INTRODUCTION
Estuary and coastal areas are important habitats as nurseries and feeding grounds for various kinds of fishes, for both resident species and temporal or migratory species that move between rivers and oceans, such as diadromous fishes (Elliot & Dewailly, Reference Elliot and Dewailly1995; Meager et al., Reference Meager, Williamson and King2005; Elliot et al., Reference Elliot, Whitfield, Potter, Blaber, Cyrus, Nordlie and Harrison2007; Whitfield, Reference Whitfield2016). Estuaries have the important function of providing numerous habitats for fish and links between rivers and ocean areas (Able, Reference Able2005). Conversely, human activities, such as reclamation for aquaculture, agriculture, and construction of artificial structures, have negatively affected estuarine and river ecosystems and their associated biodiversity (Arun, Reference Arun2005; Eriksson et al., Reference Eriksson, van der Heide, van der Koppel, Piersma, van der Veer and Olff2010). In general, rivers in islands are shorter and narrower than those on continents, and so it is likely that they are strongly affected by human activities.
The Ryukyu Archipelago is composed of 198 islands, is ~1000-km long, and is located in sub-tropical and tropical areas of southern Japan. In the archipelago, sediment pollution by laterite red soil run-off from the land due to road and dam construction has a key issue for the conservation of water ecosystems (Fujii, Reference Fujii2001).
Brackish areas are inhabited by various fish species and the suborder Gobioidei is a representative fish in these areas (Humphries & Potter, Reference Humphries and Potter1993; Yokoo et al., Reference Yokoo, Kanou, Moteki, Kohno, Tongnunui and Kurokura2012). The benthic gobioid fish genus Acanthogobius is mainly distributed in East Asia, and inhabits estuarine and coastal waters (Suzuki et al., Reference Suzuki, Shibukawa and Yano2004). Acanthogobius luridus and A. elongates are distributed in the north-west Pacific: Korean and Chinese coasts of Bohai Sea, Yellow Sea and East China Sea (Froese & Pauly, Reference Froese and Pauly2017). Four species of this genus, A. flavimanus, A. lactipes, A. hasta and A. insularis, have been recorded in Japan (Akihito et al., Reference Akihito, Sakamoto, Ikeda, Aizawa and Nakabo2013). Acanthogobius flavimanus and A. lactipes are widely distributed and one of the most common gobies found in the estuaries of mainland Japan (Takizawa, Reference Takizawa1994; Zama, Reference Zama1999; Kanou et al., Reference Kanou, Koike and Kohno2000; Okazaki et al., Reference Okazaki, Yokoo, Kanou and Kohno2012). Conversely, A. insularis is an endemic species that is distributed only in the Amami-oshima and Okinawa-jima Islands of the Ryukyu Archipelago. This species is registered as ‘Vulnerable (VU)’ in the Red Data Books of the Ministry of the Environment of Japan, and Kagoshima and Okinawa Prefectures, and is threatened with extinction (Tachihara, Reference Tachihara2005, Reference Tachihara2015; Yonezawa & Shinomiya, Reference Yonezawa and Shinomiya2016). Although this species has been recognized as a single species with A. lactipes, inhabiting mainland Japan for many years, it was described as a new species by Shibukawa & Taki (Reference Shibukawa and Taki1996). The spawning season of A. insularis has been reported based on the presence of mature females in the Taiho River in Okinawa-jima Island (Shibukawa & Taki, Reference Shibukawa and Taki1996). However, no study has focused on the reproduction of A. insularis in detail. Furthermore, little is known about their life-history traits, such as lifespan and growth. The habitat of A. insularis is strictly limited to brackish areas in several rivers in Okinawa-jima Island (Shibukawa & Taki, Reference Shibukawa and Taki1996). In addition, Tachihara (Reference Tachihara2015) noted that their populations may be decreasing due to environmental degradation following riverine and coastal developments, such as wetland drainage, resulting in increased turbidity and sedimentation, and decreased water flow. In the Taiho River, which is one of the main habitats of A. insularis and flows to the Shioya Bay in the Ogimi Village in the northern part of Okinawa-jima Island, the aquatic environment has been destroyed by red soil run-off from the land at ~657 tons per year owing to the construction of roads and the Taiho Dam (Okinawa Prefecture, 2013). Due to the narrow entrance of Shioya Bay, red soils have been continuously deposited at the bottom of the bay. Therefore, one of the main habitats of A. insularis in Okinawa-jima Island still faces severe threat. To conserve this species under such conditions, its life history and habitat use should be investigated in detail. The objective of this study was to obtain fundamental information on A. insularis by investigating its life history, such as growth, reproduction, senescence, and habitat use in Okinawa-jima Island.
MATERIALS AND METHODS
Sampling sites
The surveys were conducted in the Taiho River, Ogimi Village, northern Okinawa-jima Island in southern Japan. The river is about 10.3-km long and has an estuary that flows into Shioya Bay. The Taiho Dam was constructed in the upper reaches, 2.9 km from the entrance of Shioya Bay. Five stations were set in the brackish area along the river (Figure 1; Stations A–E). Features of each station were as follows. Station A was located on an open mud flat in the estuary. The substrate was soft mud without dead coral, debris, or sea algae, and there were bivalves, including shells of Anadara antiquata and living Isognomon ephippium. Station B had a muddy substrate with cobble flowing from the river. Filamentous algae flourished in winter. An artificial waterway was constructed in the vicinity, and a revetment was reconstructed throughout the study period. Mangroves such as Kandelia obovata and Bruguiera gymnorrhiza grew near Station C, which had a similar substrate to Station B, and filamentous algae also flourished in winter. A small tributary flowed into the Taiho River near Station C. Cobbles (~15 cm in diameter) were dotted on the pebble substrate at Station D. The substrate of Station E was finer than that of Station D.
