Introduction
Clay minerals have diverse applications across different disciplines, including agriculture, medicine, and the environment, with a notable role as repositories for radioactive waste storage (Komadel et al., Reference Komadel, Madejova and Bujdak2005; Madejová et al., Reference Madejová, Pálková and Komadel2006; Zhao et al., Reference Zhao, Li, Sun, Liu, Zhou and Xu2022a). Most clay minerals assume a layered silicate structure, and these layers can be categorised as 1:1 and 2:1 type (Cheng et al., Reference Cheng, Yang, Liu, Zhang and Frost2010; Li et al., Reference Li, Li, Li, Zhao and Peng2022). The 1:1 type includes one tetrahedral sheet and one octahedral sheet (Cao et al., Reference Cao, Wang and Cheng2021), whereas the 2:1 type includes two tetrahedral sheets with one octahedral sheet situated between them (Bodart et al., Reference Bodart, Delmotte, Rigolet, Brendlé and Gougeon2018). The octahedra come in two forms: (1) dioctahedral, wherein two thirds of the octahedral cation centres are occupied by cations; and (2) trioctahedral, where all octahedral cation positions are filled (Gao, Reference Gao2017; Wu et al., Reference Wu, Zhao, Zhou, Wang, Zuo and Cheng2022). Both illite and montmorillonite are 2:1 layer clay minerals displaying dioctahedral structures (Gournis et al., Reference Gournis, Lappas, Karakassides, Többens and Moukarika2008; Zhao et al., Reference Zhao, Ma, Yang, Cui, Xu, Luo and Wen2022b). Negative charges within the structural layer of illite and montmorillonite originate from isomorphous substitution of Al3+ in the octahedral sheet by Fe2+, Mg2+, etc., alongside limited Al3+ replacement for Si4+ in tetrahedral sheets. The resulting negative charge is offset by exchangeable cations (e.g. Na+ and K+) at sheet edges and within interlayers (Chen and Wang, Reference Chen and Wang2007; Wang et al., Reference Wang, Wang, Zeng and Wu2011; Jeldres et al., Reference Jeldres, Uribe, Cisternas, Gutierrez, Leiva and Valenzuela2019; Zhao et al., Reference Zhao, Ma, Yang, Cui, Xu, Luo and Wen2022b).
An accurate understanding of the interaction mechanism between mineral lattices and interlayer cations subsequent to heat or hydrothermal treatment is crucial for designing and employing clay-mineral-based materials, particularly in radioactive waste storage where elevated temperatures can prompt some interlayer cations to infiltrate clay structures, inducing mineral structural layer collapse and a significant reduction in containment efficacy for the radioactive waste (Theng et al., Reference Theng, Hayashi, Soma and Seyama1997; Alba et al., Reference Alba, Alvero, Becerro, Castro and Trillo1998). Studies have revealed that heating clay minerals within the range of 200–300°C can result in interlayer cation immobilisation, leading to irreparable mineral structural layer collapse, known as the Hofmann–Klemen effect (Schultz, Reference Schultz1969; Greene-Kelly, Reference Greene-Kelly1995). Three viewpoints exist regarding interlayer cation occupation sites within the Hofmann–Klemen effect: (1) cations infiltrate ditrigonal cavities in tetrahedral layers (Tettenhorst, Reference Tettenhorst1962; Beaufort et al., Reference Beaufort, Berger, Lacharpagne and Meunier2001; Bodart et al., Reference Bodart, Delmotte, Rigolet, Brendlé and Gougeon2018); (2) cations inhabit vacant octahedral positions (Farmer and Russell, Reference Farmer and Russell1967; Gates et al., Reference Gates, Komadel, Madejová, Bujdák, Stucki and Kirkpatrick2000; Stackhouse and Coveney, Reference Stackhouse and Coveney2002); and (3) cations migrate to both sites (Russell and Farmer, Reference Russell and Farmer1964; Komadel et al., Reference Komadel, Madejova and Bujdak2005) (Fig. 1). Notably, research on montmorillonite heating commonly involves investigations into interlayer cation migration locations (Karakassides et al., Reference Karakassides, Madejová, Arvaiová, Bourlinos, Petridis and Komadel1999; Madejová et al., Reference Madejová, Arvaiová and Komadel1999a). Using infrared spectroscopy, Tettenhorst (Reference Tettenhorst1962) observed interlayer Li+ migration into ditrigonal cavities within tetrahedral layers upon heating montmorillonite to 300°C, with no migration into vacant octahedra. Luca and Cardile (Reference Luca and Cardile1988) used a 57Fe atomic probe to ascertain that Li+ primarily occupied tetrahedral sites during montmorillonite heating. Infrared spectroscopy-based findings by Farmer and Russell (Reference Farmer and Russell1967) indicated Li+ migration into vacant octahedral positions during heating, with the remaining interlayer Li+ interacting with structural OH groups to release H+. Density functional theory (DFT) calculations by Ebina et al. (Reference Ebina, Iwasaki and Jee1999) postulated interlayer Li+ migration from interlayers into ditrigonal cavities and vacant octahedral positions in montmorillonite, occurring at temperatures of 250–350°C, with respective migration rates of 60% and 40%. Furthermore, studies have reported cation migration during montmorillonite heating for other cations (e.g. Cd2+, Mg2+, Cu2+ and Ni2+). Based on infrared spectroscopy, Madejová et al. (Reference Madejová, Arvaiová and Komadel1999b) investigated Cu2+ and Cd2+ migration between montmorillonite layers after heating, revealing Cd2+ is incapable of migrating to tetrahedral and octahedral structures, and Cu2+ has limited migration into ditrigonal cavities within tetrahedral layers, without further penetration into vacant octahedral positions. It is evident that cationic properties, including ionic radius and valence, significantly impact cation migration sites in clay minerals. Moreover, mineral species can also influence cation transport patterns between layers. However, few studies have examined interlayer cation occupation in distinct clay mineral types following heating. Thus, this study has selected Li+ and Na+ as representative cations and illite and montmorillonite as representative clay minerals. The thermal migration and occupation characteristics of interlayer cations in clay minerals were investigated using X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), magic angle rotating solid nuclear magnetic resonance spectroscopy (MAS NMR) and X-ray photoelectron spectroscopy (XPS).

