There is a large body of evidence on the cardiovascular benefits of n-3 long-chain PUFA (marine n-3 fatty acids), mainly EPA (20 : 5n-3) and DHA (22 : 6n-3)(Reference Lavie, Milani and Mehra1). The synthesis of these fatty acids is extremely inefficient in humans(Reference Brenna, Salem and Sinclair2), and thus they must be acquired pre-formed from dietary sources, mainly from fatty fish. Dietary EPA and DHA are readily incorporated into cell membranes where they influence membrane function, and this is believed to be one of the mechanisms underlying their anti-arrhythmic, lipid-lowering, anti-thrombotic and overall anti-atherosclerotic effects(Reference Lavie, Milani and Mehra1). The strength of the evidence favouring n-3 fatty acids has prompted many international health organisations and agencies to publish recommendations for increased n-3 fatty acid intake, typically by advising the inclusion of at least two servings/week of fatty fish to promote cardiovascular health(Reference Lucas, Asselin and Plourde3).
The blood lipid contents of EPA and DHA have been widely used as biomarkers of intake and as surrogates of their enrichment in cellular membranes(Reference Baylin and Campos4). In addition, the omega-3 index, defined as the sum of percentages of EPA+DHA in erythrocyte membranes, has been proposed as a new risk marker and risk factor for CHD, particularly sudden cardiac death(Reference Brenna, Salem and Sinclair2). Based on previous studies in Western populations, the cardioprotective target level for the omega-3 index has been tentatively set at 8 %, while values below 4 % are associated with higher cardiovascular risk(Reference Harris5). The main determinant of the omega-3 index is the consumption of EPA+DHA(Reference Sands, Reid and Windsor6–Reference Itomura, Fujioka and Hamazaki8), which explains the higher values reported in Japanese populations compared with those from the USA or other Western countries(Reference Sands, Reid and Windsor6–Reference Cohen, Garg and Ali9). In recent years, some other factors that influence the omega-3 index have been described, including age, BMI, diabetes, smoking, physical activity and socio-economic status(Reference Sands, Reid and Windsor6–Reference Cohen, Garg and Ali9). The influence of dietary fatty acids other than EPA and DHA on the omega-3 index has been little investigated but is a subject of interest. This is particularly true of the n-6 fatty acids linoleic acid (LA) and arachidonic acid since there is some controversy regarding their role in CHD risk(Reference Whelan10–Reference Ramsden, Hibbeln and Lands13), and of the plant-derived n-3 fatty acid α-linolenic acid (ALA) because it is a precursor of long-chain n-3 PUFA. To address these questions, we examined the associations between the omega-3 index and dietary fatty acids in subjects at high cardiovascular risk living in Spain, the country with the highest fish consumption both in Europe(Reference Welch, Lund and Amiano14) and among Mediterranean countries(Reference Garcia-Closas, Berenguer and González15).
Methods
Subjects
The present analysis was conducted within the Prevención con Dieta Mediterránea (PREDIMED) study, a large, multicentre, parallel-group, controlled, randomised clinical trial aimed at assessing the effects of the Mediterranean diet on the primary prevention of CVD. PREDIMED has eleven recruitment sites in nine Spanish cities, including one at the Hospital Clinic of Barcelona (Barcelona-North, Spain). The protocol has been reported in detail elsewhere(Reference Estruch, Martínez-González and Corella16). Briefly, participants were men aged between 55 and 80 years and women aged between 60 and 80 years with no prior CVD but at high cardiovascular risk. Inclusion criteria were either type 2 diabetes mellitus or at least three of the following risk factors: current smoking (>1 cigarette/d during the last month); hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or antihypertensive medication); LDL-cholesterol ≥ 1600 mg/l; HDL-cholesterol ≤ 400 mg/l in men or ≤ 500 mg/l in women, independently of lipid-lowering therapy; BMI ≥ 25 kg/m2; family history of premature CHD (definite myocardial infarction or sudden death before 55 years in male first-degree relatives or before 65 years in female first-degree relatives). Exclusion criteria were as follows: previous history of CVD; any severe chronic illness; drug or alcohol addiction; history of allergy or intolerance to olive oil or nuts (supplemental foods given in two arms of the study); low predicted likelihood of changing dietary habits. Between 2007 and 2009, 198 participants were recruited in the Barcelona-North site. At the first visit, participants provided informed consent to participate in the study and have their data about medical history, medication use and lifestyle, including dietary intake to be used. Anthropometric and blood pressure measurements were performed, and fasting blood samples were drawn. The study protocol (ISRCTN 35739639) was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by the ethics committee of the institution. Written informed consent was obtained from all subjects.
