Introduction
Information on the distribution of species is crucial to any conservation effort. Nevertheless, the paucity of information about how most Neotropical species are distributed makes this a difficult task. A fairly recent approach which attempts to overcome this lack of information is the simulation of species’ distributions based on a model of their ecological niche (Peterson Reference Peterson2001). In this paper we adopt this approach, in conjunction with expert knowledge, to predict the distributions of species in the genera Grallaria and Grallaricula in Ecuador. We then use the predicted distribution maps, along with each species’s natural history and the current and past state of vegetation in Ecuador, to make a national conservation assessment for each species.
Species in the genera Grallaria (31 species) and Grallaricula (eight species) (Grallariidae, see Remsen et al. Reference Remsen, Cadena, Jaramillo, Nores, Pacheco, Robbins, Schulenberg, Stiles, Stotz and Zimmer2008) are largely terrestrial, understorey, Neotropical insectivores, primarily confined to montane regions of the Andes. Other than a few species above the tree-line, they generally inhabit areas with dense, wet, tangled understorey (Fjeldså and Krabbe Reference Fjeldså and Krabbe1990, Ridgely and Tudor Reference Ridgely and Tudor1994, Kattan and Beltrán Reference Kattan and Beltrán1999). Both genera reach their peak diversity in the tropical Andes from central Colombia to central Peru (Ridgely and Tudor Reference Ridgely and Tudor1994). Most species of Grallaria and Grallaricula are fairly restricted both geographically and altitudinally, and are considered habitat specialists (Graves Reference Graves1988, Kattan and Beltrán Reference Kattan and Beltrán1999, Reference Kattan and Beltrán2002). While several species occur in syntopy, some replace each other altitudinally (Ridgely and Tudor Reference Ridgely and Tudor1994, Kattan and Beltrán Reference Kattan and Beltrán1999). Species of both genera are among the least known of Neotropical birds, due in part to their elusive behaviour and preference for inhabiting thick understorey (Kattan and Beltrán Reference Kattan and Beltrán1999, Reference Kattan and Beltrán2002, Dobbs et al. Reference Dobbs, Martin and Kuehn2001, Greeney et al. Reference Greeney, Dobbs, Martin and Gelis2008). This is highlighted by the fact that seven species have been described in the last five decades (Wetmore and Phelps Reference Wetmore and Phelps1956, Lowery and O’Neill Reference Lowery and O’Neill1969, Schulenberg and Williams Reference Schulenberg and Williams1982, Graves et al. Reference Graves, O’Neill and Parker1983, Graves Reference Graves1987, Stiles Reference Stiles1992, Krabbe et al. Reference Krabbe, Agro, Rice, Jácome, Navarrete and Sornoza1999), and very little is known of their breeding biology (Greeney et al. Reference Greeney, Dobbs, Martin and Gelis2008).
Six of the 20 species of Grallaria and Grallaricula occurring in Ecuador are currently ranked as globally threatened or ‘Near Threatened’ (BirdLife International 2009. At the national level, two additional species are ranked as threatened (Granizo et al. Reference Granizo, Pacheco, Ribadeneira, Guerrero and Suárez2002). However, we suspect that many more species might be susceptible to extinction than those currently recognised in these works, for two reasons. First, they prefer the understorey of forest habitats and are reluctant to abandon forests. Studies have demonstrated that most Grallaria and Grallaricula are confined to forests (Kattan and Beltrán Reference Kattan and Beltrán1999, Reference Kattan and Beltrán2002), generally able to briefly occupy neighbouring, more open habitats only under overcast weather conditions (Poulsen Reference Poulsen1993). It has also been demonstrated that ground and understorey insectivores are among the first groups of birds that vanish when forests are severely fragmented (Renjifo Reference Renjifo2001, Sekercioglu et al. Reference Sekercioglu, Ehrlich, Daily, Aygen, Goehring and Sandí2002). Second, their preferences for specific habitats along narrow altitudinal belts or small geographic regions make them more prone to disappear if those habitats are depleted (Manne et al. Reference Manne, Brooks and Pimm1999). Furthermore, taxonomy in the group remains poorly known; species such as Grallaria gigantea and G. nuchalis are represented by different races in the eastern and western Andes that may actually be specifically distinct, making local declines or extinctions more significant than is recognised under current classifications.
In this paper we evaluate the conservation status of Grallaria and Grallaricula antpittas in Ecuador using environmental niche modelling based on a compilation of expert knowledge and locality records, primarily from museum specimens and published literature. We then evaluate how their ranges have changed using pre- and post-deforestation vegetation maps (Sierra Reference Sierra1999). With this information, we use IUCN criteria for regional assessments (UICN 2003) to evaluate the national conservation status of each species, with the goal of revising the assessments recently published by Granizo et al. (Reference Granizo, Pacheco, Ribadeneira, Guerrero and Suárez2002) for Ecuadorian birds, and to better understand the threats to these poorly studied species (Freile Reference Freile2000, Greeney et al. Reference Greeney, Dobbs, Martin and Gelis2008). Where data allow, we have indicated species that may warrant reclassifying on the global IUCN Red List.
Methods
Study species and conservation status
Ecuador is home to 20 of the 39 currently recognised species in both genera (Ridgely and Greenfield Reference Ridgely and Greenfield2001). In this paper, we consider only 18 species because two – Grallaria rufocinerea and Grallaricula ferrugineipectus – were only recorded in Ecuador after the data were compiled (Freile Reference Freile2000, Nilsson et al. Reference Nilsson, Jonsson and Krabbe2001, P. Coopmans in litt. 2001).
The national conservation status of each species was assessed using the IUCN guidelines for regional assessments (Gärdenfors et al. Reference Gärdenfors, Rodríguez, Hilton-Taylor, Hyslop, Mace, Molur and Poss1999, Reference Gärdenfors, Hilton-Taylor, Mace and Rodríguez2001; UICN 2003). IUCN uses five criteria to determine a category of threat: A. reduction in population size, B. size of geographic range, C. estimated population size, D. small or reduced population, and E. quantified estimate of extinction. The poor knowledge of the ecology of most Grallaria and Grallaricula antpittas does not allow an accurate evaluation of population sizes and trends, as no current estimates of population densities exist for the vast majority of species (see Kattan and Beltrán Reference Kattan and Beltrán1999, Reference Kattan and Beltrán2002). Therefore, given the information currently available, and the evidence that tropical understorey insectivores, including Grallaria and Grallaricula, are susceptible to forest fragmentation (Stouffer et al. Reference Stouffer, Bierregaard, Strong and Lovejoy2006), we consider criterion B1 (extent of occurrence) the most applicable criterion for assessing the conservation status of Ecuadorian Grallaria and Grallaricula. We also applied criterion A3 (future population reduction) based on current deforestation rates (Stewart and Gibson 1995), an estimation of minimum and maximum deforestation over three generations, and the percentage of species ranges expected to disappear under this deforestation scenario (see species accounts). For data on these estimates, contact the senior author.