Figure 1. Schematic diagram of possible occupation sites of Li+ in clay minerals with a 2:1 layer type of two tetrahedral sheets (SiO4 tetrahedra) with one octahedral sheet in between. Three possible Li+ occupation sites are: in ditrigonal cavities; in the interlayer; and in vacant octahedral positions (Bodart et al., Reference Bodart, Delmotte, Rigolet, Brendlé and Gougeon2018; Zhao et al., Reference Zhao, Wang and Cheng2023).
Experimental
Materials
For this investigation, the clay minerals illite and montmorillonite were selected (symbols Ilt and Mnt, respectively, using Warr, Reference Warr2020). Illite was obtained from the Carboniferous Benxi Formation, located in Kou Town, Xianyang City, Shaanxi Province, whereas montmorillonite was procured from Xianding Biotechnology Co. Ltd. To eliminate soluble impurities, the clay mineral samples underwent a thorough process of triple rinsing using deionised water. Subsequently, they were dried in an oven at 50°C for 24 h and further ground in an agate mortar until reaching a powder consistency with a 200 mesh particle size. The Na+ and Li+ solutions were prepared by dissolving Na2SO4 and Li2SO4, respectively, in ultrapure water. The analytical grade Na2SO4 and Li2SO4 were purchased from Tianli chemical reagent Co., Ltd. (Tianjin, China) and Komio Chemical Reagents Co., Ltd. (Tianjin, China), respectively. To prepare Na+-saturated montmorillonite and Li+-saturated montmorillonite (Na+-Mnt and Li+-Mnt), 4.00 g of montmorillonite was added to 100 mL of Na+ (1M) and Li+ (1M) solutions, respectively. These solutions were agitated for a duration of 2 h at 25°C and 50 r/min. Subsequently, the samples underwent centrifugation at 10,000 rpm for 10 min to facilitate solid–liquid separation. The isolated solids were air-dried under natural conditions at ~25°C. The dried montmorillonite samples were then exposed to various temperatures for 24 h. The resulting specimens were designated as M-Mnt25, M-Mnt100, M-Mnt150, M-Mnt200, M-Mnt250 and M-Mnt300. In this nomenclature, M signifies either Na+ or Li+, M-Mnt25 denotes a sample that was at room temperature (not subjected to heating), and M-Mnt100 signifies a sample heated to 100°C. Additionally, control samples were produced by mixing 4.00 grams of illite with 100 mL solutions of Na+ (1M) and Li+ (1M), respectively. The resulting control samples were named M-Ilt25, M-Ilt100, M-Ilt150, M-Ilt200, M-Ilt250 and M-Ilt300.
Characterisations
The major chemical compositions of illite and montmorillonite were analysed using an X-ray fluorescence spectrometer (XRF, Shimadzu LAB CENTER XRF-1800). Cation exchange capacities (CEC) in samples were determined by extraction with ammonium acetate solution (1M) at pH 7. A 100 mg sample was rinsed triple with deionised water to remove water-soluble cations and collect solid. The solid was dispersed in 20 mL ethanol, then 20 mL ammonium acetate solution was added, and the extraction solution was collected after standing for 24 h. Subsequently, 20 mL deionised water was added to the remaining solid and the extraction solution was collected after 24 h. The extraction process was repeated three times, and all extraction solutions of the same sample were mixed. The cation concentration (Na+ and Li+) in the solution was determined by inductively coupled plasma optical emission spectrometry (ICP-OES, Agilent 5110).
