The metabolic syndrome is related to an increase in incidence of type 2 diabetes and CVD(Reference Eckel, Grundy and Zimmet1). The disturbances associated with obesity (glucose intolerance, dyslipidaemia and postprandial hyperlipaemia) are independent risk factors for endothelium dysfunction, which is considered as an early marker of atherosclerosis(Reference Luscher and Barton2).
Among fatty acids, n-3 PUFA, especially EPA and DHA found in sea fish, have been reported to reduce the risk of arrhythmia and myocardial infarction in animal models(Reference Billman, Kang and Leaf3). Effect of n-3 PUFA on lipid metabolism is widely reported in rodents(Reference Rustan, Christiansen and Drevon4, Reference Flachs, Mohamed-Ali and Horakova5) and in human subjects(Reference Woodman, Mori and Burke6, Reference Balk, Lichtenstein and Chung7), with various findings on LDL- and HDL-cholesterol (HDLCH) depending on the type of study and animal species. Postprandial lipaemia response to n-3 PUFA also showed inconsistent data(Reference Hanwell, Kay and Lampe8, Reference Harris, Lu and Rambjor9). Glucose intolerance and insulin resistance are associated with a high risk of developing diabetes(Reference Balkau, Charles and Drivsholm10). Results from human epidemiological studies indicated that n-3 PUFA reduce the development of insulin resistance and diabetes, whereas those from intervention studies in animal models present some divergences(Reference Fedor and Kelley11, Reference Abate, Chandalia and Snell12).
Although controversial, clinical studies with fish or fish oils have also shown a decrease in cardiovascular events in human subjects(Reference Siscovick, Raghunathan and King13). Several mechanisms have been reported to explain this effect(Reference von Schacky, Angerer and Kothny14, Reference Ruidavets, Bongard and Dallongeville15). Whether or not fish intake is associated with future development of the metabolic syndrome and its related endothelium dysfunction has not been carefully evaluated. Endothelium dysfunction is related to the metabolic syndrome, and a diet enriched with DHA has been reported to enhance vasodilation mechanisms and to attenuate constriction responses in hyperlipidaemic, overweight men(Reference Mori, Watts and Burke16). However, in experimental models, the influence of EPA and DHA on endothelium-dependent vasorelaxation has been controversial and seems to depend mostly on the experimental conditions and the animal species. Preincubation of aortic rings with EPA reduced the vessel-relaxing response to carbachol in healthy rats(Reference Christon, Marette and Badeau17). On the same line, DHA-enriched diet failed to improve the altered endothelium-dependent aortic vasorelaxation in spontaneously hypertensive rats(Reference Abeywardena and Head18) and in streptozotocin diabetic rats(Reference Goirand, Ovide-Bordeaux and Renaud19), whereas EPA improved the endothelium-dependent relaxation of Otsuka Long-Evans Tokushima Fatty rats(Reference Kusunoki, Tsutsumi and Hara20).
The effect of n-3 has already been assessed in metabolic disturbances associated with obesity. However, few studies evaluated the involved parameters in the same animal and in the same conditions, and none of these used the hamster model. The present study is the first one that integrated different metabolic disturbances associated with obesity and the metabolic syndrome, in the same condition and using the hamster animal model. The hamster has been used as a valuable model because of its similarities to human subjects with regard to lipoprotein cholesterol metabolism(Reference Briand21) and development of atherosclerotic lesions(Reference Nistor, Bulla and Filip22).
In this study, we investigated the effect of n-3 PUFA on the prevention of some clinical and metabolic disturbances associated with obesity (dyslipidaemia, glucose intolerance, body composition) as well as endothelial dysfunction in hamsters fed with a high-fat diet (HFD).
Materials and methods
Animals
A total of thirty-six male golden Syrian hamsters were obtained from Janvier (Le Genest-St-Isle, France) at 8 weeks of age, weighing 80–90 g. They were housed in colony cages with wood litter (four hamsters per cage) in a controlled environment (22°C, 12 h light–12 h dark cycle) and received water and diet ad libitum. All experiments were performed according to the regulations for animal welfare of the French Ministry of Food, Agriculture and Fisheries. The experimental protocol adhered to European Union guidelines and was approved by the local animal use and care advisory committee.