Even though we estimated percentage of range loss, criterion A2 (population size reduction) could not be straightforwardly applied as this loss occurred over more than three generations and deforestation rates were not linear. Similarly, criterion B2 was not used as we did not estimate extent of suitable habitat within the range of each species. There was insufficient information to even guess population sizes. Our assessments of conservation status were discussed with local experts and compared with threat categories from Granizo et al. (Reference Granizo, Pacheco, Ribadeneira, Guerrero and Suárez2002) and BirdLife International (2009) for endemics or near-endemics.
Data compilation and data cleaning
In total, 980 Ecuadorian records were compiled from museum specimens, published literature (e.g. Parker and Carr Reference Parker and Carr1992, Poulsen and Krabbe Reference Poulsen and Krabbe1998), field work carried out by JFF, and personal contributions from a number of experts on the avifauna of Ecuador.
Museum data were collected through visits to several collections [Museo Ecuatoriano de Ciencias Naturales (MECN), Museo de Zoología del Departamento de Biología de la Escuela Politécnica Nacional (MEPN), Museo de Zoología, Departamento de Ciencias Biológicas, Pontificia Universidad Católica del Ecuador (QCAZ), Museum of Zoology, Louisiana State University (LSUMZ), Museum of Zoology, University of Kansas (KU), and British Museum of Natural History, Tring (BMNH)] and by examining catalogues of the major museum collections in North America known to hold specimens from Ecuador, including: Academy of Natural Sciences of Philadelphia (ANSP), Carnegie Museum of Natural History (CMNH), Field Museum of Natural History (FMNH), Smithsonian Institution (USNM) and Western Foundation of Vertebrate Zoology (WFVZ).
As we were not able to verify the taxonomic identity of all the specimen data gathered, a conservative approach was used to evaluate data accuracy and records located well outside the known distribution range of a given species (e.g. Andean species with a tropical collection site or vice versa) were not included in the analyses. Similarly, records with insufficient information (e.g. eastern Ecuador, Pichincha Province) were not included in the modelling and assessments. The geographic location of most records was verified against topographic and ornithological gazetteers (U.S. Board on Geographic Names 1957, Instituto Geográfico Militar 1978–1982, Paynter and Traylor Reference Paynter and Traylor1977, Paynter Reference Paynter1993). These three filters left us with a total of 719 points. The number of points per species was highly variable, ranging from four (Grallaria ridgelyi) to 178 (Grallaria quitensis) (mean 39.9 sites). See Table 1 for number of localities per species.
Table 1. Landscape metrics used for model construction and conservation assessment of Grallaria and Grallaricula species in Ecuador.

1 indicates metrics before deforestation, while 2 indicates metrics after deforestation (see Sierra Reference Sierra1999). All area metrics are in hectares: TA= total area (km2); NP= number of patches; MPS= mean patch size; PSCOV= patch size coefficient of variation; PSSD= patch size standard deviation; TE= total edge; NL= number of localities. Species are presented in alphabetical order.
In total, 26 field trips were carried out in order to fill gaps in the knowledge of potential distributions. On each field trip, a combination of three methods was used to locate Grallaria and Grallaricula species: playback transects, dawn chorus recordings, and mist-netting (see Freile Reference Freile2000 for details). All the information gathered was stored in a Microsoft Access database, and is available on request through the senior author.
Species distribution modelling
We used BIOCLIM (Nix Reference Nix1986) to create models. BIOCLIM performs well with presence-only data (Parra et al. Reference Parra, Graham and Freile2004) and was the most straightforward modelling method. This method extracts the values of the environmental data layers at each point locality for a given species and generates a bioclimatic profile that consists of the ranges of all climate variables used (e.g. 95th percentiles). The model matches the bioclimatic profile of each species with climate estimates at other sites on a grid to determine other locations with similar values, thereby producing a map of the predicted potential distribution of a given species. To evaluate models, we split the data into training (80% of points) and testing (20% of points) datasets. We ran BIOCLIM models with the training dataset and then calculated the percentage of withheld (i.e. testing) points that were predicted to be suitable. We only ran evaluation for species where we had 18 or more points. Note that all points were used in final models used in conservation assessment. We also generated models with a machine-learning technique (Maxent; Phillips et al. Reference Phillips, Anderson and Schapire2006) in order to ensure that the performance of BIOCLIM models was reasonable and similar to that of Maxent. This was done because recent criticisms have been made of BIOCLIM results and machine learning methods have been shown to perform better (Elith et al. Reference Elith, Graham, Anderson, Dudik, Ferrier, Guisan, Hijmans, Huettman, Leathwick, Lehmann, Li, Lohmann, Loiselle, Manion, Moritz, Nakamura, Nakazawa, Overton, Peterson, Phillips, Richardson, Scachetti-Pereira, Schapire, Soberón, Williams, Wisz and Zimmermann2006, Tsoar et al. Reference Tsoar, Allouche, Steinitz, Rotem and Kadmon2007). We found that methods provided similar outputs (see Fig. 1 for a representative sampling of four species).

Figure 1. Comparison between BIOCLIM and Maxent models for a representative set of species, three Grallaria and one Grallaricula species. Note that the main differences (BIOCLIM smaller predicted ranges) are the result of expert knowledge cutting and that our models (Figs. 2–10) present only areas with highest presence probability.
We used the following environmental variables in the model: 1. annual mean monthly temperature, 2. total annual rainfall, 3. coefficient of variation in temperature, 4. coefficient of variation in rainfall, 5. driest consecutive three months of the year and 6. water budget. These variables, recently made available by CHG after a compilation of weather station data for Ecuador, were chosen because, in concert, they should be representative of a major axis of the birds’ ecological niche (i.e. factors that might limit Grallaria and Grallaricula distributions). Assuming we have a representative sample of the range of climatic variables important for a given species, final models will likely represent the potential or historic distribution of the species, as they are based only on climatic parameters and do not reflect recent land-cover changes (deforestation). These predicted geographic distributions were further modified based on expert knowledge (JFF, N. Krabbe, P. Greenfield, R. Ridgely, F. Ortiz-Crespo, P. Coopmans). Major modifications were based on (i) suitability of vegetation, (ii) exclusion by competing species and (iii) elevational limits. To evaluate how recent deforestation might have influenced species’ ranges, we identified all deforested areas within each predicted distribution based on a recent map of deforestation of Ecuador (Sierra Reference Sierra1999), removing these areas from the modelled distribution. This resulted in two modelled distributions for each species: one that represents the historic distribution and one that represents the current distribution with deforested areas removed (Figs. 2–10).