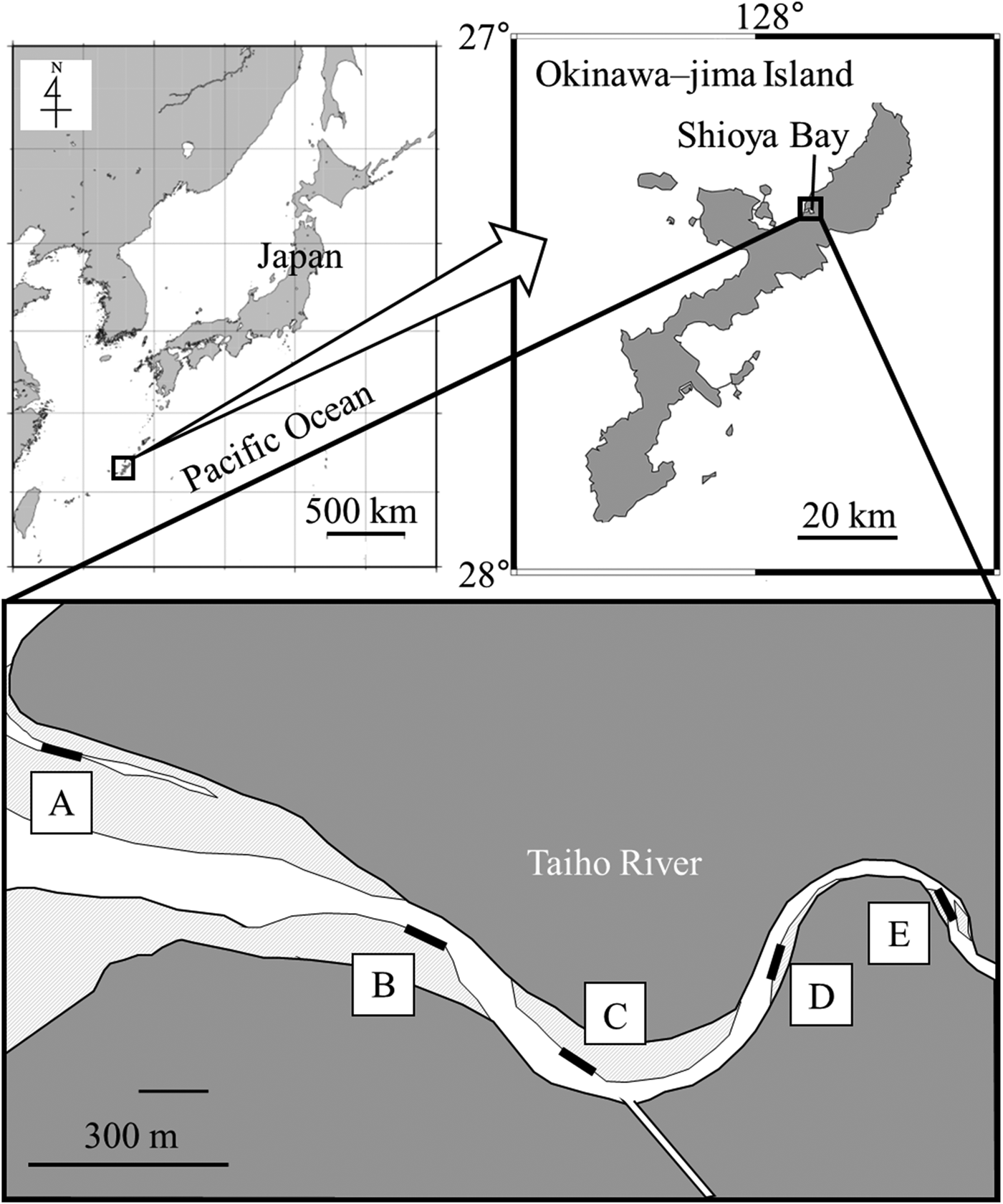
Fig. 1. Maps showing the study site at the Taiho River in Okinawa–jima Island, Japan. Black bars show sampling stations. Each colour shows the following; grey areas: land; light grey areas: sand or mud exposed at low tide; white areas: water at low tide.
Fish collection and environmental measurement
Acanthogobius insularis individuals were collected using a small seine net with a sinker attached to reach the bottom during the daytime (1.0-mm mesh; 0.8 m in height; 3.5 m in width; with a bag 1.0-m long and 0.7-m diameter; Supplementary Material 1). Sampling was performed at each station monthly from November 2014 to November 2015. The seine net was hauled by two people using poles set at each end of the net along the shoreline during the low tides of spring tides during the daytime. At each station, the net was hauled once for 5.0 m to minimize the impact of sampling on the population. Additional samplings were conducted using a hand net when the body size ranges of monthly samples became biased due to low sample sizes. Water temperature to the nearest 0.1°C and salinity (S; UNESCO, 1981) were measured at each sampling using a mercury thermometer and a refractometer (S/Mill-E or PAL-06S, ATAGO), respectively.
Since A. insularis is registered as ‘Vulnerable’ (VU) in the Red Data Book by the Ministry of the Environment of Japan (Tachihara, Reference Tachihara2015), a maximum of 25 individuals were randomly subsampled each month and taken to the laboratory. Other individuals in each station were placed in a container attached to a grid sheet (10-mm mesh) in the field (Supplementary Material 2), and a photograph was taken using a digital camera (Stylus TG-4, Olympus). Then, fish were released at each site after the number of individuals was determined. Subsampled individuals were euthanized using ice in the field and brought to the laboratory. Total length (TL) and standard length (SL) of collected specimens were measured to the nearest 0.1 mm using a digital calliper (Digimatic Calliper, Mitsutoyo) in the laboratory. TLs of released fish were estimated to the nearest 0.1 mm from images using the software Image J 1.48i (Schneider et al., Reference Schneider, Rasband and Eliceiri2012) and converted to SL by the following formula, generated based on the relationship between TL and SL in the samples: TL = 0.8355 × SL + 0.1363, R 2 = 0.9922, N = 303 (except for two specimens with a broken caudal fin).
Gonadosomatic index and histological observation of ovary
Body weight (BW) and gonad weight (GW) were measured to the nearest 0.001 g for each specimen. Sex was mainly determined by histological observation of the gonadal tissue during dissection. Moreover, the sex was determined by direct observations of the gonadal morphology during the spawning season (January to May, see details in the Results) as follows; ovary: rounded and yellowish; testes: folded and whitish. Individuals smaller than 13.9 mm were determined as sex-indistinct owing to undeveloped gonads. The spawning season was estimated based on monthly changes in the female gonadosomatic index (GSI), which was calculated as follows: GSI = (GW/BW−GW) × 100, and based on histological observations of ovaries. However, GSI values of females caught between June and November were assumed to be zero, because GW could not be measured owing to non-developed gonads during this period. The gonads were fixed using 10% buffered formalin and dehydrated in paraffin. Thin sections of ovaries (7 µm) were stained with Mayer's haematoxylin and eosin using routine histological procedures to examine the developmental stages of the oocytes in the ovary. The number of specimens used for histological analyses varied from one to 24 each month. Many individuals had no clear gonad during the non-spawning season; thus, the number of samples during the non-spawning season was low (N = 2–9). Oocyte stages were classified based on the methodology described by Kuno & Takita (Reference Kuno and Takita1997) and Kitano et al. (Reference Kitano, Hatakeyama, Akiyama and Ueno2003). In the present study, the mature stage of each individual was determined based on the most developed oocytes in the ovary. Sexually mature females were defined as having an ovary in the late development stage (tertiary yolk globule to maturation stage; see Results). Based on the morphological observations, individuals smaller than 10 mm SL were classified as juvenile (see details in the Results).