Diets
Two HFD (21 % fat, w/w), either enriched (HFn-3) or not (HF) with n-3 PUFA, and a control chow diet (5 % fat, w/w; Control) were used. Each of the HFD were designed with the same composition and contained 38·7 % starch, 24·7 % proteins, 21 % lipids, 8·5 % minerals, 6 % cellulose and 1·2 % vitamins. The lipid mixture consisted of 15 % lard, 3·5 % palm oil and 2·5 % maize oil for HF. In the HFn-3 diet, 10 % of lard (1·5 g) was replaced by an equivalent amount of n-3 PUFA oil mixture (Pierre Fabre Santé, Castres, France). In both diets, lipids provided about 45 % of total energy content. The Control diet contained 23 % protein, 58 % starch, 5 % lipids (2 % maize oil and 3 % palm oil), 8·5 % minerals, 6 % cellulose and 1·2 % vitamins, and 13 % of energy intake was provided by lipids. The experiments were performed in three groups of animals for a period of 20 weeks of consumption of one of the three diets. The fatty acid composition of the diets was analysed using a gas chromatograph with DB225 capillary column (J&W Scientific, Agilent Technologies, Santa Clara, CA, USA; 60 m × 0·25 mm × 0·25 μm; Table 1).
Table 1 Lipid diet composition (% of total fatty acids) measured by GC
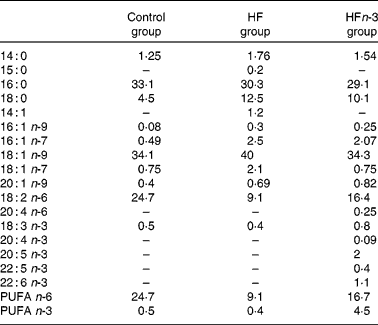
Control, control diet; HF, high-fat diet; HFn-3, high-fat diet enriched with n-3 PUFA.
Body composition
Hamsters were intraperitoneally injected with 150 mg/kg body weight (BW) 2H-labelled water (99·9 % D/H; Euriso-top, Gif-sur-Yvette, France). Blood samples were collected before and 2 h after administering tracer injection. Plasma samples were frozen at − 20°C until analysis. Plasma 2H enrichment was analysed by Fourier-transformed IR spectroscopy liquid using the sampling cell system (Perkin-Elmer, Les Ulis, France). Total body water was calculated from the dilution space of the isotope. A 74·4 % fat-free mass hydration rate was assumed. Fat-free mass was thus calculated as total body water/0·744(Reference Ferrier, Robert and Dumon23).
Glucose tolerance test
An intraperitoneal glucose tolerance test was performed at 09.00 hours after being unfed for 18 h. Eight hamsters of each group received 1 g of glucose per kg BW intraperitoneally. Blood samples were obtained by retro-orbital puncture (under isofluran-induced anaesthesia) at 0 (basal), 10, 20, 30, 60 and 120 min and glycaemia was measured immediately using fresh blood (glucometer: Accu-Chek active; Roche Diagnostics, Mannhein, Germany). Areas under the curves (AUC) were calculated using GraphPad Prism (GraphPad Software, La Jolla, CA, USA).
Plasma lipids
Hamsters were food deprived for 18 h and blood was obtained by retro-orbital puncture to determine plasma lipids. Plasma was then separated by centrifugation (4°C, 10 min, 3000 g). The following components were quantified in plasma: TAG, cholesterol and NEFA using enzymatic kits (Bio-Merieux, Marcy-l'Etoile, France and Wako Chemicals, Richmond, VA, USA). Cholesterol and TAG profiles were performed using fast protein liquid chromatography (AKTA FPLC SYSTEM; GE Healthcare, Piscataway, NJ, USA)(Reference Briand, Magot and Krempf24).
Post-prandial lipaemia
A measure of 450 μl of olive oil per 100 g BW was given to animals unfed for 18 h. Blood was taken from the eye orbital venous plexus every 2 h for 10 h. Plasma was separated, TAG concentration was measured and AUC were calculated.