Figure 2. Historical and current distribution ranges of Ochre-striped Grallaria dignissima and Moustached G. alleni Antpittas in Ecuador. Green dots represent actual locations.

Figure 3. Historical and current distribution ranges of Watkin's Grallaria watkinsi and Rufous-crowned G. ruficapilla Antpittas in Ecuador. Green dots represent actual locations.

Figure 4. Historical and current distribution ranges of Scaled Grallaria guatimalensis and Plain-backed G. haplonota Antpittas in Ecuador. Green dots represent actual locations.

Figure 5. Historical and current distribution ranges of White-bellied Grallaria hypoleuca and Yellow-breasted G. flavotincta Antpittas in Ecuador. Green dots represent actual locations.

Figure 6. Historical and current distribution ranges of Undulated Grallaria squamigera and Giant G. gigantea Antpittas in Ecuador. Green dots represent actual locations.

Figure 7. Historical and current distribution ranges of Rufous Grallaria rufula and Tawny G. quitensis Antpittas in Ecuador. Green dots represent actual locations.

Figure 8. Historical and current distribution ranges of Chestnut-naped Grallaria nuchalis and Jocotoco G. ridgelyi Antpittas in Ecuador. Green dots represent actual locations.

Figure 9. Historical and current distribution ranges of Slate-crowned Grallaricula nana and Ochre-breasted G. flavirostris Antpittas in Ecuador. Green dots represent actual locations.

Figure 10. Historical and current distribution ranges of Crescent-faced Grallaricula lineifrons and Peruvian G. peruviana Antpittas in Ecuador. Green dots represent actual locations.
Landscape metrics
We calculated a series of landscape metrics for each modelled distribution to quantify how ranges have changed with deforestation. We used the software Fragstats (McGarigal et al. Reference McGarigal, Cushman, Neel and Ene2002) to calculate the following landscape metrics: total area, number of patches, mean patch size (with coefficient of variation and standard deviation), and total edge (Table 1). We performed a two-factor ANOVA to assess the significance of changes along the distribution ranges using SPSS software (SPSS Inc. 2001).
Results and Discussion
General distribution patterns
The predicted range maps are generally consistent with previously known general distributions (Krabbe et al. Reference Krabbe, Skov, Fjeldså and Petersen1998, Ridgely and Greenfield Reference Ridgely and Greenfield2001, Ridgely et al. Reference Ridgely, Allnut, Brooks, McNicol, Mehlman, Young and Zook2003) (Figs. 2–10). BIOCLIM generally predicted withheld data well. For the 10 species with 18 locality points or more, 82% (standard deviation 17%) of withheld points were predicted to be suitable. The higher level of detail in our models provides a more accurate depiction of the distribution ranges and differs in some relevant features from broader models, as discussed below (Ridgely and Greenfield Reference Ridgely and Greenfield2001, Ridgely et al. Reference Ridgely, Allnut, Brooks, McNicol, Mehlman, Young and Zook2003).
Fifteen species, including all Grallaricula species, are confined to the Andes. Two species occur in the Amazonian lowlands: Grallaria dignissima is confined to the Amazon, whereas Grallaria guatimalensis is rather widespread in the lower portions of both slopes of the Andes and more local in the eastern lowlands (Figs. 2, 4). Another species is restricted to the lowlands, foothills and slopes of the dry south-western lowlands (G. watkinsi, Fig. 3).
Among the Andean species, five are confined to the eastern slope, one is confined to the Pacific slope, five occur on both slopes of the Andes, and four are widespread throughout the Ecuadorian Andes including the inter-Andean valleys and slopes. Furthermore, eight species also inhabit isolated Amazonian volcanoes and mountain ranges (Sumaco volcano, Kutukú and del Cóndor cordilleras), and two species (Grallaria guatimalensis and G. watkinsi) are found in the southern Cordillera de la Costa (Chongón-Colonche region in western provinces of Santa Elena and Manabí); only Grallaria guatimalensis is also present in the northern portion of these isolated and discontinuous mountain ranges (Mache-Chindul) (Figs. 2–10).
The western Andes has 12 species and the eastern Andes 15 species. Distributions in the western Andes show three distinctive patterns (Figs. 2–9). Four species are restricted to the north (south to Cotopaxi province), four species are continuously distributed, and one is confined to the dry southern slopes (Loja province). Meanwhile, two species (Grallaria haplonota and Grallaricula flavirostris) share a discontinuous distribution, being absent from approximately 1°25′S to 3°30′S (between southern Pichincha and western Azuay provinces; Figs. 4, 9). It has been suggested for other taxa (Krabbe et al. Reference Krabbe, Skov, Fjeldså and Petersen1998, Freile Reference Freile2004) that poor knowledge of the avifauna of this ‘distributional gap’ might explain the patterns found and that ranges might be continuous along this slope. Interestingly, our modelled distributions suggest that these areas have a bioclimatic profile different to the areas to the north and south, supporting the idea that the break in distributions might be real. Distributions of species restricted to one side of the Andes are generally not predicted to occur on the other side based on climatic niche modelling, suggesting that each slope has a different climatic profile, an observation also made in other studies (Kattan et al. Reference Kattan, Franco, Rojas and Morales2004). This situation might be mirrored on the western slope, with eco-climatic differences accounting for discontinuous distributions of some species. One additional species (Grallaria guatimalensis) appears to have a similar discontinuous range in the western Andes (Fig. 4), but this might only be a result of poor sampling in the central portion (Cotopaxi through northern Azuay) (N. Krabbe pers. comm.) as it apparently tolerates habitat fragmentation and inhabits a range of humid to semi-deciduous forests.
Species’ ranges in the eastern Andes tend to be more contiguous: nine species have uninterrupted ranges from the Colombian to the Peruvian border, three occur fairly sparsely along nearly the whole cordillera (Grallaricula flavirostris, G. lineifrons and G. peruviana, Figs. 9-10), while two are restricted to the north. Grallaria alleni extends south to the Pastaza river depression and G. gigantea probably occurs south to the Paute river depression (Figs. 2, 6). These two fairly deep river depressions, which dissect the eastern Andes, are also considered potential barriers to the distribution of other Andean taxa (Krabbe et al. Reference Krabbe, Skov, Fjeldså and Petersen1998, Krabbe Reference Krabbe2008). It should be noted though that widespread species such as Grallaria squamigera and G. ruficapilla, apparently occur only locally along the eastern Andes (N. Krabbe in litt., contra our predicted distributions in Figs. 3 and 6). Recently, the known distribution of Grallaricula peruviana changed following a new record nearly 200 km northward (Greeney et al. Reference Greeney, Hannelly and Lysinger2004). Bioclimatic profiles were similar along the whole eastern cordillera, supporting the evenness of species’ distributions.