Monthly growth
As a preliminary study, age in days was estimated using the sagittal otolith. Otoliths were extracted from each specimen under a stereomicroscope, mounted on glass slides using clear nail varnish, and examined under an optical microscope (NIKON, ECLIPSE Ni-u). The rings were observed and assumed to have been deposited daily; two people counted the number of rings independently. Next, the hatching date of specimens was calculated based on sampling dates and estimated ages (N = 166). However, the estimated ages differed largely between the two readers, and the hatching dates calculated did not match the spawning season (see Results), which was estimated from monthly GSI values and histological observations. Therefore, we concluded that the use of otoliths for age determination was not appropriate for A. insularis. Thus, we estimated their growth from monthly changes in the average SLs. As there were bimodal SL distributions in some months (see Results), the average for each cohort was calculated instead.
Statistical analyses
Differences in SL between males and females were analysed by Mann–Whitney U-test. Kruskal–Wallis test was used to compare SL among stations. When significant differences were determined by Kruskal–Wallis test, post-hoc multiple comparison tests were carried out using the Holm method. The sex ratio was tested by a Fisher's exact test. Significant differences were determined at the 0.05 probability level. All statistical analyses were conducted using R version 3.0.2 (R Core Team, 2013).
RESULTS
Water temperature and salinity
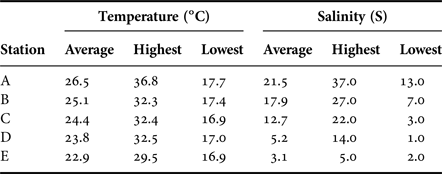
The lowest mean water temperature recorded was 17.1°C in January 2015, following which it rapidly increased from March (Figure 2), reaching the highest mean temperature of 32.6°C in July before starting to decrease in August or September. When observed at each station, Station A had higher water temperatures than the other stations throughout the year (Table 1). Salinity was also higher in the lower stations (Stations A and B) than in stations in the upper reaches.
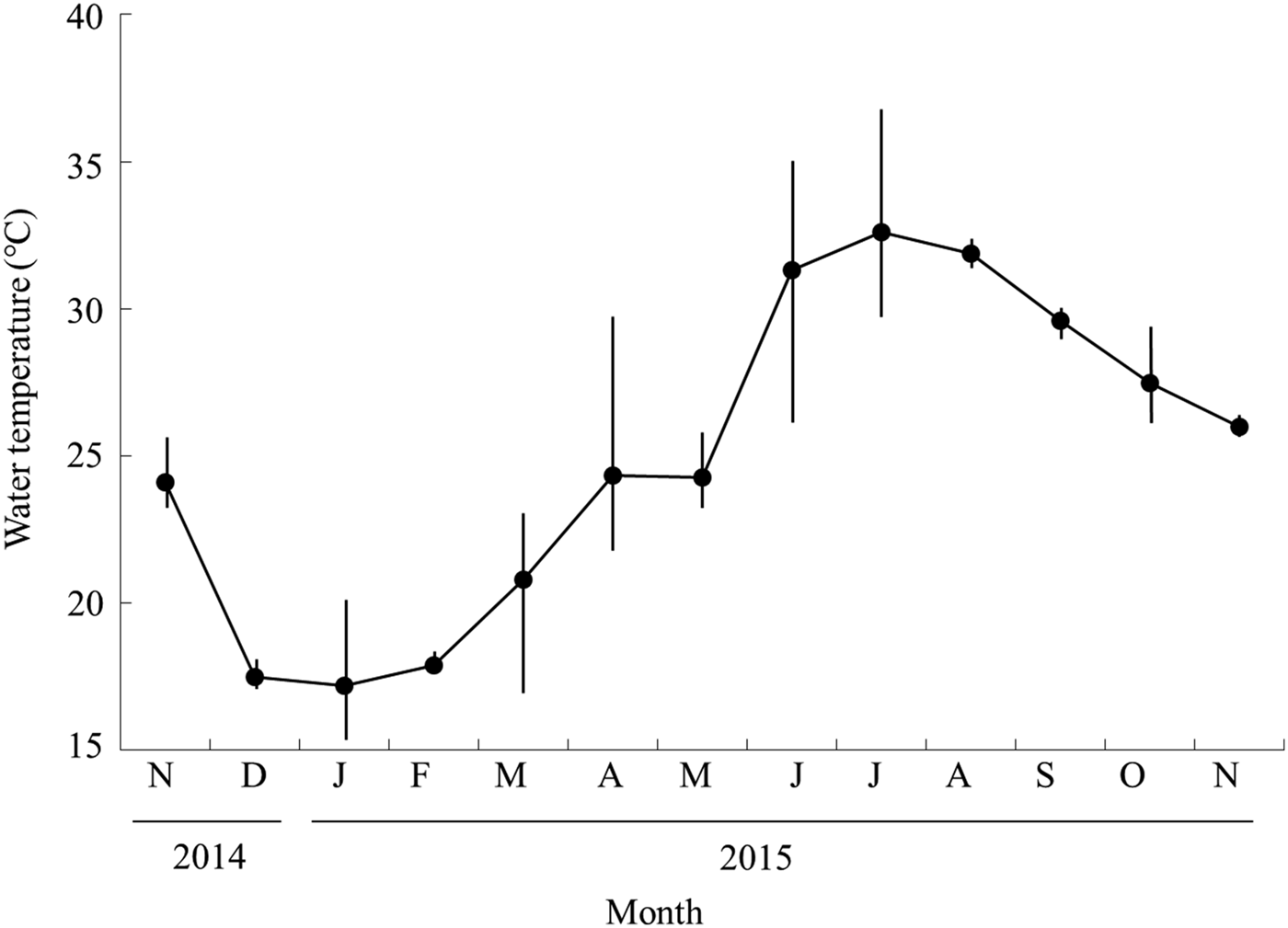
Fig. 2. Monthly changes in water temperature of the Taiho River in Okinawa-jima Island. Closed circles and bars show the average and standard deviation, respectively.
Table 1. Water temperature and salinity at each station at the Taiho River from November 2014 to November 2015.
Standard length ranges
In total, 636 individuals were collected, among which 331 individuals were released in the field, and their SLs were estimated from photographs. Out of 305 individuals collected and brought to the laboratory, 271 and 34 were caught using the small seine net and hand net, respectively.
The SL of sampled individuals was 13.9–54.5 mm (mean ± S.D.: 39.8 ± 10.8 mm, N = 115) for females, 19.7–46.4 mm (30.5 ± 7.2 mm, N = 29) for males, and 9.6–52.2 mm (25.9 ± 8.4 mm, N = 161) for sex-indistinct individuals, and 9.6–54.5 mm (24.4 ± 10.4 mm, N = 636) for all individuals including those released. Females were significantly larger than males (Mann–Whitney U-test, U = 2499.5, P < 0.01). The smallest individual had a SL of 9.6 mm and was considered a juvenile just after recruiting based on its body size and low amount of pigmented colouration. Juvenile A. insularis were distinguished from Favonigobius sp. using the number of spiny-rays on the first dorsal fin (8), which is a unique feature of this species. The SL frequency distribution of A. insularis collected by the seine and hand nets over 1 year showed clear monthly variation (Figure 3). Unimodal patterns were observed in all months except for April 2015, when juveniles smaller than 10 mm SL appeared. After May 2015, large individuals (≥40 mm SL) disappeared and remained absent until the end of the survey in November 2015. The average SL slowly but continuously increased from April to November. Monthly changes in the SL were greater from December onwards, and the mean SL increased to over 40 mm in January or February. Then, it increased slowly until March. Monthly growth, which was estimated by the average SL in each month, was slow from April to November (up to 6.8 mm/month), following which it became rapid to about 10 mm per month between November and January (Figure 3).
