as means with their standard errors. For statistical analysis, data were analysed by a one-way ANOVA, followed by Bonferroni's post hoc test. A significant difference was defined as a P value < 0·05.
Results and discussion
In the present study, the potential of L137 to inhibit pro-inflammatory mediator production was investigated in vitro by using LPS-stimulated adherent human monocytes. LPS treatment caused a strong increase in prostanoid production, and L137 significantly inhibited the production of PGE2 and TXA2 starting from 1 and 10 μm, respectively (Table 1). To confirm that the inhibiting effect of L137 is not due to cytoxicity, we used lactate dehydrogenase and methylthiazoletrazolium tests. Results showed that at the employed concentrations, cell viability was not affected by L137 (data not shown).
Table 1 Inhibiting effect of 1-phenyl-6,7-dihydroxy-isochroman (L137) on prostanoid production induced by lipopolysaccharide (LPS) in human monocytes pre-treated or not with aspirin (ASA)
(Mean values with their standard errors)†
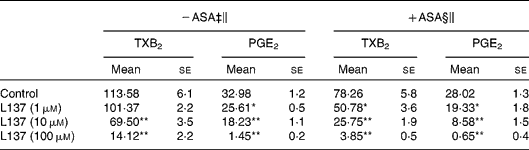
TX, thromboxane.
Mean values were significantly different from the control: *P < 0·01, **P < 0·001.
† Performed in triplicate on cells from four different donors.
‡ Monocytes were treated with L137 or buffer solution (control) 30 min before LPS (50 ng/ml).
§ Monocytes were pre-treated with ASA (0·05 mm) for 30 min, then washed twice and stimulated with LPS (50 ng/ml) in the presence or absence of indicated concentrations of L137.
∥ After 24 h incubation, the medium was removed, and prostanoid concentration (ng/mg protein) was determined by RIA.
As has been clearly described(Reference Brock, McNish and Peters-Golden23), synthesis of some prostanoid products depends on different COX isoforms: monocyte TXA2 on both COX-1 and COX-2 and PGE2 mainly on COX-2 activity, respectively. In order to quantify the inhibitory potential of L137 on COX-2 activity, experiments were carried out on monocytes pre-treated with aspirin so as to block the prostanoid production derived from the constitutive isoform of COX (COX-1). Aspirin pre-treatment, in fact, followed by several washings is able to inhibit COX-1 activity, but does not significantly affect COX-2-derived PGE2 and TXB2 production induced by LPS(Reference Demasi, Caughey and James21). As reported in Table 1, the inhibitory effect of L137 on COX-2-derived prostanoid production reached statistical significance even at the lowest concentration assayed (1 μm), suggesting that its inhibitory effect is mainly referred to COX-2 activity. Results obtained with NS-398, the specific inhibitor of COX-2 activity, confirmed this possibility: L137 did not modify the COX-1-derived TXB2 production (3·52 (se 0·36) in control v. 4·60 (se 0·88) in 100 μm-L137-treated monocytes).
COX-2 peroxidase activity was not affected by the treatment with the isochroman. Indeed, L137 did not modify the PGE2 production when PGG2 was used as the substrate (10·8 (se 2·2) ng/ml in controls v. 12·07 (se 1·4) and 13·87 (se 2·5) in human monocytes treated with L137 at 10 and 50 μm, respectively).
The production of TNF-α, one of the major pro-inflammatory cytokines involved in the pathogenesis of chronic inflammatory diseases and modulated by oxidative stress(Reference Calamia24), was also impaired by L137. The amount of TNF-α produced over 24 h by LPS-stimulated, but untreated, cells was 0·8–5·2 ng/ml, and L137 treatment at 0·5, 10 and 100 μm decreased the TNF-α production by about 30, 60 and 80 %, respectively (P < 0·01).
The effect of L137 on LPS-induced COX-2 protein expression was examined by Western blotting. Furthermore, since COX-2 is a NF-κB-regulated gene, we investigated whether L137 is able to suppress LPS-induced NF-κB activation. Human monocytes both untreated and pre-treated with L137 were primed with LPS. Whole-cell extracts were prepared and analysed by Western blotting. As shown in Fig. 1(A), L137 only at 100 μm significantly decreased LPS-induced COX-2 expression through the suppression of NF-κB activation (Fig. 1(B)). Because the inhibitory effect on COX-2-mediated prostanoid release was recorded even at the lowest concentration (1 μm), whereas only at 100 μm, COX-2 expression was significantly reduced, the effect of L137 seems to depend mainly on a direct inhibition of the COX activity of COX-2 and to involve a decrease in COX-2 synthesis only at the highest concentration.