According to our models, the two Amazonian species (Grallaria guatimalensis and G. dignissima) are widespread across the whole region, but records of both species south of the Pastaza river are sparse (Figs. 2, 4). Further, records of G. guatimalensis from the whole Amazonian lowlands are scanty, making thorough field work necessary to corroborate our modelled distribution (Fig. 4). In the humid western lowlands, no Grallaria or Grallaricula species is known to occur. Grallaria watkinsi, the only species in the Pacific lowland region, occurs mostly along the foothills and slopes of the Andes and isolated ranges in semi-deciduous and deciduous forests (see discussion under species account) (Fig. 3).
Estimated historic range sizes in Ecuador vary from 112,745 km2 for Grallaria guatimalensis to 56 km2 for Grallaria ridgelyi (mean range size 20,594 km2, SD 27,695 km2) (Figs. 4, 8). Four species have predicted Ecuadorian ranges < 2,000 km2, eight species have ranges between 4,000 and 15,000 km2, and six have ranges > 20,000 km2 (Table 1).
Conservation assessments
The amount of habitat loss throughout the country has affected all Ecuadorian Grallaria and Grallaricula antpittas. A statistical analysis of the degree of range reduction suggests that historic ranges within Ecuador were significantly larger than current ranges as measured by total area (two factor ANOVA; F = 37.75, df = 17.00, P < 0.01). Range reduction was employed as a measure of range and population decline for the application of IUCN criteria to assess conservation status in Ecuador, as discussed below.
In our regional assessment, three species were ranked as ‘Endangered’, one ‘Vulnerable’ and three as ‘Near Threatened’ in Ecuador (Table 2). All Grallaria and Grallaricula species ranked as threatened or ‘Near Threatened’ (see below) have small distributional ranges and are confined to particular centres of endemism (Long et al. Reference Long, Crosby, Stattersfield and Wege1996). In a previous assessment of the conservation status of Ecuadorian birds (Granizo et al. Reference Granizo, Pacheco, Ribadeneira, Guerrero and Suárez2002), four species were ranked as ‘Endangered’, one as ‘Vulnerable’, and two as ‘Near Threatened’. Those species included in Granizo et al. (Reference Granizo, Pacheco, Ribadeneira, Guerrero and Suárez2002) are also considered here as threatened or ‘Near Threatened’, but four species are included in different categories. These differences in categories assigned to each species at national level arise (i) because of the more precise distributional data and recent historic vegetation maps we used to make more precise distribution maps, and (ii) because we applied the most recent IUCN criteria and guidelines for regional assessments (UICN 2003), whereas Granizo et al. (Reference Granizo, Pacheco, Ribadeneira, Guerrero and Suárez2002) applied an older version for global (rather than regional) assessments (UICN 2001). Further discussions on the categorisation of each species are presented in the species accounts below. Ridgely and Greenfield (Reference Ridgely and Greenfield2001) also provide a list of threatened species within Ecuador, in which they include two Grallaria species as ‘Endangered’ (one of them, G. rufocinerea, not considered in our assessment), two as ‘Vulnerable’ and one Grallaricula as ‘Near Threatened’. These differences will also be discussed below.
Table 2. Species of Grallaria and Grallaricula occurring in Ecuador, with their conservation categories in Ecuador and supporting information and criteria.
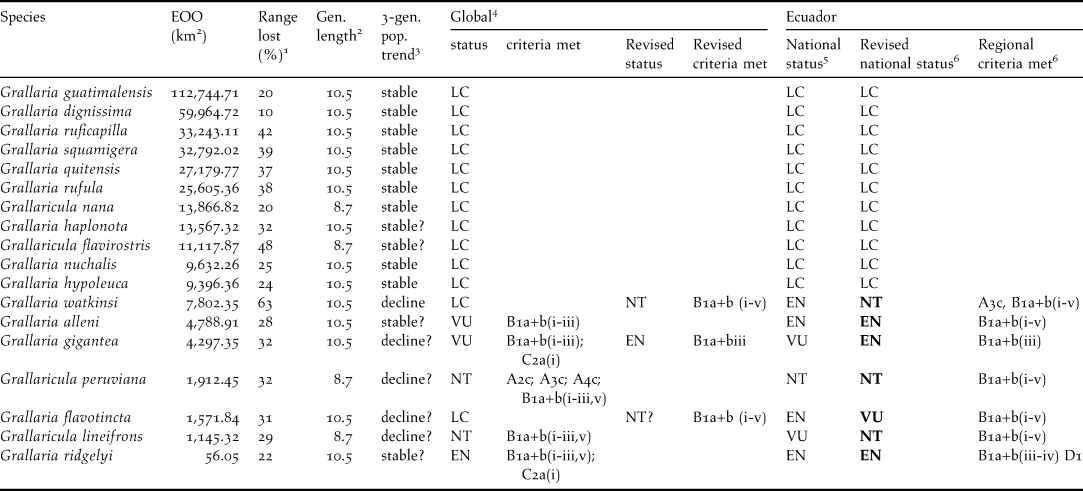
Species are organised according to range extent (EOO) within Ecuador, as estimated in the software Fragstats (McGarigal et al. Reference McGarigal, Cushman, Neel and Ene2002). Range loss and fragmentation were also calculated using this software. Proportion of range loss1 occurred over a long time frame since species original historic range extent to present time. Generation length2 and IUCN Global Red List status and criteria4 from BirdLife International (2009). National status in Ecuador5 assessed by Granizo et al. (Reference Granizo, Pacheco, Ribadeneira, Guerrero and Suárez2002). Population trend3 extrapolated from total forest loss within species ranges over a long undetermined time frame. Revised national status6 from this study. Grallaria rufocinerea and Grallaricula ferrugineipectus were not included in this analysis as both were discovered in Ecuador after data compilation for this study was finished.
The remaining 11 species, not considered as threatened in our evaluation, might nevertheless be of conservation concern at different levels. Grallaria dignissima is restricted to western Amazonia north of the Marañón river, within the Napo and Pastaza river basins (Napo Endemic Bird Area fide Stattersfield et al. Reference Stattersfield, Crosby, Long and Wege1998, Fig. 2). Forest is still extensive in the region, but deforestation, logging, oil extraction, and agricultural expansion are rapidly devastating large tracts of forest in the northern provinces of Orellana and Sucumbíos (Vallejo Reference Vallejo2003).