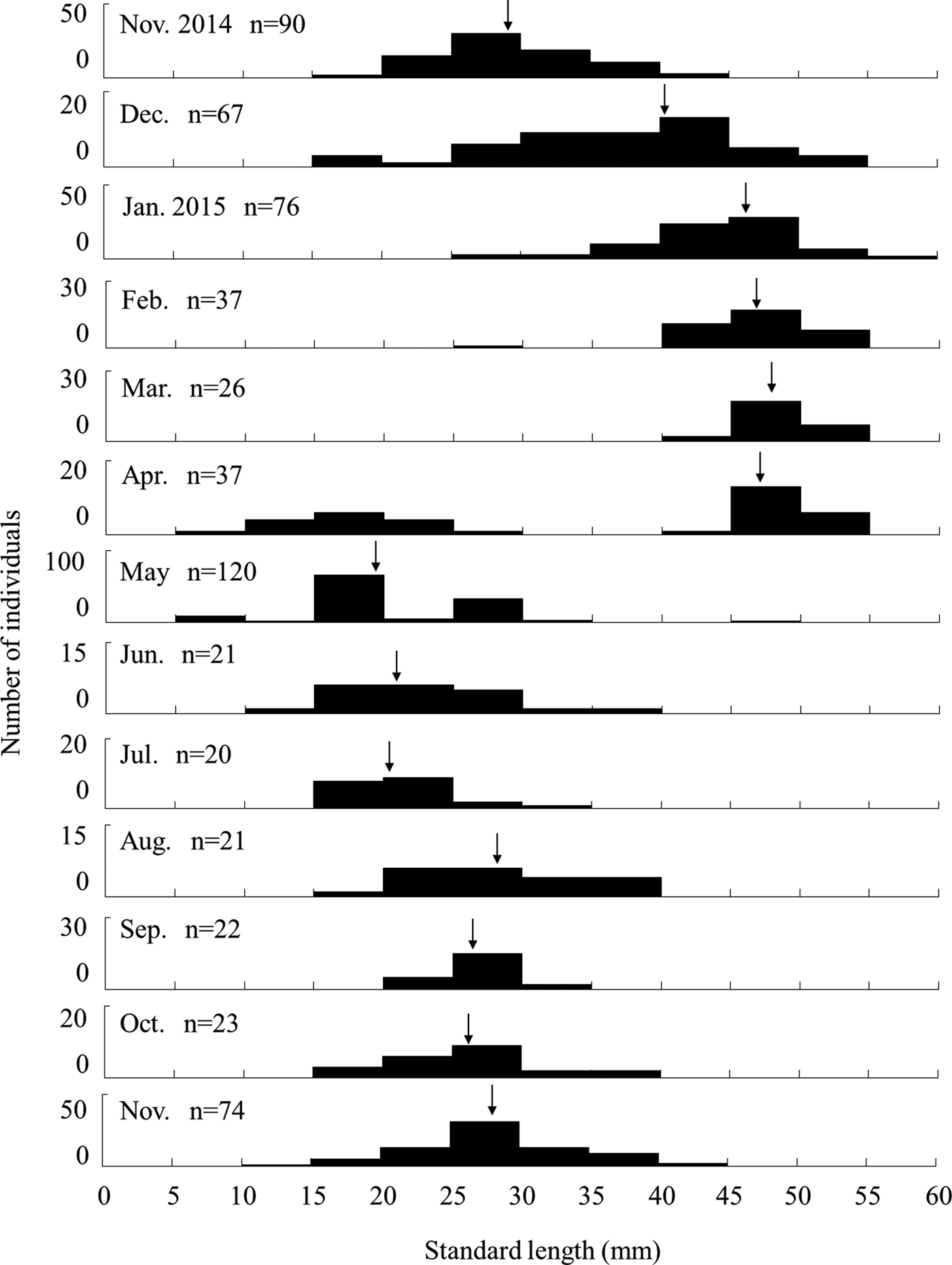
Fig. 3. Monthly changes in standard length frequency of Acanthogobius insularis with whole samples collected by seine and hand nets from the Taiho River, Okinawa-jima Island. Arrows show the average values of SL.
Reproduction
Monthly variation in GSI for females was highest during the winter season (Figure 4). The average GSI of females began to increase in December (mean ± S.D.: 0.8 ± 0.9), reaching a peak in March (10.3 ± 4.5), and rapidly decreasing in May (1.0 ± 2.5). Then, GSI values were almost zero from June to November 2015.