X-ray diffraction patterns were obtained using a Shimadzu XRD–6100 powder diffractometer. The instrument employed a Cu target as the source (40 kV voltage and 30 mA current) and scanned within the 2θ range of 2–70° at a scanning speed of 4°/min. FTIR spectra were acquired using the KBr pressing method. A Thermo Fisher Nicolet iS5 FTIR spectrometer was employed for data collection, utilising 1 mg of sample powder and 100 mg of spectrographic pure KBr powder. This mixture was ground thoroughly for 15 min and subsequently pressed into transparent flakes. Infrared spectral data were gathered across the range of 4000–400 cm–1, involving 32 scans at a resolution of 4 cm–1. MAS NMR spectral data for 27Al, 29Si and 7Li were obtained using a JEOL ECZ600R/S3 NMR spectrometer from JEOL RESONANCE, Japan. Resonance frequencies were 156.39 MHz for 27Al, 119.20 MHz for 29Si, and 233.08 MHz for 7Li. Single-pulse decoupled MAS NMR experiments were conducted with a 3.2 mm dual resonance probe, employing magic angle rotation speeds of 15 kHz for 27Al, 12 kHz for 29Si, and 8 kHz for 7Li. The pulse widths for 27Al, 29Si and 7Li were set at π/6, π/2 and π/4, respectively, with corresponding pulse delays. Chemical shifts for 27Al, 29Si and 7Li were referenced externally to solutions of aluminium nitrate (Al(NO3)3), tetramethylsilane (TMS) and lithium chloride (LiCl), respectively. XPS was conducted using a Thermo Fisher Scientific ESCALAB Xi+ X-ray photoelectron spectrometer, equipped with a monochromatised AlKα X-ray source. The full-spectral pass energy is 100 eV with a step size of 1 eV, and the narrow-spectral pass energy is 30 eV with a step size of 0.1 eV. The typical elements Al, Si and Li were examined in this study.
Results and discussion
X-ray diffraction data
The XRD patterns demonstrate the high purity of illite and montmorillonite samples (Fig. 2, Supplementary Fig. S1). Generally, the d values of the 00l reflections in minerals are influenced by the radius and hydration status of interlayer cations, whereas the hkl reflections are influenced by mineral layer structures (Alvero et al., Reference Alvero, Alba, Castro and Trillo1994; Alba et al., Reference Alba, Alvero, Becerro, Castro and Trillo1998). The positions and intensities of hkl reflections for illite and montmorillonite, heated to different temperatures, remain consistent with their unheated counterparts (Fig. 2). This indicates the absence of changes in the crystal structure of these minerals. The 00l reflections for illite show little change when heated to various temperatures. In contrast, the 00l reflections for montmorillonite display significant shifts after exposure to different temperatures.

Figure 2. XRD patterns of M-Ilt and M-Mnt subjected to various temperatures: (a) Na+-Ilt; (b) Li+-Ilt; (c) Na+-Mnt; and (d) Li+-Mnt.
For illite, the d (001) value remains ≈10.0 Å before and after heating, implying that the hydration status and migration behaviour of interlayer cations in illite are not affected by temperature (Fig. 2a,b) (Jeldres et al., Reference Jeldres, Uribe, Cisternas, Gutierrez, Leiva and Valenzuela2019). In the case of montmorillonite, the layer spacing of Na+-saturated montmorillonite (~15.3 Å) is slightly larger than that of Li+-saturated montmorillonite (~14.9 Å) in the unheated state. This difference can be attributed to the lower hydration level of Li+ compared to Na+, resulting in slightly larger layer spacing when Na+ occupies the interlayer. The d values of 00l reflections for montmorillonite experience noticeable alterations after exposure to varying temperatures, with a decrease observed in d values as temperature increases (Fig. 2c,d). This may be due to interlayer cations losing ligand water and/or migrating into the montmorillonite lattice. Specifically, for Na+-saturated montmorillonite, the d (001) values decrease to ~12.5, ~12.4, ~12.4, ~12.4 and ~12.4 Å, whereas for Li+-saturate montmorillonite, the values decrease to approximately ~12.4, ~10.9, ~9.9, ~9.6 and ~9.6 Å after heating to 100, 150, 200, 250 and 300°C, respectively. At 200°C, the layer spacing of Li+-saturated montmorillonite falls below 10.0 Å, aligning with the d (001) value for completely collapsed montmorillonite. Conversely, Na+-saturated montmorillonite maintains a layer spacing exceeding 10.0 Å after exposure to varying temperatures, indicating that heating does not lead to complete crystal structure collapse. Electrostatic interaction exists between silicon–aluminate layers and interlayer cations. Heating can prompt interlayer cations to infiltrate mineral crystal structures, resulting in reduced structural layer negativity, low electrostatic repulsion between layers, and decreased layer spacing. The extent of decrease in the d (001) value suggests that Li+ exhibits more readiness to infiltrate montmorillonite crystal structures compared to Na+.