Additionally, all Andean species are threatened by ongoing deforestation, caused primarily by agricultural expansion. Some species seem to be especially susceptible, even to moderate habitat modification. While some species inhabit open areas (Grallaria quitensis), others have fairly limited altitudinal distributions and/or fragmented ranges (Figs. 4, 6, 8, 9) and tend to prefer less disturbed forests (Grallaria squamigera, G. nuchalis, G. haplonota and Grallaricula flavirostris). Similarly, Grallaria hypoleuca, G. rufula and Grallaricula nana are linked to somewhat naturally disturbed areas, especially dense secondary growth and bamboos stands, but are still reliant on ‘good’ forest coverage. On the other hand, Grallaria ruficapilla tends to prefer more disturbed areas (though not fully open), which might make it more tolerant of human-induced habitat modification (Figs. 3, 5, 7, 9).
Of the species studied here, Grallaria quitensis is probably the most tolerant of moderate disturbance. The only paramo species in Ecuador can survive in fairly degraded paramos, even in the vicinity of small agricultural towns, exotic tree plantations, and agricultural fields. It should be noted that, even though it tolerates a certain level of habitat modification, it is one of the Andean species with the highest overall amount of range loss (c. 37%; Fig. 7). Finally, Grallaria guatimalensis, the most widespread species in Ecuador, is not currently considered threatened due to its large extent of occurrence. Grallaria guatimalensis inhabits several different ecosystems (from Amazonian terra firme forest through humid cloud forest on Andean slopes). Forests in some areas within its range are extensively fragmented (e.g. south-western Andes in Loja and El Oro provinces). Even though this species regularly occurs in fragmented habitats (N. Krabbe, pers. comm.) ongoing fragmentation might adversely affect population dynamics and breeding success (Tewksbury et al. Reference Tewksbury, Garner, Garner, Lloyd, Saab and Martin2006).
There are notable differences in the conservation status of populations on the western and eastern Andean slopes. The populations of three species not considered as threatened (Grallaria nuchalis, G. haplonota, and Grallaricula flavirostris) and two ranked as ‘Endangered’ (Grallaria alleni and G. gigantea) occurring in the Cordillera Occidental are likely to be facing severe declines due to deforestation and habitat fragmentation. In fact, Grallaria nuchalis, G. haplonota and Grallaricula flavirostris nearly met the quantitative threshold for listing nationally as ‘Vulnerable’ under criterion B (i.e. estimated EOO < 20,000 km2 within which it occurs at no more than ten locations or habitat is severely fragmented), particularly for their western populations. It is important to highlight that, except for G. alleni, all these species have different subspecies on the eastern and western slopes (Grallaricula flavirostris has two races in the west and one in the east). From an evolutionary standpoint, differences in the conservation status of these subspecies may be critical (Moritz Reference Moritz2002). Studies have shown that within a single species, eastern and western subspecies are genetically divergent (Chaves et al. Reference Chaves, Pollinger, Smith and LeBuhn2007). It has even been suggested that the hylodroma (western) race of G. gigantea is a valid species (Ridgely and Greenfield Reference Ridgely and Greenfield2001). If this is confirmed, the conservation status of G. hylodroma, both at national and global levels, will need careful evaluation, as it would likely qualify as ‘Critically Endangered’.
Species accounts
Giant Antpitta Grallaria gigantea - Revised national status: EN
The national conservation status of the two subspecies of G. gigantea occurring in northern Ecuador differ. In eastern Ecuador, G. gigantea has only been recorded at a few sites (only two of them recent), but forests are still extensive along its predicted range, and there is a high level of protection in most of its range (Fig. 6). There are two Ecological Reserves (Cayambe-Coca and Antisana) and three National Parks (Sumaco-Napo Galeras, Llanganates and Sangay), covering c. 60% of the species’s range in Ecuador (Freile Reference Freile2000). On the other hand, the western race (hylodroma) has been recorded from several localities in Ecuador, most of them confined to the western slopes of Volcán Pichincha. While there are no records from the large Cotacachi-Cayapas Ecological Reserve, north of Pichincha, and only two recent records from the surrounding areas of Illinizas Ecological Reserve, our model (Fig. 6) predicts its presence in both protected areas. These differences in the protection status, the number of known localities, and the degree of forest fragmentation between the ranges of the eastern and western subspecies of G. gigantea complicate an assessment of its national conservation status. This can only be assessed by combining the two ranges, populations, and trends, to conduct a pooled but not necessarily accurate evaluation. Granizo et al. (Reference Granizo, Pacheco, Ribadeneira, Guerrero and Suárez2002) and BirdLife International (2009) found that G. gigantea qualifies as ‘Vulnerable’ owing to its small and declining range size. Our modelled distribution of its current range suggests it is ‘Endangered’ (EOO < 5,000 km2 and habitat is severely fragmented) at national scale. Neighbouring Colombian populations might not represent a potential source for immigration (see IUCN 2003 guidelines for regional assessments) as it is also threatened in Colombia (EN), where its range is minute, and no populations are known near the Colombia-Ecuador boundary to act as a source of immigrants (Renjifo et al. Reference Renjifo, Franco, Kattan, Amaya and Gómez2002). Given the small global range of G. gigantea and the critical situation of Colombian subspecies lehmanni (Renjifo et al. Reference Renjifo, Franco, Kattan, Amaya and Gómez2002) we suggest it is uplisted globally to ‘Endangered’ (meeting criterion B1a+biii).
Moustached Antpitta Grallaria alleni - Revised national status: EN
The distribution and status of this species is similar to G. gigantea (Fig. 2). While in the east, forests are still extensive, with large areas almost inaccessible and protected (e.g. Sumaco National Park), in the western Andes forests are more fragmented. Similarly, protection in the west is deficient; there are no records from Cotacachi-Cayapas Ecological Reserve and only two records from the vicinity of Illinizas Ecological Reserve. Several private preserves hold populations of G. alleni, and while it seems to be fairly common in adequate, forested habitat (e.g., Maquipucuna and Otonga Reserves), few of these are large enough for long-term conservation. The occurrence of G. alleni in Ecuador was only recently confirmed (Krabbe and Coopmans Reference Krabbe and Coopmans2000) because its vocalisations were previously unknown. Since tape-recordings became available, several new localities have been discovered and its natural history has become better understood (Freile and Renjifo Reference Freile and Renjifo2003, Greeney and Gelis Reference Greeney and Gelis2006). Quantitative data suggest that ‘Endangered’ status in Ecuador is appropriate (Table 2, see also Granizo et al. Reference Granizo, Pacheco, Ribadeneira, Guerrero and Suárez2002). This category might be overestimating its actual conservation status in Ecuador, particularly in the eastern Andes, but is the result of a conservative application of IUCN criteria. Potential immigration from Colombian populations is rather unlikely as it has also been ranked as EN in that country (Renjifo et al. Reference Renjifo, Franco, Kattan, Amaya and Gómez2002).