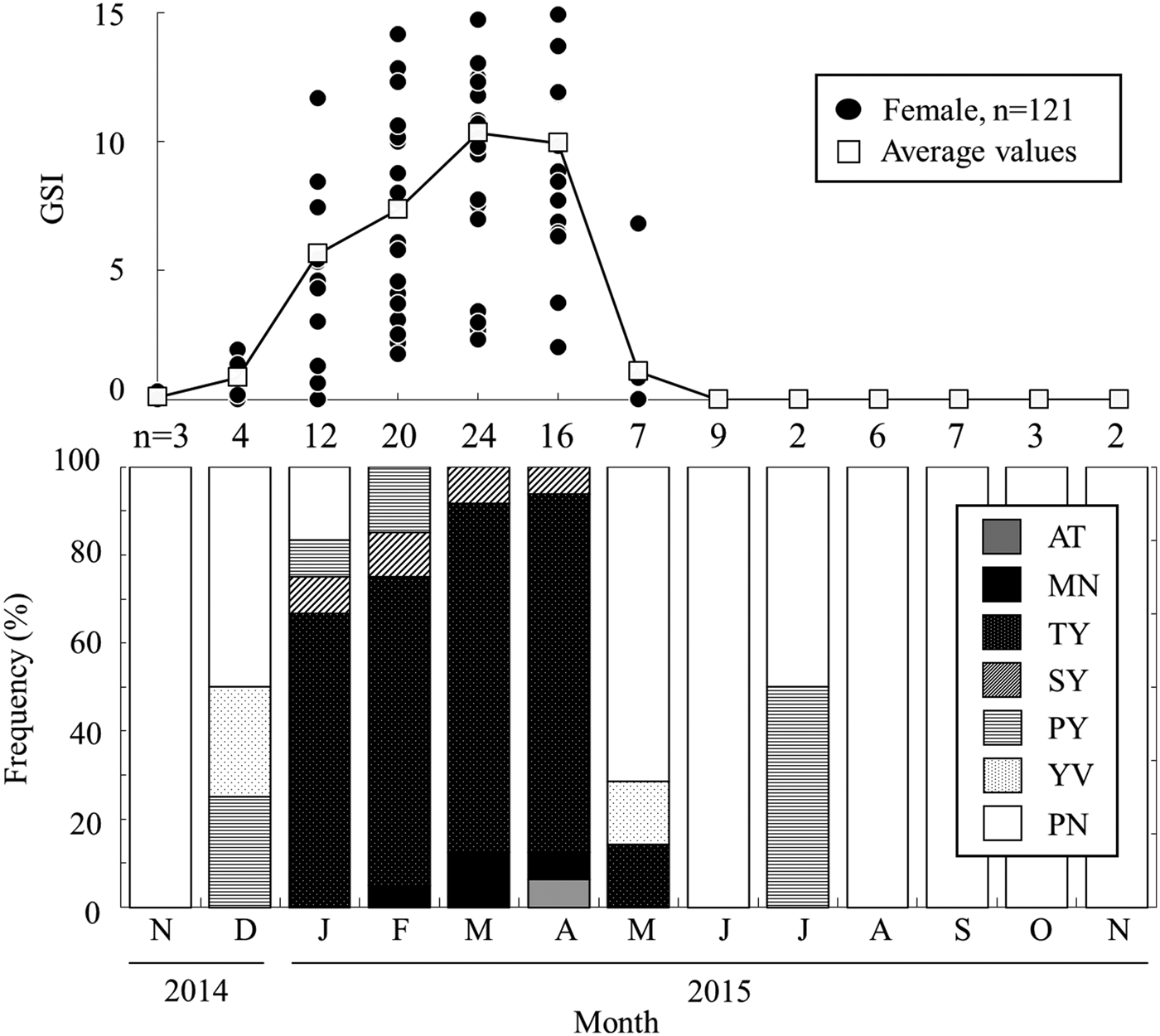
Fig. 4. Monthly changes in gonadosomatic index (above) and the frequency of various gonadal maturity stages (below) occurring in female Acanthogobius insularis collected at the Taiho River in Okinawa-jima Island. AT: atretic stage; MN: migratory stage; TY: tertiary yolk stage; SY: secondary yolk globule stage; PY: primary yolk globule stage; YV: yolk vesicle stage; PN: peri-nucleus stage.
Histological observations of A. insularis ovaries revealed that maturity occurred in the following stages (Supplementary Material 3): peri-nucleus stage (PN, N = 40); yolk vesicle stage (YV, N = 2); primary yolk globule stage (PY, N = 3); secondary yolk globule stage (SY, N = 6); tertiary yolk globule stage (TY, N = 55); migratory nucleus stage (MN, N = 5); and atretic stage (AT, N = 1). No post-ovulatory follicles (POF) were observed in this study. Moreover, as various stage oocytes were observed in one section of many individuals, oocyte development in A. insularis was asynchronous. Matured females from TY to MN were observed from January to May, and accounted for approximately 80% of individuals in March and April (Figure 4). Conversely, all females caught from June to December were immature. Results of GSI analysis and histological observations indicated that their spawning season occurs between January and May in the Taiho River, Okinawa-jima Island.
The relationship between SL and GSI in females during the spawning season showed that the GSI values was greater in individuals over 40 mm SL, and the smallest mature female estimated by the histological observation was at 40.8 mm SL with a GSI value of 3.0 (Figure 5). The lowest GSI value in a mature female based on the histological observation was 2.5, with a SL of 48.9 mm.
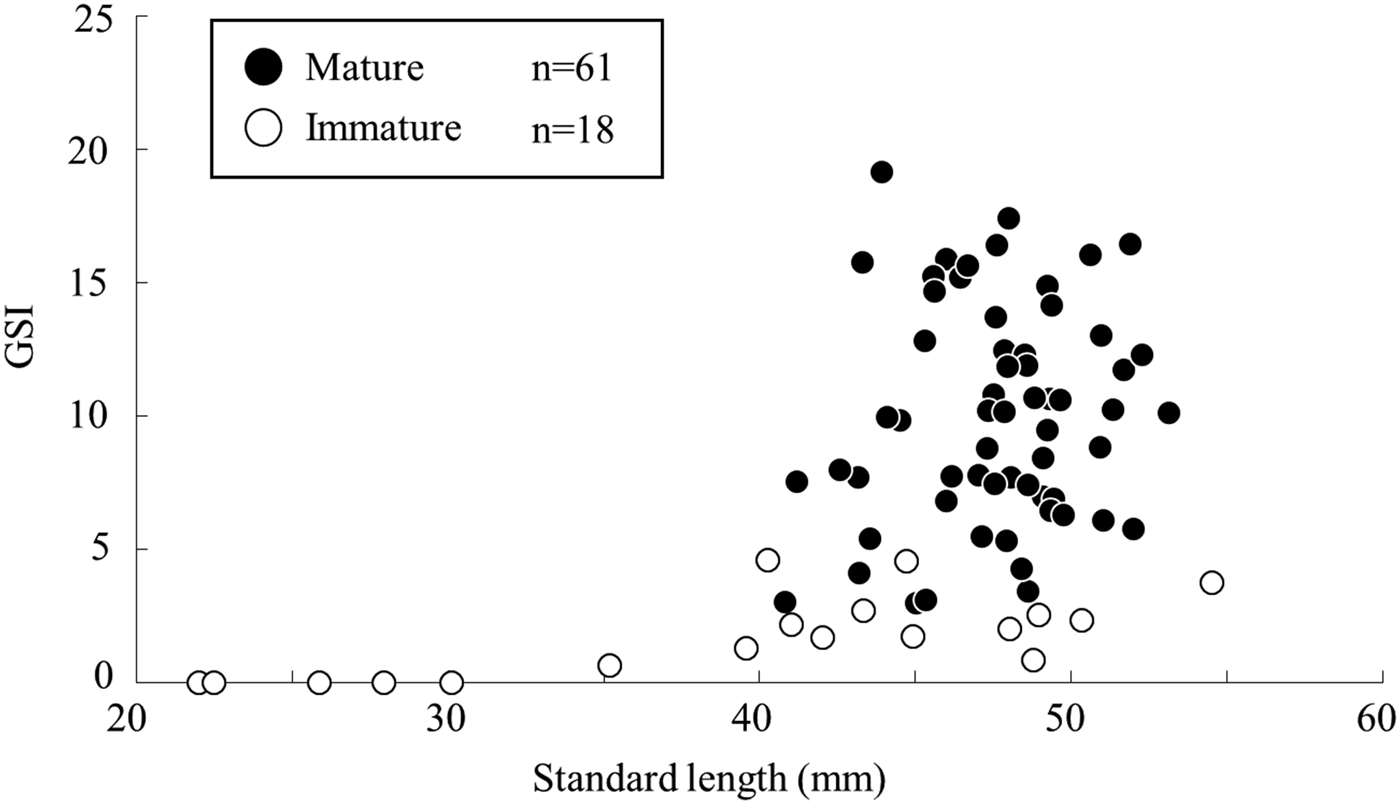
Fig. 5. Relationship between gonadosomatic index and standard length of female Acanthogobius insularis during the spawning season, collected at the Taiho River in Okinawa-jima Island.