Assessment of risk factors
Participants were considered as diabetic, hyperlipidaemic or hypertensive if they had a previous diagnosis of these conditions and/or they were treated with antidiabetic, cholesterol-lowering or antihypertensive agents, respectively. Smoking status was categorised into never, current or past smoking according to self-reports. Physical activity was determined with the validated Spanish version of the Minnesota questionnaire(Reference Elosua, Marrugat and Molina17, Reference Elosua, Garcia and Aguilar18). Height, weight and waist circumference were measured with standard methods. Trained personnel measured systolic and diastolic blood pressure in triplicate with a validated semi-automatic oscillometer (Omron HEM-705CP; Omron Healthcare Europe, Hoofddorp, The Netherlands).
Dietary intake
The dietary habits of participants were assessed using a validated 137-item FFQ(Reference Fernández-Ballart, Piñol and Zazpe19). At the inclusion visit, the FFQ was completed by a trained dietitian in face-to-face interviews. Participants were asked about the frequency of consumption of each food item during the past year, specifying usual portion sizes (semi-quantitative assessment). A total of nine possibilities of frequency were offered, from never to more than six times per d. Information on seafood products was collected in eight items of the FFQ (uncanned fatty fish; lean fish; smoked/salted fish; molluscs; shrimp, prawn and crayfish; octopus, baby squid and squid; fatty fish canned in oil; fatty fish canned in salted water). Nutrient intakes were computed using Spanish food composition tables(Reference Moreiras, Carbajal and Cabrera20) and were adjusted for energy intake by the residual method(Reference Willett, Howe and Kushi21).
Laboratory analyses
Both fasting serum and 0·1 % EDTA blood were collected and processed immediately. Serum lipid and glucose concentrations were determined by standard enzymatic methods in the hospital clinical laboratory. A 100 μl aliquot of EDTA-collected blood was transferred into a chloroform-resistant eppendorf containing 1400 μl of distilled water. Once cells were haemolysed, they were spun for 5 min at 4°C at 2800 g in a microcentrifuge (Hermle Z 233 MK-2; Midwest Scientific, St Louis, MO, USA). The supernatant fluid (containing Hb and serum lipids) was discarded, and the pellet (almost entirely composed of erythrocyte membranes) was extracted with chloroform–methanol (2:1, v/v) containing butylated hydroxytoluene (50 mg/ml) and evaporated to dryness under N2. The lipid extract was stored at − 80°C until analysed. The lipid extract was redissolved in 1 ml boron trifluoride–methanol and transferred to a screw-cap test-tube, which was heated for 10 min at 100°C to hydrolyse and methylate the membrane glycerophospholipid fatty acids. The extracts were cooled at 25°C, and fatty acid methyl esters were isolated by adding 300 μl of n-hexane. After shaking for 1 min, 1 ml of a saturated NaCl solution was added, and the tubes were centrifuged for 10 min at 2200 g at room temperature to separate the layers. The upper (hexane) layer was removed, dried with anhydrous sodium sulphate, and a 50 μl aliquot was transferred into an automatic injector vial equipped with a volume adapter of 300 μl. Fatty acid methyl esters were separated by GC using a Perkin Elmer Clarus 500 apparatus (Perkin Elmer España, Madrid, Spain) equipped with a 30 m × 0·25 μm × 0·25 mm SupraWAX-280 capillary column (Teknokroma, Barcelona, Spain), an autosampler and a flame ionisation detector. Each fatty acid is expressed as a percentage of total identified fatty acids in the whole blood sample. The omega-3 index was calculated by the sum of percentages of EPA+DHA. To ensure the comparability of the omega-3 indices measured in the laboratory of the Hospital Clinic of Barcelona with that of other centres, ten dried blood samples were analysed, and the results were compared with those obtained in a reference centre (OmegaQuant, LLC, Sioux Falls, SD, USA). The regression coefficient (r 2) between the omega-3 index values was 0·96 (P < 0·0001), and mean values for EPA+DHA were 3·84 (sd 2·22) % at OmegaQuant v. 3·89 (sd 2·11) % in Barcelona.