Watkin’s Antpitta Grallaria watkinsi - Revised national status: NT
The Tumbes endemic G. watkinsi is not currently considered globally threatened despite its small range, rather specific habitat requirements, and the extent of habitat loss throughout its range. Nonetheless, early evaluations included it in lists of candidate species for the Red Data Book of the Americas (Best et al. Reference Best, Clarke, Checker, Broom, Thewlis, Duckworth and McNab1993). This species is primarily restricted to foothill forests and scrub in the Andean slopes of south-western Ecuador (Loja and El Oro provinces), as well as in the low coastal ranges of Guayas, Santa Elena, and Manabí provinces (Fig. 3). There is a gap in the distribution of G. watkinsi between the Andean slopes of El Oro-Loja and the coastal cordillera of Guayas-Santa Elena-Manabí. It is not clear if this distributional gap is natural or is a consequence of the high level of habitat alteration that exists in the intervening area. Bioclimatic differences shown in our model suggest that this may be a real discontinuity. Grallaria watkinsi has the highest percentage of range loss (63%) of all the studied species, reflecting the current status of deciduous and semi-deciduous forests, and even scrub, in the Pacific lowlands of southern Ecuador (Sierra Reference Sierra1999). This author demonstrated that most forest types in the region (eight) are facing critical rates of deforestation. Even though, G. watkinsi ranges from forest understorey to dense regenerating scrub, the critical degree of habitat degradation throughout its range over the last decades has wholly devastated all vegetation cover from large areas, particularly in Manabí, Guayas, Santa Elena, and El Oro lowlands, and in El Oro and Loja lower slopes (Sierra Reference Sierra1999).
Remnant forests in western Ecuador (c. 10% of original cover) are generally small and fragmented. Moreover, land protection is low. There are three fairly small protected areas in the region (Machalilla National Park, Manglares-Churute and Arenillas Ecological Reserves), but there are no confirmed records from Manglares-Churute. A few scattered, privately protected reserves, much smaller than these national reserves, also exist. Their limited extension, combined with habitat destruction in surrounding areas, makes long term conservation seem dubious.
Grallaria watkinsi appears to tolerate a moderate to high degree of habitat alteration, inhabiting dense regenerating scrub and secondary forest edges, but being absent from completely modified areas or from forest patches where domestic animals (e.g. goats, pigs) forage and trample all understorey vegetation (an important threat to Tumbesian dry forests) (Bonaccorso et al. Reference Bonaccorso, Santander, Freile, Tinoco and Rodas2007). The critical level of range loss, as well as loss of suitable habitat within current extent of occurrence, suggest that its conservation status has been underestimated. However, the degree of tolerance to habitat alteration of this species precluded a threatened category even though it might fit quantitative thresholds for ‘Vulnerable’ in the country under criteria B1 and A3 (Table 2). We estimate a 10–30% reduction in population size over the next three generations (10.5 years) based on an educated guess of 288.9–866.7 km2 lost in this span of time, as a result of a national deforestation rate calculated in 1,000–3,000 km2 annually (Stewart and Gibson 1995). For further data on these estimates, contact the senior author.
We suggest, therefore, the ‘Near Threatened’ category for G. watkinsi in Ecuador (contra Granizo et al. Reference Granizo, Pacheco, Ribadeneira, Guerrero and Suárez2002). As G. watkinsi is near-endemic to Ecuador, we recommend re-assessing the global species status as ‘Near Threatened’ (with a small range < 20,000 km2 but found at more than 10 locations, being fairly tolerant to habitat disturbance and fragmentation).
Yellow-breasted Antpitta Grallaria flavotincta - Revised national status: VU
This species is not considered as globally threatened (BirdLife International 2009), but was also a candidate species for the Red Data Book of the Americas (Collar et al. Reference Collar, Gonzaga, Krabbe, Madroño Nieto, Naranjo, Parker and Wege1992; N. Krabbe, pers. comm. 2000). In Ecuador it was accorded ‘Endangered’ status by Granizo et al. (Reference Granizo, Pacheco, Ribadeneira, Guerrero and Suárez2002). It is confined to very wet, steep slopes and ravines along the Pacific slope of Colombia and north-western Ecuador (Ridgely and Tudor Reference Ridgely and Tudor1994) (Fig. 5). Its range is small, and its area of occupancy (i.e. areas within its distributional range where the species actually occurs = potential niche) might be even more limited considering microhabitat preferences. Within the Ecuadorian part of its range, G. flavotincta is fairly uncommon and local, and has not been recorded continuously from the Colombian border to the southern end of its range in western Pichincha province, as shown in map in Ridgely and Greenfield (Reference Ridgely and Greenfield2001). It shares a similar distributional pattern with other forest-confined Chocó endemics, which are currently ranked as threatened or ‘Near Threatened’ in Ecuador (e.g. Star-chested Treerunner Margarornis stellatus VU, Tanager-Finch Oreothraupis arremonops VU; Granizo et al. Reference Granizo, Pacheco, Ribadeneira, Guerrero and Suárez2002) and consequently might also be facing similar threats. The status of G. flavotincta in Colombia is apparently less critical than in Ecuador, in part due to a larger amount of continuous forest (not included in Renjifo et al. Reference Renjifo, Franco, Kattan, Amaya and Gómez2002). However, based on the deforestation map of Sierra (Reference Sierra1999) we estimate that less than 50% of the original cover of Andean forests in western Ecuador remains (Sierra Reference Sierra1999). There are two officially protected areas along the Ecuadorian range of G. flavotincta (Cotacachi-Cayapas Ecological Reserve and Pululahua Geobotanic Reserve), but the species has not yet been recorded in either, though our model predicts its occurrence in both. We feel confident that the category we assigned to G. flavotincta reflects the actual status of this species in Ecuador, and that previous assessments (Ridgely and Greenfield Reference Ridgely and Greenfield2001) failed to acknowledge the significant habitat loss and fragmentation which may affect this species. Further, we suggest that its global status needs to be reassessed, taking into account the categorisation of other species with similar distributions and habitat preferences. Even though the species approaches the threshold for EN in Ecuador under criterion B1a+b(i-v), Colombian populations might represent a source of immigrants for declining populations in Ecuador, making the VU category more likely.