X-ray fluorescence and cation exchange capacity
The main chemical compositions of illite and montmorillonite are SiO2 and Al2O3, ranging from 45.8 to 59.5 wt.%, and from 25.7 to 36.1 wt.%, respectively (Table 1). In addition, the K2O content in illite is higher than that in montmorillonite. By contrast, the contents of Fe2O3(tot) and MgO in illite are lower than that in montmorillonite.
Table 1. Major chemical compositions (wt.%) of illite and montmorillonite samples.

The CEC of Na+ and Li+ in M-Ilt and M-Mnt are shown in Table 2. The amount of exchangeable Na+ and Li+ in illite is lower than that in montmorillonite, because the CEC of illite is lower than that of montmorillonite (Zhao and Zhang, Reference Zhao and Zhang1990). With the increase of temperature, the exchangeable Na+ and Li+ in Na+-Ilt, Li+-Ilt, and Na+-Mnt remain almost unchanged. However, exchangeable Li+ in Li+-Mnt gradually decreases, indicating that Li+ is fixed by montmorillonite upon heating, consistent with the results from XRD.
Table 2. CEC values (mmol/g) of Na+ and Li+ in M-Ilt and M-Mnt subjected to varying temperatures.

Fourier-transform infrared spectroscopy
To investigate the migration of cations within illite and montmorillonite, mid-infrared spectroscopy was employed to study the shifts in OH group and Si–O vibrational modes. These vibrational modes include stretching and bending vibrations (Liu et al., Reference Liu, Yao, Cheng and Frost2012; Ai et al., Reference Ai, Yang, Zeng, Zheng and Hu2013; Qi et al., Reference Qi, Yin, Wu, Tao, Wang, Cheng, Liu and Chen2022).
OH stretching and bending vibrations
The FTIR spectra of the unheated samples (Na+-Ilt25, Li+-Ilt25, Na+-Mnt25 and Li+-Mnt25) show a prominent absorption band at ~3630 cm–1, attributed to the stretching vibration of the OH group coordinated with the cations in octahedral positions (Fig. 3). The stretching vibrational behaviour of the OH group in minerals can be attributed to two factors: (1) the nature of the central atoms in the octahedral coordination with the OH group; and (2) the isomorphic substitution in the lattice structure. In this context, the presence of the tetrahedral top oxygen (Oap) generates local negative charges, which can be counterbalanced by cations entering the lattice. This interplay influences the OH group's stretching vibrations (Madejová et al., Reference Madejová, Arvaiová and Komadel1999a). During the heating process of Na+-Ilt, Li+-Ilt, and Na+-Mnt samples, the OH-stretching vibrational bands remain relatively constant at ~3630 cm–1 (Fig. 3a–c). However, the OH stretching vibrational characteristics of Li+-Mnt differed noticeably from those of Na+-Ilt, Li+-Ilt and Na+-Mnt. For Li+-Mnt heated to 100°C, there is no significant shift in the OH stretching vibration. With further temperature increase, the OH stretching vibrational bands of Li+-Mnt150, Li+-Mnt200, Li+-Mnt250, and Li+-Mnt300 shift to ~3633, ~3634, ~3638 and ~3641 cm–1, respectively. Previous studies have shown that the entry of interlayer cations into the ditrigonal cavities of the tetrahedral layers leads to a shift in OH stretching vibrational bands towards higher wavenumbers (Madejová et al., Reference Madejová, Pálková and Komadel2006; Gao, Reference Gao2017). For the Li+-Mnt25 sample, the interactions between OH and Oap form OH⋅⋅⋅Oap configurations. Upon cation migration into the ditrigonal cavities the interaction between OH and Oap weakens, whereas the interaction between O and H in OH strengthens, leading to a shift in stretching vibrations towards higher wavenumbers (Madejová et al., Reference Madejová, Arvaiová and Komadel1999a). Therefore, it is inferred that interlayer Li+ migrate into the montmorillonite's ditrigonal cavities when heated to 150°C.

Figure 3. FTIR spectra of OH stretching vibrations for the samples heated to different temperatures: (a) Na+-Ilt; (b) Li+-Ilt; (c) Na+-Mnt; and (d) Li+-Mnt.
Notably, a new vibrational band appears in the FTIR spectrum at ~3671 cm–1 upon heating montmorillonite at 200°C (Fig. 3d). Previous studies have indicated that during the heating process, interlayer hydrated Li+ could undergo dehydration into the vacant octahedral positions of montmorillonite, forming a local trioctahedral structure (AlMgLi–OH) and giving rise to the emergence of a new OH stretching vibrational band at ~3671 cm–1 (Madejová et al., Reference Madejová, Pálková and Komadel2006; Jeldres et al., Reference Jeldres, Uribe, Cisternas, Gutierrez, Leiva and Valenzuela2019).