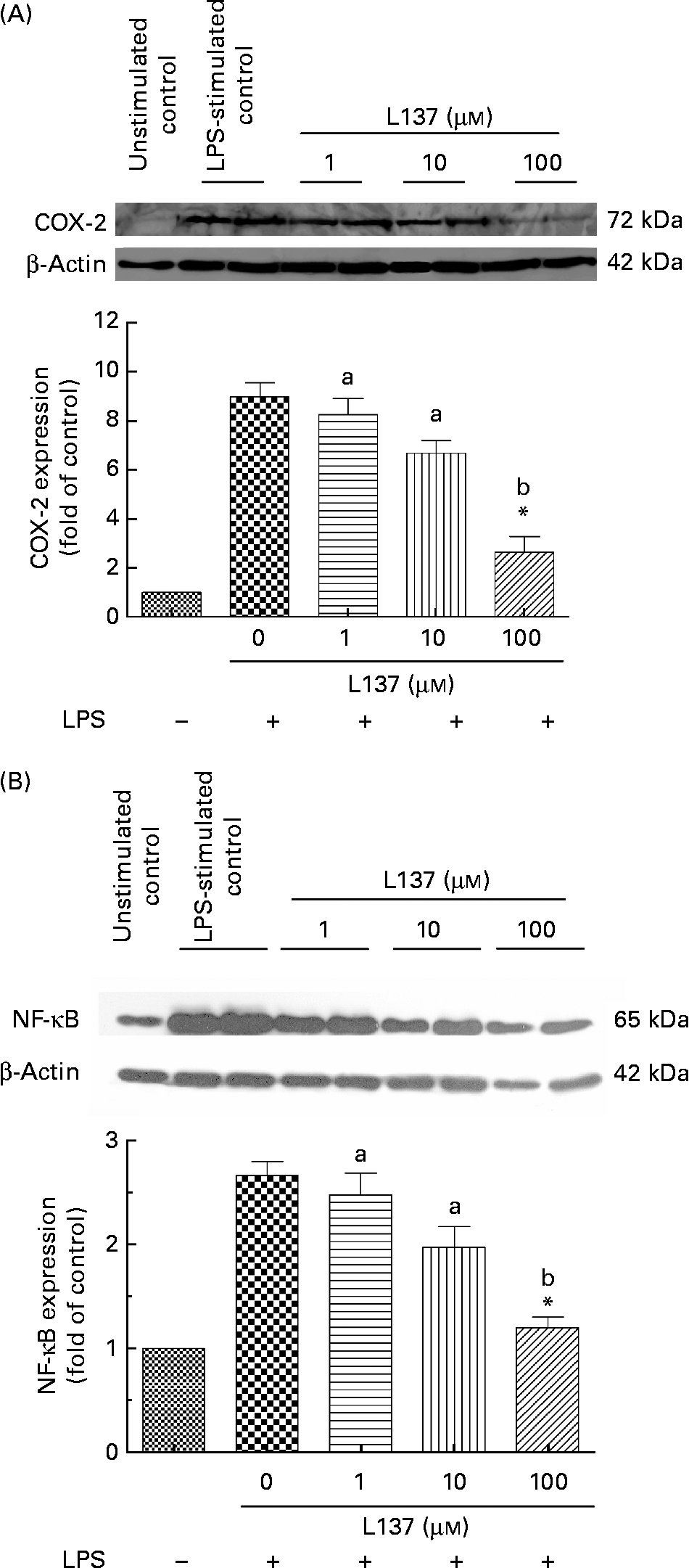
Fig. 1 Effect of 1-phenyl-6,7-dihydroxy-isochroman (L137) on cyclo-oxygenase-2 (COX-2) (A) and NF-κB (B) protein expression in human monocytes stimulated with lipopolysaccharide (LPS; 50 ng/ml). The densitometric data were calculated as the fold decrease of the value for the LPS-stimulated group. Values are means from three different experiments, with standard errors represented by vertical bars. * Significant inhibition v. LPS-stimulated cells (P < 0·001). a,b Mean values with unlike letters were significantly different (P < 0·01).
In conclusion, in the present study, we demonstrated that L137 suppresses LPS-induced pro-inflammatory mediator production and COX-2 expression by inhibiting the activation of the NF-κB signal transduction pathway. This effect, in addition to the reported antioxidant and anti-platelet activity(Reference Togna, Togna and Franconi14, Reference Lorenz, Zeh and Martens-Lobenhoffer25), indicates that the isochroman compounds may also contribute to the anti-atherogenic and anti-inflammatory properties of the extra-virgin olive oil.
Acknowledgements
We gratefully acknowledge Paola Patrignani for providing specific PGE2 antiserum. The present study was partially supported by a grant to G. I. T. from the ‘Sapienza’ University of Rome. None of the authors has any conflicts of interest. The contribution of the authors was as follows: G. T., A. R. T. and G. I. T. designed the study; G. T., A. R. T. and V. L. contributed to the execution of the experimental work; M. G. and C. M. synthesised, purified and characterised the isochroman. All authors contributed to and approved the final manuscript.