Jocotoco Antpitta Grallaria ridgelyi - Revised national status: EN
This recently described species (Krabbe et al. Reference Krabbe, Agro, Rice, Jácome, Navarrete and Sornoza1999) is the only Grallaria almost endemic to Ecuador (recently found in extreme northern Peru, T. S. Schulenberg in litt. 2007). The very limited distributional range of G. ridgelyi is unique among Andean birds in Ecuador, being confined to steep slopes in a very narrow elevational range of the Cordillera de Lagunillas region of the extreme southern portion of the eastern Andes (Fig. 8). It is apparently replaced by one of its hypothesised close relatives (G. carrikeri) south of the Marañón river depression. Another close relative (G. nuchalis) occurs in sympatry with G. ridgelyi (Krabbe et al. Reference Krabbe, Agro, Rice, Jácome, Navarrete and Sornoza1999), with territories regularly found in close proximity (JFF unpubl. data). Its known global extent of occurrence encompasses less than 60 km2 (Table 2), but a recent record (Heinz et al. Reference Heinz, Schmidt and Schaefer2005) suggests that it might cover at least 180 km2. Its area of occupancy is even smaller as it is known to range only locally along this tiny global range (possibly around 25–36 km2). Recent efforts to locate new populations further north, south and east of the few currently known localities resulted in only one new site out of several areas visited (Heinz et al. Reference Heinz, Schmidt and Schaefer2005). Additionally, N. Krabbe (in litt. 2008) recently found it in San Luis, 20 km south of Tapichalaca, in an area about to be deforested. So far, this species seems to be genuinely rare, and as such, its vulnerability to extinction even under natural (not human-induced) catastrophes is very high (Lande Reference Lande, Mace, Balmford and Ginsberg1998). Forests are still fairly continuous along the limited range of G. ridgelyi, and deforestation is similarly restricted (Sierra Reference Sierra1999). Further, two of its four known localities in Ecuador are currently under protection within Tapichalaca Private Reserve and Podocarpus National Park, and new sites might be discovered within Podocarpus and neighbouring Colambo-Yacuri Protected Forest (Freile and Santander Reference Freile, Santander, Boyla and Estrada2005). Changes in the conservation status of forests, in the face of new mining concessions and ongoing deforestation, even in protected areas (López et al. Reference López, Torres and Beltrán2003) might severely affect the small range where G. ridgelyi occurs. Currently available data might even justify ranking G. ridgelyi as ‘Critically Endangered’ (under B1a+b(iii) with an EOO < 100 km2, severely fragmented and declining) both at national and global scales. However, as its global range may be larger than 100 km2, it occurs at more than one location, and because Podocarpus National Park and Tapichalaca Reserve might effectively protect the majority of the global population, fragmentation is unlikely to be severe and, hence the species should be listed as ‘Endangered’ EN B1a+b(iii,iv) (Granizo et al. Reference Granizo, Pacheco, Ribadeneira, Guerrero and Suárez2002, BirdLife International 2009).
Peruvian Antpitta Grallaricula peruviana - Revised national status: NT
Grallaricula peruviana has a small geographic range and a limited altitudinal distribution, being endemic of the Amazonian Andean slope and isolated mountain ranges of eastern Ecuador and extreme northern Peru (Ridgely and Tudor Reference Ridgely and Tudor1994) (Fig. 10). Until recently, it was only known from a handful of sites in southern Ecuador, and nothing was known about its natural history (even its vocalisations were unknown). Greeney et al. (Reference Greeney, Hannelly and Lysinger2004) described the nest and vocalisations of this species and provided a notable range extension that increased its very limited distribution. Its range is potentially continuous at least from the northernmost site (Cordillera de Guacamayos area, Napo province) south to northern Peru. Within this range, several protected areas exist (Antisana Ecological Reserve, Sumaco-Napo Galeras, Llanganates, Sangay and Podocarpus National Parks), but G. peruviana has only been certainly recorded from Podocarpus NP. Foothill forests where the species occurs are fairly extensive, particularly in Llanganates and Sangay National Parks, and in the Cordilleras de Kutukú and del Cóndor (Sierra Reference Sierra1999). It seems plausible that the lack of records in the area between the northern and southern localities is a result of insufficient ornithological research in this region (Sangay National Park is one of the least known protected areas of Ecuador). Grallaricula peruviana approaches the threshold for listing as EN within Ecuador under criteria B1 and B2 based on its restricted distribution but it does not meet the subpopulation qualifiers (severe fragmentation and ongoing population declines). It qualifies as ‘Near Threatened’ in Ecuador, in accordance with previous national and global assessments (Granizo et al. Reference Granizo, Pacheco, Ribadeneira, Guerrero and Suárez2002, BirdLife International 2009). Recent surveys suggest that the species has disappeared from the Cordillera de Guacamayos region (San Isidro Reserve) (N. Krabbe pers. comm.).
Crescent-faced Antpitta Grallaricula lineifrons - Revised national status: NT
The altitudinal range of this species is very restricted, as is its overall distribution (Ridgely and Tudor Reference Ridgely and Tudor1994) (Fig. 10). Until recently, it was known from a few sites in northern (Napo and Carchi provinces) and southern Ecuador (Loja province), but recent records in the intervening area suggest a continuous distribution (Ridgely and Greenfield Reference Ridgely and Greenfield2001). It occurs in the Amazonian and inter-Andean slopes of the Cordillera Oriental, but inter-Andean forests are almost completely gone because of agricultural expansion (Sierra Reference Sierra1999). Ridgely and Greenfield (Reference Ridgely and Greenfield2001) suggest that G. lineifrons is not currently at risk of extinction due to its capability to inhabit naturally altered areas like bamboo stands and secondary growth. However, the species is absent from highly degraded areas, and some areas of its range are threatened by agricultural expansion, burning and timber extraction for charcoal (Freile and Santander Reference Freile, Santander, Boyla and Estrada2005). In a study carried out at Guandera Biological Station, Cresswell et al. (Reference Cresswell, Hughes, Mellanby, Bright, Catry, Chaves, Freile, Gabela, Martineau, MacLeod, McPhie, Anderson, Holt, Barabas, Chapel and Sánchez1999) found G. lineifrons to be seemingly rare in secondary forest patches with re-grown canopy (but records were too scarce to allow abundance quantification). The species qualifies as NT in Ecuador (contra Granizo et al. Reference Granizo, Pacheco, Ribadeneira, Guerrero and Suárez2002 but in line with its global status categorised by BirdLife International 2009), but approaching the thresholds for listing as VU under criterion B1.