The characteristics of hydroxyl-group bending vibrations provide further insights into the behaviour of cation migration during the heating of illite and montmorillonite. The infrared spectra of Na+-Ilt25 and Li+-Ilt25 show obvious absorption bands at ~760, ~832 and ~933 cm–1 (Fig. 4a,b). Na+-Mnt25 and Li+-Mnt25 exhibit pronounced absorption bands at ~797, ~844 and ~912 cm–1 (Fig. 4c,d), which are presumed to correspond to OH bending vibrations of FeMg–OH, AlMg–OH and AlAl–OH, respectively, in the mineral structure (Gates et al., Reference Gates, Komadel, Madejová, Bujdák, Stucki and Kirkpatrick2000; Madejová et al., Reference Madejová, Bujdák, Petit and Komadel2000; Skoubris et al., Reference Skoubris, Chryssikos, Christidis and Gionis2013). The positions and intensities of the OH bending vibrational bands remain unchanged during the heating of Na+-Ilt, Li+-Ilt and Na+-Mnt samples (Fig. 4a–c). Conversely, for Li+-Mnt, the OH bending vibrational band corresponding to AlAl–OH is not observed after heating at 150°C, and the bending vibrational band intensity of OH in AlMg–OH decrease or even disappear with temperature increasing (Fig. 4d), indicating that Li+ can potentially migrate into the montmorillonite lattice.

Figure 4. FTIR spectra of OH bending vibrations and Si–O stretching vibrations for the samples heated at different temperatures: (a) Na+-Ilt; (b) Li+-Ilt; (c) Na+-Mnt; and (d) Li+-Mnt.
As mentioned above, the interlayer cations (Na+ and Li+) do not exhibit migration into the illite lattice. The montmorillonite lattice does not accommodate Na+, but can accept inwards migration by Li+. This behaviour is predominantly influenced by interlayer cation properties, including ionic radius and valence. Specifically, the ionic radii of Na+ and Li+ are 0.95 and 0.60 Å, respectively (Madejová et al., Reference Madejová, Arvaiová and Komadel1999a). In comparison to Li+, Na+ possesses a larger ionic radius, rendering it less prone to entering the montmorillonite lattice. Furthermore, the occupation site of Li+ is not limited to a single location and can include both ditrigonal cavities and vacant octahedral sites. Skoubris et al. (Reference Skoubris, Chryssikos, Christidis and Gionis2013) reported that upon heating, montmorillonite undergoes changes in its hydrated Li+ within the interlayers. These ions dehydrate and transition from interlayer positions to occupy ditrigonal cavities, followed by vacant octahedrons.
Si–O stretching and bending vibrations
The migration of interlayer cations from minerals into ditrigonal cavities of tetrahedral layers leads to changes in Si–O stretching and bending vibrational bands. (Clementz and Mortland, Reference Clementz and Mortland1974; Alvero et al., Reference Alvero, Alba, Castro and Trillo1994). This migration also neutralises layer charges, rendering montmorillonite similar to pyrophyllite, a typical uncharged dioctahedral mineral (Karakassides et al., Reference Karakassides, Madejová, Arvaiová, Bourlinos, Petridis and Komadel1999).
Both Na+-Ilt25 and Li+-Ilt25 exhibit Si–O stretching vibrational bands ≈1028 cm–1 (Fig. 4a,b). Meanwhile, Na+-Mnt25 and Li+-Mnt25 display Si–O stretching vibrational bands in the range of 1035–1150 cm–1, with bands at ~1039 and ~1093 cm–1, respectively (Fig. 4c,d). Notably, heated Na+-Ilt and Li+-Ilt samples maintain their Si–O stretching vibrational bands, signifying the absence of interlayer cations migrating into the ilmenite lattice structure (Fig. 4a,b). Similarly, the Si–O stretching vibrational bands of Na+-Mnt remain unchanged during heating, suggesting no migration of interlayer Na+ into the ditrigonal cavities of montmorillonite's tetrahedral layers (Fig. 4c). In contrast, Si–O stretching vibrational bands in the Li+-Mnt series shift to higher values after heating (Fig. 4d). Specifically, the ~1039 cm–1 band remains relatively stable at 100°C; it shifts to a higher value (~1043 cm–1) at 150°C; then gradually shifts to ~1052 cm–1 at 300°C. This alteration indicates the resemblance of montmorillonite's structure to that of pyrophyllite, facilitated by Li+ migration during heating. The Si–O stretching band of pyrophyllite is at ~1050 cm–1 (Madejová et al., Reference Madejová, Pálková and Komadel2006; Xia et al., Reference Xia, Zhong, Liu, Huang, Chang and Li2009). Moreover, the band at ~1093 cm–1 shifts to ~1115 cm–1 at 100°C, further shifting to ~1127 cm–1 at 200°C, and eventually progressing to ~1130 cm–1 with increasing temperature.