Instream distribution
The SL of individuals collected by the small seine net each month varied among stations (Kruskal–Wallis test, P < 0.05; Figure 6). The number of individuals was lower in Station A and higher in Stations C and D. Juveniles (<10 mm SL) occurred at Stations B and C (Holm method, each P < 0.05). Large individuals (≥40 mm SL) were abundant in the upper stations. The SL of individuals at Station D was significantly larger, and of Station A was smaller, than that at other stations.
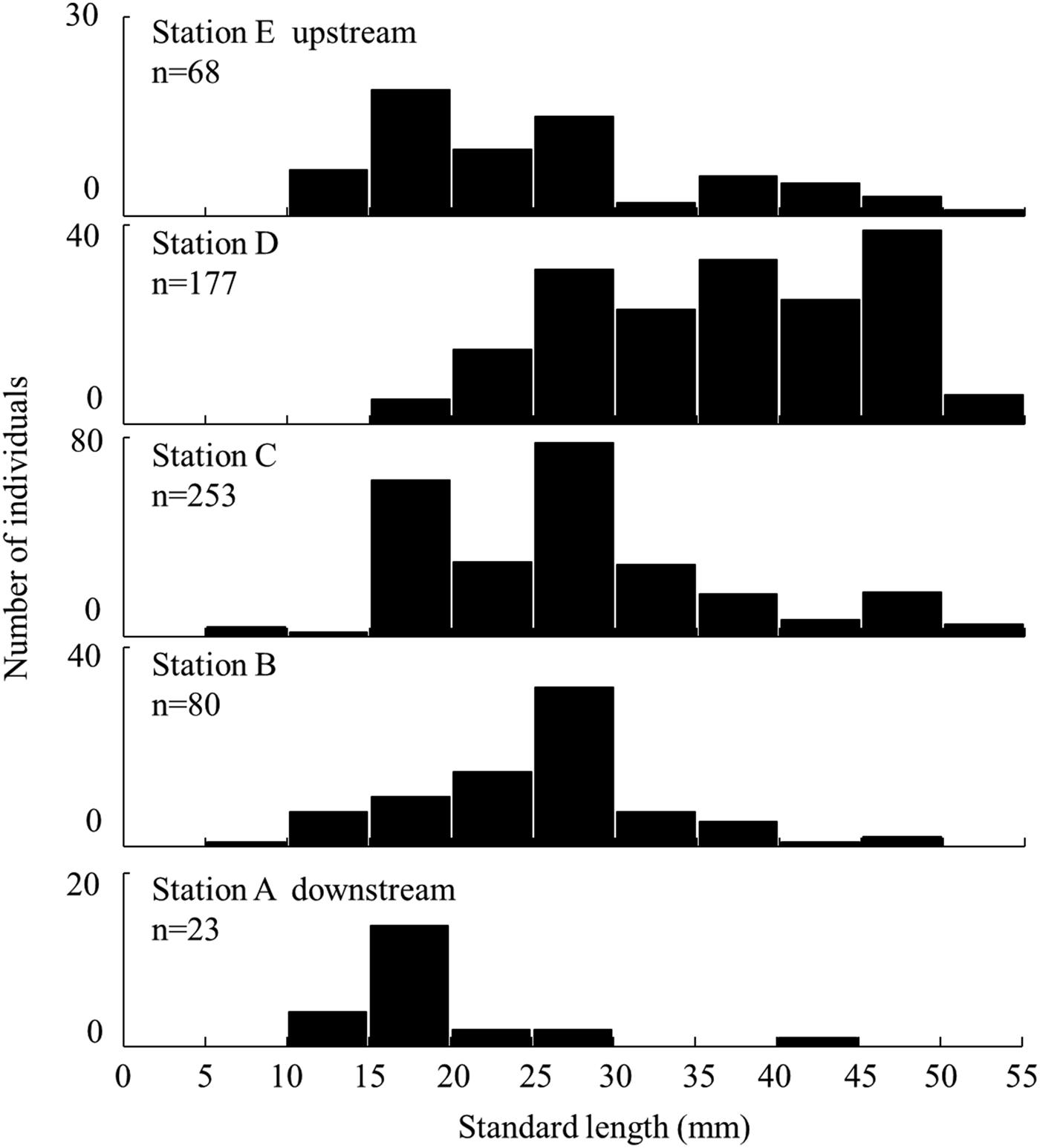
Fig. 6. Standard length distribution of Acanthogobius insularis collected by seine net at each station at the Taiho River in Okinawa-jima Island.
For the monthly changes in SL frequency at each station, small individuals, including juveniles, were widely distributed in the Taiho River during the non-spawning season from June to December (Figure 7). Larger individuals (≥40 mm SL), which were probably matured, according to their size, mainly appeared in the upper area of Stations C and D during the spawning season and were present at lower numbers in May.