Statistical analyses
Univariate regression and one-way ANOVA models were used to determine the effects of each patient's characteristics on the EPA+DHA of whole blood cells. The ANOVA models included independent determinants known to be predictors of the omega-3 index, chosen based on previous literature, i.e. sex, age, being a current smoker, treatment for diabetes, hypertension or dyslipidaemia, physical activity, BMI, and energy-adjusted dietary intakes of total fat and alcohol (g/d)(Reference Sands, Reid and Windsor6–Reference Cohen, Garg and Ali9, Reference di Giuseppe, de Lorgeril and Salen22). Some variables known to be related to the omega-3 index, such as fasting serum TAG and hypertension(Reference Block, Harris and Pottala7), were left out of the model, as they were considered to be more a consequence than a determinant of the omega-3 index.
In addition, independent associations between the omega-3 index and the intake of fatty acids other than EPA+DHA, i.e. SFA, MUFA, LA, arachidonic acid and ALA, were also assessed by multivariate regression models for the Z-transformed scores of each fatty acid of interest. After constructing an unadjusted model, we adjusted for energy-adjusted EPA+DHA intake in a second model; finally, in a third model, we further considered as confounders those predictors with P < 0·05 univariate associations (current smoking and physical activity). Statistical significance was defined as P < 0·05. Analyses were performed using SPSS software, release 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
The study population included 102 men and 96 women, aged 66 (sd 6) years. None of the study subjects had suffered prior CHD, but all of them were at high cardiovascular risk, as attested by the prevalence of overweight or obesity (92·9 %), family history of premature CHD (41·4 %), and/or treatment with antidiabetic, cholesterol-lowering and/or antihypertensive agents (85·4 %). Table 1 shows detailed information on clinical and anthropometric variables. The whole blood cell membrane fatty acid composition is presented in Table 2. The average omega-3 index was 7·1 % (Fig. 1), and was above 8 % in 25·8 % of the study group and below 4 % in 3·5 %. Consumption of the fatty acids of interest is shown in Table 3. The intake of specific seafood items and their EPA+DHA content (Table 4) confirms the high consumption of total seafood of the study population. No significant differences were observed by sex or age regarding consumption of any of the seafood items (data not shown). None of the participants reported consumption of fish oil supplements. Attesting to the validity of the questionnaire used in the present study, Pearson's correlation coefficient values between the omega-3 index and both the total seafood intake and the calculated intake of EPA+DHA from seafood were 0·384 and 0·350, respectively (P < 0·000001, both).
Table 1 Characteristics of the study population
(Mean values, standard deviations, number of subjects, percentages, ranges, medians and interquartile ranges, n 198)

* MET-min, minutes at a given metabolic equivalent level (units of energy expenditure in physical activity; 1 MET-min is roughly equivalent to 4·2 kJ (1 kcal)).
Table 2 Proportions of the main fatty acids in whole blood cell membranes of the study population
(Medians and interquartile ranges, n 198)
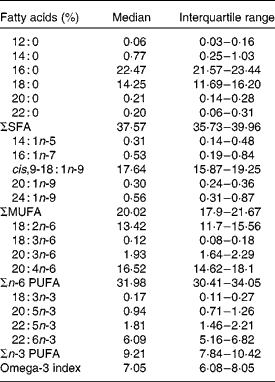

Fig. 1 Distribution of the percentage of whole blood cell EPA+DHA values (omega-3 index) in the study population (n 198). The omega-3 index at 8 and 4 % indicates proposed low- and high-risk horizons (—), respectively, while that at 7·1 % is the population average (····).