In ilmenite, bending vibrational bands of Si–O are mainly located at ~537 (Si–O–Al) and ~484 cm–1 (Si–O–Si). These band positions and intensities remain largely unchanged as temperature increases (Fig. 5a,b). The Si–O–Si and Si–O–Al bending vibrational bands in montmorillonite appear at ~467 and ~520 cm–1, respectively (Fig. 5c,d). The absorption bands of Na+-Mnt remain constant during heating, whereas the Si–O–Al absorption bands of Li+-Mnt slightly decrease in intensity with rising temperature.

Figure 5. FTIR spectra of Si–O bending vibrations for the samples heated at different temperatures: (a) Na+-Ilt; (b) Li+-Ilt; (c) Na+-Mnt; and (d) Li+-Mnt.
In summary, interlayer cations in ilmenite do not migrate into its crystal structure. However Na+ remains within montmorillonite's interlayers and Li+ can penetrate ditrigonal cavities of tetrahedral layers.
Nuclear magnetic resonance and X-ray photoelectron spectroscopy
The XRD and FTIR results indicate that the interlayer Li+ in montmorillonite may lose their ligand water when the heating temperature reaches 100°C, and the interlayer Li+ migrates into the ditrigonal cavities within the tetrahedral layers when the heating temperature reaches 150°C. To further demonstrate whether Li+ can migrate into the vacant octahedral sites of montmorillonite upon heating to 200°C, Li+-Mnt25 and Li+-Mnt200 were selected for finer characterisation techniques (MAS NMR and XPS).
The 29Si–MAS NMR patterns for Li+-Mnt25 and Li+-Mnt200 samples are illustrated in Fig. 6a. The 29Si signal of Li+-Mnt25 displays a peak at approximately –92.7 ppm, indicative of tetrahedral silicon atoms connected to three other silicon atoms via oxygen (Alba et al., Reference Alba, Alvero, Becerro, Castro and Trillo1998; Pavón and Alba, Reference Pavón and Alba2021). Upon heating to 200°C, the 29Si signal's peak of Li+-Mnt200 shifts to approximately –95.3 ppm due to interlayer cations neutralising negative charges within structural unit layers and integrating into the crystal structure (Steudel et al., Reference Steudel, Heinzmann, Indris and Emmerich2015). Moreover, the 29Si peak widens, reflecting interlayer cation migration into tetrahedral positions post-heat treatment, leading to Si–O–Si angle changes (Alba et al., Reference Alba, Alvero, Becerro, Castro and Trillo1998). The 27Al–MAS NMR spectra for Li+-Mnt25 and Li+-Mnt200 are shown in Fig. 6b. The 27Al signal in Li+-Mnt25 appears at ~7.5 and ~67.9 ppm, corresponding to octahedral Al (VIAl) and tetrahedral Al (AlIV), respectively (Takahashi et al., Reference Takahashi, Kanhhashi and Saito2008; Steudel et al., Reference Steudel, Heinzmann, Indris and Emmerich2015). Additionally, the AlIV signal intensity is notably lower than AlVI due to the higher abundance of AlVI (Reinholdt et al., Reference Reinholdt, Miehe-Brendle, Delmotte, Le Dred and Tuilier2005; Steudel et al., Reference Steudel, Heinzmann, Indris and Emmerich2015). Post-heating at 200°C, the AlIV signal disappears, attributed to tetrahedral structure distortion from interlayer Li+ migration into the lattice (Steudel et al., Reference Steudel, Heinzmann, Indris and Emmerich2015). Conversely, this effect is less pronounced with Li+ occupying octahedral vacancies, as a result of minimal interference from a small amount of migrating Li+ (Steudel et al., Reference Steudel, Heinzmann, Indris and Emmerich2015). The spectra from 7Li–MAS NMR for Li+-Mnt25 and Li+-Mnt200 are shown in Fig. 6c. Both samples exhibit symmetrical powder sideband patterns, demonstrating a first-order quadrupolar interaction effect (Bodart et al., Reference Bodart, Delmotte, Rigolet, Brendlé and Gougeon2018). For finer crystal structure insight, Fig. 6d shows an enlarged view of the 7Li–MAS NMR spectra. The main resonance signal of Li+-Mnt25 appears at ~0.2 ppm, consistent with interlayer Li+ (Pistiner and Henderson, Reference Pistiner and Henderson2003). Post-heating to 200°C, the 7Li signal of Li+-Mnt200 shifts by approximately –0.6 ppm, indicating octahedral Li+ presence (Hindshaw et al., Reference Hindshaw, Tosca, Goût, Farnan, Tosca and Tipper2019).

Figure 6. MAS NMR spectra of Li+-Mnt25 and Li+-Mnt200: (a) 29Si–MAS NMR spectra; (b) 27Al–MAS NMR spectra, the reference mark (※) indicates spinning sidebands; (c) 7Li–MAS NMR spectra, the reference mark (※) signifies spinning sidebands; and (d) partial amplification from the 7Li–MAS NMR spectra.