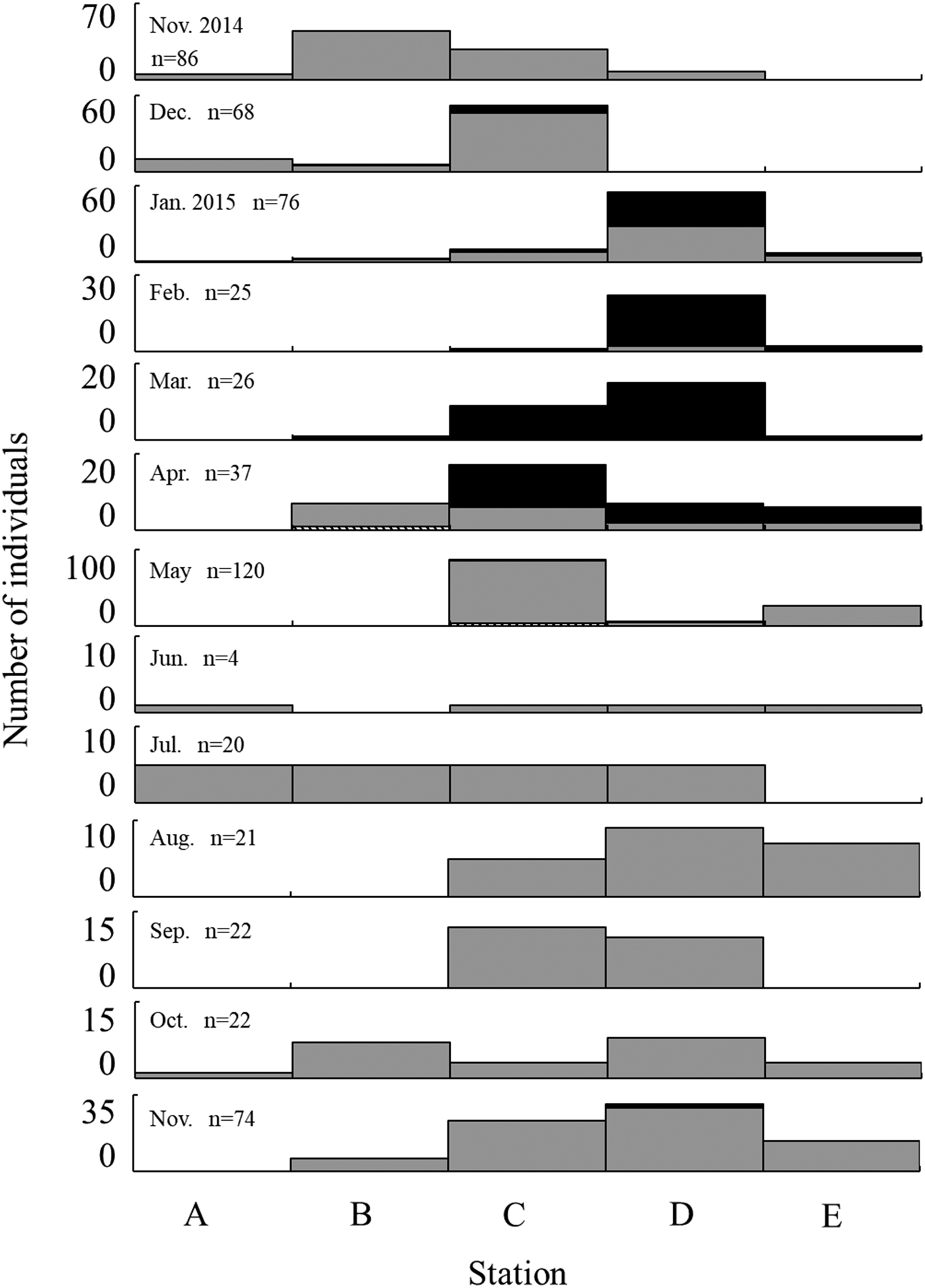
Fig. 7. Monthly changes in standard length of Acanthogobius insularis collected by seine net at each station. Stripe: ≤10 mm SL, grey: 10.1~39.9 mm SL, black: ≥40 mm SL.
DISCUSSIONS
Lifespan and growth
The results of a yearlong survey of the endemic endangered goby A. insularis on the Okinawa-jima Island showed that this species is annual and exhibits rapid growth during the winter season. Based on monthly changes in length frequency distribution, A. insularis individuals were recruited as juveniles in April and May and grew to over 50 mm SL, with large individuals disappearing after May (Figure 3). Moreover, monthly SL frequency distributions were unimodal, except during their recruiting period in April. Therefore, we could conclude that this species is annual. Compared with congener species, although A. lactipes is also reported to be annual (Takizawa et al., Reference Takizawa, Maruta, Ikawa, Nakamura and Nakamoto1994; Zama, Reference Zama1999), the senescence of A. flavimanus and A. hasta is 1 or 2 years, and occasionally 3 years for A. flavimanus (Miyazaki, Reference Miyazaki1940; Hoshino et al., Reference Hoshino, Kinoshita and Kanno1993; Kuno & Takita, Reference Kuno and Takita1997; Zama, Reference Zama1999; Nakamura, Reference Nakamura2002). Acanthogobius insularis individuals have a relatively short life cycle compared with other species in the genus.
Monthly growth was slow from April to November, and rapid from November to January (Figure 3). Recruitment occurred only in April and May suggesting the slow growth during April to November was not caused by underestimation in size frequency by new recruits. At the beginning of the rapid growth period, the mean water temperature declined from 24.0°C in November to 17.4°C in December (Figure 2). The rapid growth of A. insularis could be related to decreasing water temperature. Conversely, in mainland Japan, A. flavimanus and A. lactipes mainly grow from spring to autumn, and their growth slows in winter (Takizawa et al., Reference Takizawa, Maruta, Ikawa, Nakamura and Nakamoto1994; Zama, Reference Zama1999). Although most of the Acanthogobius species inhabiting Japan, are distributed in temperate region in East Asia area, A. insularis is the most southern species, distributed only in the sub-tropical area of the Ryukyu Archipelago (Nakabo, Reference Nakabo2013). The water temperature of Okinawa-jima Island was higher than that of mainland Japan over the course of a year. For example, the monthly average water temperature ranged from 17.1°C in January to 32.6°C in July in this study, and from 3.2°C in January to 25.5°C in August in Miyagi Prefecture, northern Japan in a previous study (Zama, Reference Zama1999). High water temperature of sub-tropical areas in the summer would not be suitable for A. insularis, which might account for their slow growth in the summer. Furthermore, water temperature in winter in Okinawa-jima Island would be suitable for them, and the decrease in water temperature may promote the initiation of rapid growth.
Reproduction
The spawning season of A. insularis was estimated to occur from January to May, peaking in March and April in the Okinawa-jima Island based on a combination of monthly changes in GSI values and histological observations of gonadal sections (Figure 4). Shibukawa & Taki (Reference Shibukawa and Taki1996) previously estimated the spawning season of this species through its sexual dimorphism and the occurrence of females with matured eggs in Okinawa-jima Island, when they first described this species. However, this was the first study to estimate the spawning season of A. insularis by histological observation. In the present study, it was not clarified whether A. insularis is a multiple spawner or not since we did not observe any direct evidences of multiple spawning such as POF by the histological observation. However, it was observed that oocyte development of A. insularis was asynchronous in the present study. From such aspects, they might be multiple spawners.
A relationship might exist between the frequency of mature females, the GSI value of females, and mean water temperature. The frequency of mature females and GSI values rapidly increased when the mean water temperature dropped 6.6°C from November (24.0°C) to December (17.4°C) (Figures 2 & 4). Then, mature females disappeared in June, when there was a sudden elevation of 7.1°C in the mean water temperature from May (24.2°C) to June (31.3°C). These results suggest that the maturity of female A. insularis may be related to the low water temperature observed in the winter season. Suzuki et al. (Reference Suzuki, Sakurai and Sugihara1989) indicated that the maturity of A. flavimanus individuals was inhibited by water temperatures above 20°C, and a low temperature period of 11–14°C is needed to progress their maturity in the laboratory. Notably, the spawning season of A. flavimanus starts in winter (Dotsu & Mito, Reference Dotsu and Mito1955; Zama, Reference Zama1999, Table 2). Although little is known about the relationship between the maturity of A. hasta and water temperature, one is likely because the spawning season of A. hasta occurs during winter from Nagasaki Prefecture in western Japan (February to March: Kuno & Takita, Reference Kuno and Takita1997; Table 2). Nevertheless, A. insularis is the only sub-tropical species within the genus Acanthogobius, in which the spawning season started in winter, the same as with the other Acanthogobius species. Conversely, A. lactipes in Kyusyu in western Japan and Miyagi Prefecture in northern Japan is the only species known to spawn during the summer (May to August in Kyushu: Dotsu, Reference Dotsu1959; June to August in Miyagi Prefecture: Zama, Reference Zama1999; Table 2). The difference between the spawning season of A. lactipes and that of congeners might be the result of some competition in the spawning ground and/or timing with other sympatric Acanthogobius species.