Table 3 Intake of fatty acids of interest in the study group
(Mean values, standard deviations and ranges)
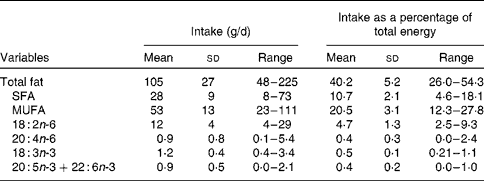
Table 4 Intake of seafood products and their associated EPA+DHA content
(Mean values, standard deviations and ranges)

The univariate associations between the omega-3 index and several clinical, anthropometric and dietary variables are shown in Table 5. The only factors significantly associated with the omega-3 index were serum TAG and smoking status (inversely), and leisure-time physical activity and EPA+DHA intake (directly). Alcohol intake was not significantly associated with the omega-3 index. For every additional grams of EPA+DHA consumed per day, the omega-3 index increased by 1·17 units. However, the most potent determinant of the omega-3 index, the EPA+DHA intake, explained only 12 % (P < 0·001) of the index's variability. The inclusion of another ten potential predictors to the model (sex, age, being a current smoker, treatment for diabetes, hypertension or dyslipidaemia, physical activity, BMI, and energy-adjusted dietary intakes of alcohol and total fat) increased the explanatory value of the index's variability to 19 %.
Table 5 Univariate associations of the omega-3 index with clinical, anthropometric and dietary variables*

* Results obtained by regression and ANOVA.
† Value corresponding to a 1 sd increase.
‡ MET-min, minutes at a given metabolic equivalent level (units of energy expenditure in physical activity; 1 MET-min is roughly equivalent to 4.2 kJ (1 kcal)).
Table 6 shows the associations between the omega-3 index and the intake of different fatty acids after adjustment for energy-adjusted intake of EPA+DHA. (Since other subjects' characteristics were not significant predictors in the multivariate model, they were not included in this model.) Consumption of EPA+DHA continued to be strongly related to the omega-3 index, while MUFA, LA, arachidonic acid and ALA were unrelated even in the unadjusted models. In contrast, the sum of dietary SFA showed a significant inverse association with the omega-3 index in the unadjusted model. However, when the model was further adjusted for energy-adjusted intake of EPA+DHA, current smoking and physical activity, the statistical significance of the association was blunted, although a trend for an inverse association was still present (P = 0·095).
Table 6 Multivariate associations between energy-adjusted fatty acid intake and the omega-3 index in the study population (n 198)*

LA, linoleic acid; AA, arachidonic acid; ALA, α-linolenic acid.
* Data are presented for a 1 standard deviation unit increase in the fatty acid of interest, examined by multiple linear regression analyses.
† Unadjusted.
‡ Adjusted for EPA+DHA intake (energy-adjusted).
§ Further adjustment for current smoking and total physical activity (increase of 293·7 MET-min/d). MET-min, minutes at a given metabolic equivalent level (units of energy expenditure in physical activity; 1 MET-min is roughly equivalent to 4.2 kJ (1 kcal)).
Discussion
In the present cross-sectional study, we searched for dietary, anthropometric and lifestyle determinants of the omega-3 index in a population at high risk of CHD living in Spain, the country with customarily high intakes of total fat, MUFA (supplied by olive oil), and EPA and DHA from seafood. Consistent with previous data, intake of EPA and DHA was the main predictor of the omega-3 index, but explained only 12 % of its variability. None of the other dietary fatty acids was related to the omega-3 index.
Recognising that there are methodological differences in measuring the omega-3 index, the mean value found in the present study group (7·1 %) is noticeably higher than those described in Western populations, particularly in the USA, where it has been consistently reported to be about 5 %(Reference Sands, Reid and Windsor6, Reference Block, Harris and Pottala7, Reference Cohen, Garg and Ali9). The cross-validation of our method against the method used in the original definition of the omega-3 index (proposed by Harris and von Schacky) indicates that methodological differences do not explain the higher omega-3 index values seen in Spain. However, the value for the index reported here is still lower than those described in similar studies conducted in Japan(Reference Itomura, Fujioka and Hamazaki8), Korea(Reference Park, Park and Yi23) or Alaska (USA)(Reference Ebbesson, Devereux and Cole24). The obvious explanation for the high omega-3 index in the present study population is the high intake of EPA and DHA (mean 0·9 g/d), which is approximately the American Heart Association recommendation for subjects in secondary prevention(Reference Lucas, Asselin and Plourde3). This, in turn, is due to the high consumption of seafood (mean 115 g/d), a value that concurs with those reported in recent surveys for different Spanish population groups(Reference Welch, Lund and Amiano14, Reference Garcia-Closas, Berenguer and González15). This high intake of EPA and DHA could in part explain the paradox of low rates of both incident CHD and cardiac death in Spain despite a high background prevalence of cardiovascular risk factors(Reference Marrugat, D'Agostino and Sullivan25). A similar paradox has been described in Japan(Reference Ueshima26), the country leading in global fish consumption(Reference Iso, Kobayashi and Ishihara27). In this regard, only 3·5 % of the present study subjects showed an omega-3 index below 4 %, the level associated with an increased risk of fatal CHD, while 26·1 % displayed an index above 8 %, the level considered to be protective against CHD death(Reference Harris5).