The spectra from XPS depicting Li+-Mnt25 and Li+-Mnt200 are presented in Fig. 7. Notably, alterations in the chemical state on the mineral surface significantly influence the positions of the photoelectron peaks. The full spectrum analysis reveals peaks corresponding to O 1s, Si 2p, and Al 2p before and after heating (Fig. 7a). In the case of Li+-Mnt25, the Si 2p peak appears at ~102.7 eV, whereas after heating to 200°C, it is shifted to ~103.3 eV (Li+-Mnt200, Fig. 7b). This shift is attributed to the presumed entry of Li+ into the ditrigonal cavities of the tetrahedral layers, leading to an upwards shift in the Si 2p peak. The binding energies of Al 2p for Li+-Mnt25 and Li+-Mnt200 are situated at ~74.5 and ~75.4 eV, respectively (Fig. 7c). Earlier investigations suggest that the incorporation of Li+ into the aluminium–oxygen octahedron results in the shift of the Al 2p peak towards higher values (Zhong et al., Reference Zhong, Lin and Yu2021). The Li 1s peak in Li+-Mnt25, located at ~57.3 eV, is hypothesised to represent interlayer hydrated Li+ (Fig. 7d). Upon heating to 200°C, the Li 1s peaks in Li+-Mnt200 appear at ~56.2 and ~58.3 eV, respectively (Fig. 7d). This discrepancy indicates that the former peak arises due to the dehydration of a portion of interlayer hydrated Li+ into Li+ at elevated temperatures. The latter phenomenon is attributed to the migration of Li+ into the montmorillonite lattice, leading to an elevated binding energy of Li 1s (Shen et al., Reference Shen, Hrbek, Sham and Xu1990; Connell et al., Reference Connell, Fuchs, Hartmann, Krauskopf, Zhu, Sann, Garcia-Mendez, Sakamoto, Tepavcevic and Janek2020).

Figure 7. The XPS spectra of Li+-Mnt25 and Li+-Mnt200: (a) XPS survey; (b) Si 2p; (c) Al 2p; and (d) Li 1s.
Consequently, combined with the emerging OH stretching vibration (AlMgLi–OH) at ~3671 cm–1 upon heating montmorillonite at 200°C (Fig. 3d), the observed results of MAS NMR and XPS confirm that interlayer Li+ can migrate to the ditrigonal cavities and vacant octahedral sites of montmorillonite when heating at 200°C. In addition, it is explained that when the heating temperature reaches 200°C, Li+ can migrate to vacant octahedral sites of montmorillonite through the ditrigonal cavities.
Migration and occupation of Li+ in montmorillonite
The data from XRD, FTIR, MAS NMR and XPS concerning the migration and occupation of Li+ in montmorillonite establishes that Li+ within the montmorillonite interlayer can infiltrate the montmorillonite lattice upon reaching a specific temperature (Fig. 8). This migration of Li+ occurs through the three-step process. Initially, upon heating to 100°C, interlayer hydrated Li+ undergoes dehydration; upon heating to 150°C, partial Li+ can migrate into the ditrigonal cavities of the montmorillonite tetrahedral layers. With further temperature increase to 200°C, some Li+ within the ditrigonal cavities proceeds to occupy previously vacant octahedrons in the montmorillonite, forming a local trioctahedral structure and leading to the complete collapse of the montmorillonite structural layer (<10.0 Å) (Fig. 8).