Table 2. Summary of spawning season of Acanthogobius species from references.

Instream distribution
Monthly quantitative collection using the small seine net showed that A. insularis moves upstream, following recruitment on tidal flats in the estuary, and is then widely distributed in the Taiho River. Larger individuals over 40 mm SL gathered around stones, which were used as spawning beds in the reproductive season. Yonezawa & Shinomiya (Reference Yonezawa and Shinomiya2016) reported that this species might move to locations containing cobbles during the spawning season in the Amami-oshima Island, Ryukyu Archipelago. However, the results of the present study provide the first quantitative evidence of their movement in the river. Small individuals (≤15 mm SL) appeared at Station B in April 2015 (Figure 7). From July 2015, the number of mid-sized individuals (16 to 39 mm SL) increased in the middle to upper reaches. Finally, adults (≥40 mm SL) appeared in the upper reaches (Stations C to E) during the spawning season. Several gobies inhabiting brackish, estuary and freshwater areas are known to move upstream according to their growth stage, and change their habitat (Maeda & Tachihara, Reference Maeda and Tachihara2005; Yokoo et al., Reference Yokoo, Sakamoto, Kanou, Moteki, Kohno, Tongnunui and Kurokura2009, Reference Yokoo, Kanou, Moteki, Kohno, Tongnunui and Kurokura2012; Kondo et al., Reference Kondo, Maeda, Yamasaki and Tachihara2012; Iida et al., Reference Iida, Watanabe and Tsukamoto2013). Acanthogobius insularis might also exhibit this type of movement. Mature-sized individuals occurred in the upper reaches during the spawning season, especially in Station D (Figures 6 & 7). Acanthogobius insularis females spawn their eggs underneath stones and males care for the egg mass in the nest (Tachihara, Reference Tachihara2015; Yonezawa & Shinomiya, Reference Yonezawa and Shinomiya2016); therefore, a base for a spawning bed, such as stones and shells, is needed for successful spawning. Actually, cobbles of about 15 cm in diameter were abundant in Station D compared with the other stations (Kunishima et al., unpublished). Therefore, it is quite probable that A. insularis individuals moved to Station D, where cobbles were dotted on the pebble substrate, and used the cobbles for their spawning nest.
In this study, the SL of females was higher than that of males. In general, males are larger in species where males guard the nest. In addition, in the congeneric species A. lactipes, the male is larger than the female (Dotsu, Reference Dotsu1959; Zama, Reference Zama1999). During the survey, we were unable to find egg clutches, even during the spawning period, although adult males of A. insularis have been reported to take care of egg clutches that are attached to the underside of stones (Tachihara, Reference Tachihara2015; Yonezawa & Shinomiya, Reference Yonezawa and Shinomiya2016). Sex differences in SL might be biased due to the low capture probability of large males guarding their nests under the cobbles.
Life history
The life history of A. insularis is almost completely described in the present study. The larvae hatch at 3.1 mm TL, and settle after 25 days (Tachihara, Reference Tachihara2015). They are recruited and settle into the estuary as juveniles at ~9.6 mm SL from April to May. After recruitment, they move upstream to grow. When the water temperature decreases, rapid growth and maturation occur from December to January. During the spawning season between January and May, mature adults (≥40 mm SL) gather in places containing cobbles for their spawning ground. Females spawn on the underside of stones and males care for the egg mass (Tachihara, Reference Tachihara2015; Yonezawa & Shinomiya, Reference Yonezawa and Shinomiya2016). After spawning, adults complete their annual life cycle and die.
Conservation
Acanthogobius insularis is ranked as ‘vulnerable’ in the Red Data Book of Ministry of the Environment of Japan (Tachihara, Reference Tachihara2015). Due to its limited distribution, there is an urgent need to conserve the estuarine and brackish areas inhabited by A. insularis, which are restricted to the river systems of Amami-oshima and Okinawa-jima Islands of the Ryukyu Archipelago (Shibukawa & Taki, Reference Shibukawa and Taki1996). Moreover, recently this species has been found to sparsely inhabit the southern part of Okinawa-jima Island (Kunishima et al., Reference Kunishima, Maeda and Tachihara2017). New populations of this species also need to be conserved. Hood (Reference Hood2004) and Inui & Koyama (Reference Inui and Koyama2014) suggested there is a need to take measures to conserve brackish areas, considering the effects of direct anthropogenic activities on habitat loss as well as indirect effects such as habitat changes in the surrounding areas.
In this study, low water temperature was shown to be an important factor for the maturity and growth of A. insularis. The maturity and growth of fishes are often affected by water temperature, and there is a concern that elevated water temperatures may inhibit fish reproduction by shifting spawning timing (Lehtonen, Reference Lehtonen1996; Pankhurst & Munday, Reference Pankhurst and Munday2011). If the water temperature increased as the result of climate change, or due to inflow of warm water discharge, this would threaten A. insularis.
Juveniles of this species recruit in tidal flats and estuaries. However, the number of juveniles collected in this study was low, five individuals, even during a recruiting period. Red soil sediments were found to be deposited in lower reaches (Stations A, B), and run-off occurred following heavy rain. O'Connor et al. (Reference O'Connor, Lecchini, Beck, Cadioul, Lecellier, Booth and Nakamura2016) highlighted the complex effects of sediment pollution on the ability of larval coral reef fish to find a suitable habitat, affecting their post-settlement performance and recruitment success. Recruitment of A. insularis might thus be affected by the disturbance of red soil pollution. After recruitment in tidal flats and estuaries, they moved upstream for growth and gathered in areas containing an abundance of cobbles for spawning during the reproductive season. Thus, A. insularis utilized various habitats from tidal flats to the upper brackish area throughout their life cycle. Considering their life history, sustaining aquatic environments, including tidal flats, estuaries, rivers, and substrate for use as an appropriate upstream spawning ground, is fundamental for the conservation of this vulnerable species.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315417002053.
ACKNOWLEDGEMENTS
We are grateful to the members of the Tachihara Laboratory, University of the Ryukyus for their help during the survey and laboratory works. We also thank Fabienne Ziadi-Künzli (OIST, Okinawa Institute of Science and Technology Graduate University) for proofreading this manuscript and the anonymous reviewers who commented on an early version of the manuscript.
FINANCIAL SUPPORT
T.K. was partly supported by Research Fellowships of the Japan Society for the Promotion of Science (JSPS). This study was partly supported by the Sasakawa Scientific Research Grant from The Japan Science Society (Research number: 27–706) and JSPS KAKENHI Grant (JP16J07695) to T.K. All surveys in the present study complied with the current laws of Japan.