A unique feature of the present study was the examination of the potential effects on the omega-3 index of dietary fatty acids other than EPA+DHA. Of particular interest were LA and arachidonic acid because there is a controversy on whether increased consumption of these n-6 fatty acids would decrease the proportions of EPA and DHA in membranes(Reference Kris-Etherton, Fleming and Harris11–Reference Ramsden, Hibbeln and Lands13). ALA intake was of interest since it is the precursor of EPA and DHA(Reference Brenna, Salem and Sinclair2). We found that the intake of none of these fatty acids was related to the omega-3 index. It is possible that ALA might have had more influence on the omega-3 index had the EPA intake not been so high, since EPA can inhibit the activity of δ-5 and δ-6 desaturases, which are required for the conversion of ALA into longer-chain derivatives(Reference Brenna, Salem and Sinclair2). On the other hand, the relatively low intake of LA (mean 12 g/d, owing to the low consumption of seed oils, margarines and shortenings in Spain) would be expected to enhance the conversion of ALA to EPA by reduced competition for the desaturases.
There were significant associations of current smoking and physical activity with the omega-3 index, findings that concur with data from prior studies(Reference Itomura, Fujioka and Hamazaki8). The inverse relationship between the omega-3 index and serum TAG has also been reported previously(Reference Block, Harris and Pottala7), and probably reflects the well-known effects of EPA and DHA on this serum lipid fraction(Reference Harris and Bulchandani28). Finally, in contrast to the findings of the IMMIDIET study(Reference di Giuseppe, de Lorgeril and Salen22), the omega-3 index was unrelated to alcohol intake, even though average consumption was similar in the two studies. The higher EPA+DHA intakes in Spain compared with those in the countries included in the IMMIDIET study suggest that alcohol intake may only affect the omega-3 index in the context of low n-3 intakes.
The present study has limitations. First, it was a single-centre study with a relatively small sample size. Second, given the cross-sectional design of the study, temporal relationships cannot be established, and we cannot exclude the possibility of residual confounding. Strengths of the study included the use of a validated FFQ that is comprehensive regarding seafood intake, the validation of the fatty acid analysis method against a reference laboratory and the specific focus on individuals at increased risk for cardiac death. To our knowledge, this is the first examination of the omega-3 index in a Spanish cohort.
In conclusion, in a population at high risk of CHD with high intakes of total fat, MUFA and EPA+DHA, the omega-3 index was primarily predicted by the intake of EPA+DHA, not by known demographic, metabolic or other lifestyle factors. Further research is needed to more clearly define the environmental and genetic determinants of this emerging risk factor for CHD.
Acknowledgements
Emili Corbella provided expert assistance with statistical analyses. CIBERobn is an initiative of ISCIII, Spain. W. S. H. is a scientific advisor to companies with interests in fatty acids including Monsanto, Unilever, Omthera and GlaxoSmithKline, and has been a speaker for the latter. In addition, he is the owner of OmegaQuant, LLC, a company that offers blood n-3 fatty testing. E. R. is a scientific advisor to Ferrer International, the company that commercialises Omacor (a brand of n-3 fatty acid supplements) in Spain. None of the other authors has any potential conflicts to disclose. The present study was supported by grants FIS PI06/0365 from the Spanish Health Ministry, Centro Nacional de Investigaciones Cardiovasculares 2008 and Fundació Privada Catalana de Nutrició i Lípids, Barcelona, Spain. A. S.-V. was supported by post-doctoral contract FIS CD07/0083. The author's responsibilities were as follows: A. S.-V., W. S. H. and E. R. designed the study; A. S.-V. and M. C. determined the fatty acid composition of whole blood cell membranes; A. S.-V., W. S. H. and E. R. drafted the manuscript; all other authors substantially contributed to the analysis and/or interpretation of the data and revised the manuscript critically for important intellectual content.