Concluding remarks
In general, rates of habitat loss and fragmentation are likely to have impacted all species of Grallaria and Grallaricula antpittas in Ecuador, with some species more severely affected due to their limited distributions in areas with high levels of human-induced habitat degradation. The conservation status of the species studied here was primarily assessed on the basis of distributional data, predicted range distributions based on these data, expert knowledge on the distribution and ecology of the species, and patterns of deforestation and extent and location of protected areas. However, IUCN criteria also take account of population size and population trends, information that is scarce for all Grallaria and Grallaricula species in Ecuador (Ridgely and Tudor Reference Ridgely and Tudor1994), and that might only be inferred. The IUCN guidelines (UICN 2003) suggest that inferences and projections can be used to evaluate the conservation status of species, but the application of the IUCN criteria is subject to the availability of information, biasing species’ status assessments to certain types of information which are more easily attained.
We reinforce the fact that the integration of environmental niche modelling, expert knowledge and knowledge of habitat degradation is a useful means of assessing the conservation status of a species (Renjifo et al. Reference Renjifo, Franco, Kattan, Amaya and Gómez2002, Graham et al. Reference Graham, Ferrier, Huettman, Moritz and Peterson2004, Gelfand et al. Reference Gelfand, Schimdt, Wu, Silander, Latimer and Rebelo2005). Although models are influenced by the quantity and quality of both locality and environmental information, and their performance varies with the technique used (e.g., Loiselle et al. Reference Loiselle, Howell, Graham, Goerck, Brooks, Smith and Williams2003, Elith et al. Reference Elith, Graham, Anderson, Dudik, Ferrier, Guisan, Hijmans, Huettman, Leathwick, Lehmann, Li, Lohmann, Loiselle, Manion, Moritz, Nakamura, Nakazawa, Overton, Peterson, Phillips, Richardson, Scachetti-Pereira, Schapire, Soberón, Williams, Wisz and Zimmermann2006, Graham et al. Reference Graham, Moritz and Williams2006, Hernández et al. Reference Hernández, Graham, Master and Albert2006), models can provide useful predictors of species’ ranges, especially for poorly known tropical species.
Serious recent criticisms have been made of the performance of BIOCLIM to generate predictive models (e.g. Tsoar et al. Reference Tsoar, Allouche, Steinitz, Rotem and Kadmon2007). Nonetheless, after rerunning models for four species (Fig. 1) using Maxent, a highly recommended technique (Elith et al. Reference Elith, Graham, Anderson, Dudik, Ferrier, Guisan, Hijmans, Huettman, Leathwick, Lehmann, Li, Lohmann, Loiselle, Manion, Moritz, Nakamura, Nakazawa, Overton, Peterson, Phillips, Richardson, Scachetti-Pereira, Schapire, Soberón, Williams, Wisz and Zimmermann2006), we found that while slight improvements might be possible in our models, differences among methods is qualitative and our interpretation of species declines (i.e. deforestation trends, threat level) will not change. Strong climatic and precipitation gradients in the Andes, to which species tend to respond, make it relatively straightforward to capture reasonable species-environment relationships even with simple models such as BIOCLIM (Parra et al. Reference Parra, Graham and Freile2004). Further, much of our interpretation of ranges (and range changes) was a function of bioclimatic modelling plus expert cutting and a deforestation map, which are not influenced by the original modelling method used. Given that this post-processing of each model influenced our results more than the relatively small differences expected based on what particular method was used, we are confident that our results would be qualitatively similar regardless of the original model choice.
Further information on population sizes, responses to habitat alteration, and the general ecology of antpittas is clearly needed to accurately assess their conservation status. Recently, knowledge about the natural history of both genera has notably increased (Robbins et al. Reference Robbins, Krabbe, Rosenberg, Ridgely and Sornoza1994, Dobbs et al. Reference Dobbs, Martin and Kuehn2001, Price Reference Price2003, Freile and Renjifo Reference Freile and Renjifo2003, Dobbs et al. Reference Dobbs, Martin, Batista, Montag and Greeney2003, Greeney et al. Reference Greeney, Hannelly and Lysinger2004, Reference Greeney, Dobbs, Martin and Gelis2008, Martin and Dobbs Reference Martin and Dobbs2004, Geeney and Martin 2005, Greeney and Sornoza Reference Greeney and Sornoza2005), but information is still mostly anecdotal. Aside from a single study (Heinz Reference Heinz2002), nothing is known of the population dynamics of Grallaria and Grallaricula species in Ecuador. Research on habitat preferences and population trends are needed, parallel to those recently carried out in Colombia (Kattan and Beltrán Reference Kattan and Beltrán1999, Reference Kattan and Beltrán2002). Further, current assessment methods, including ours, do not include subspecies, and the conservation of eastern and western Andean subspecies is a critical component which should be factored into future models. It is particularly important for isolated races (e.g. G. nuchalis obsoleta, G. gigantea hylodroma, G. haplonota parambae, G. flavirostris zarumae), which likely represent significant evolutionary units (Moritz Reference Moritz2002).
Acknowledgements
Thanks are due to Programa de Becas de Investigación para la Conservación, Fundación EcoCiencia, and William Belton Donations Program, American Bird Conservancy-ABC (Grant #P-98D019) for funding, and to Ministerio del Ambiente for research permits. Collection managers and curators of the following institutions kindly provided access or data: Leo Joseph (ANSP), Frank Steinheimer and Mark Adams (BMNH), Robin K. Panza and Kenneth Parkes (CMNH), Jorge Palacios (MEPN), David Willard (FMNH), Mark Robbins (KU), James Van Remsen (LSUMZ), Marco Jácome (MECN), Craig Ludwig and Gary Graves (NMNH), Manuel Marín (WFVZ), Jon Fjeldså (ZMUC), Robert Ridgely (ANSP), Mauricio Guerrero (Fundación Arcoiris), and Greg Budney, Andrea Priori and Nancy Schempf (Library of Natural Sounds, Cornell University). This research was presented as graduate thesis at the Department of Biology of Universidad Católica del Ecuador, wherein Tjitte de Vries was tutor. J.P. was funded by the Museum of Vertebrate Zoology during this work. Comments, suggestions and data provided by Niels Krabbe, Luis Suárez, Paul Greenfield, Mark Robbins, Paulo Catry, Morton Isler, Santiago Ron, Felipe Campos, Luis Coloma, Bette Loiselle and Jon Fjeldså were crucial. Many people and institutions kindly helped during fieldwork and are fully acknowledged in Freile (Reference Freile2000). This paper greatly benefited from insightful comments by Niels Krabbe, Harold Greeney, Jez Bird, and an anonymous reviewer, and the thorough advice of Elisa Bonaccorso and Olaf Jahn.