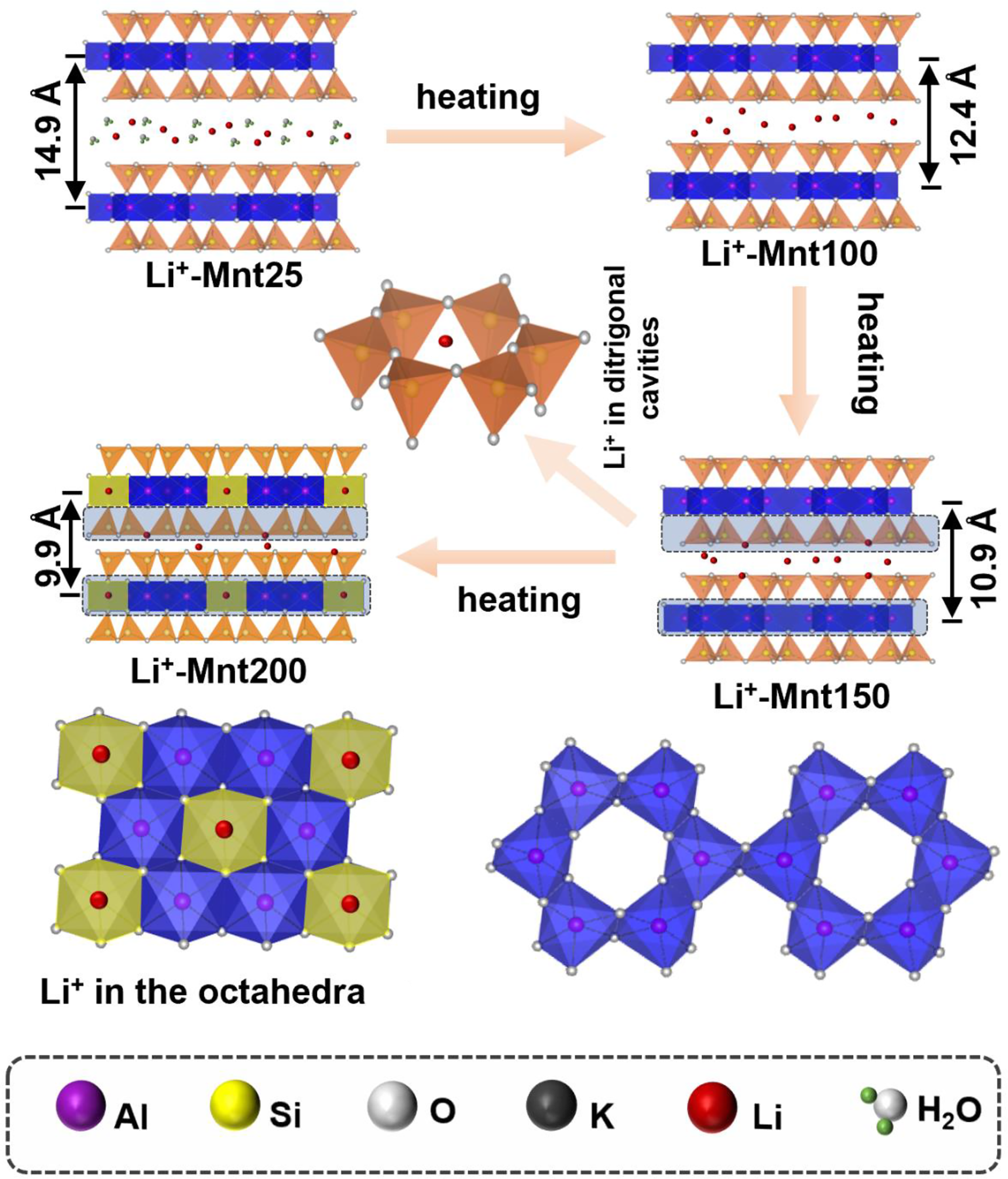
Figure 8. Diagram of Li+ migration and occupation in montmorillonite during the heating process.
Conclusions
Under heating conditions, the mineral types and interlayer cations collectively influence the migration and occupation attributes of cations within minerals, thereby having an impact on mineral lattice structures. In the case of ilmenite, the 00l reflections exhibit little change after heating, whereas the d (001) values of montmorillonite display pronounced alterations. Specifically, the layer spacing of Li+-Mnt decreases to <10.0 Å, consistent with the complete collapse of montmorillonite, and the exchangeable Li+ in Li+-Mnt gradually decreases with the increase of temperature, signifying Li+ fixation by montmorillonite. Moreover, within the temperature range of 100–300°C, the OH vibrational bands and Si–O vibrational bands of Na+-Ilt, Li+-Ilt and Na+-Mnt remain relatively stable, whereas those of Li+-Mnt undergo modifications. Specifically, as the heating temperature reaches 150°C, the OH stretching and Si–O stretching vibrational bands of the Li+-Mnt series, shift towards higher values, accompanied by the intensity decrease of OH bending and Si–O bending vibrational bands. These changes are due to the entry of Li+ into the ditrigonal cavities of montmorillonite. At 200°C, the emergence of a new OH stretching vibrational peak at ~3671 cm–1 is observed, possibly indicating the entry of Li+ into montmorillonite vacant octahedrons and the formation of a local trioctahedral structure (AlMgLi–OH). MAS NMR analysis demonstrates that the chemical shifts of the 29Si and 7Li signals of Li+-Mnt200 are more negative in comparison to the Li+-Mnt25 sample. The 27Al–MAS NMR spectrum reveals the disappearance of the AlIV signal upon heating to 200°C. Furthermore, XPS spectra illustrate that, relative to the Li+-Mnt25 sample, Al 2p and Si 2p peaks in Li+-Mnt200 shift towards higher values, and the Li 1s peak emerges at ~58.3 eV. These findings strongly support the migration of Li+ into the crystal structure of montmorillonite under heating conditions.
Acknowledgements
The authors thank the financial support provided by the National Natural Science Foundation of China (42172043, 42202148) and the Fundamental Research Funds for the Central Universities, CHD (300102263301, 300102273203).
Competing interests
The authors declare none.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1180/mgm.2024.27.