Measurement of the in vivo secretion of VLDL TAG
The effect of n-3 PUFA on the secretion of VLDL TAG by the liver was determined using tyloxapol (Sigma-Aldrich Chemicals, Lyon, France), a non-ionic surfactant that inhibits lipoprotein lipase (LPL) enzyme(Reference Schotz, Scanu and Page25). After being unfed for 18 h, the hamsters were anaesthetised with isofluran and blood samples were taken from the eye orbital venous plexus. Tyloxapol (500 mg/kg) was injected via a jugular vein. Blood samples were taken at t0, just before injection of tyloxapol and then at 30 min (t30), 120 min (t120) and at 180 min (t180). The plasma TAG concentration was determined. The first 30 min after the injection of tyloxapol are required to reach detergent equilibration and to initiate lipoprotein accumulation(Reference Millar, Maugeais and Fuki26). The TAG accumulation is proportional to VLDL TAG secretion. TAG secretion rate for individual hamsters was therefore calculated using the linear increment between t30 and t180(Reference Siri, Candela and Zhang27).
Measurement of lipid content in liver and muscle
About 30 mg of hepatic tissue and 50 mg of muscle were used to extract lipid using the Folch method(Reference Folch, Lees and Sloane Stanley28). Briefly, the tissue was homogenised with chloroform–methanol (2:1) and then centrifuged to recover the liquid phase. The solvent was washed with 0·9 % NaCl solution. After centrifugation, the lower chloroform phase containing lipids was evaporated and lipids were suspended in 0·5 ml of ethanol. TAG and cholesterol were then measured using Biomérieux kit reagents (TAG: PAP 150, cholesterol RTU; Biomérieux, Marcy-l'Etoile, France).
Evaluation of lipid peroxidation
The measurement of malondialdehyde (MDA) concentration in plasma was performed by using an MDA kit (Sobioda, Montbonnot-Saint-Martin, France).
Preparation of aortic rings and functional procedures
A total of three or four hamsters from each group were anaesthetised with pentobarbital (60 mg/kg BW). The thoracic aorta was dissected. The blood vessels were cleaned of connective tissue and cut into 2-mm rings. The rings were suspended in organ chambers of Mulvany myograph (Danish Mio Technology, Aarhus, Denmark). The arterial rings were challenged with KCl (60 mmol/l) to evaluate their functional integrity and then contracted with increasing doses of phenylephrine (PE, 10− 9 to 10− 4 mol/l; Sigma-Aldrich Chemicals). The concentration of PE inducing 90 % maximal contraction of the rings was used to contract the arteries. The rings were then relaxed with increasing doses of carbachol (10− 9 to 10− 4 mol/l; Sigma-Aldrich Chemicals).
Separation of adipose tissue components
Gonadal depots were transferred in a Falcon tube filled with Dulbecco's modified Eagle's media. The adipose tissue was then chopped thoroughly and resuspended in 10 ml digestion solution (7 ml Hanks' solution, 3 ml of 7·5 % BSA and 20 mg collagenase type II; Sigma-Aldrich Chemicals). The digestion was performed at 37°C using a shaker at 100 rpm for 20 min. Then, the samples were kept at room temperature for 5 min. After that, the adipocyte fraction (floating) was isolated, and the solution containing the stroma vascular fraction was filtered through a 100-mm cell strainer (BD Falcon, Isère, France), collected in a new tube and then centrifuged at 1500 rpm, 4°C for 5 min. The stroma vascular fraction pellet was suspended in 1 ml of selection buffer (PBS, 2 mm-EDTA, 0·5 % BSA), centrifuged again and then the cluster of differentiation molecule 11b (CD11b)-positive cells were selected using CD11b micro-beads (Miltenyi Biotec, Bergisch Gladbach, Germany). Typically, 10 ml of beads were used per ten million cells in 90 ml selection buffer, and the cells were incubated for 20 min at 4°C. After selection, the cells were washed with 1 ml of selection buffer, centrifuged and then suspended in 50 ml of selection buffer. The isolation of the positive fraction was performed using autoMACS Pro Separator (Miltenyi Biotec).
RNA extraction from liver, adipocyte and macrophage and gene expression analysis
Tissue samples for mRNA analysis were homogenised, and RNA was isolated using Trizol reagent (Invitrogen, Villebon sur Yvette, France). Real-time quantitative PCR analysis was performed as follows: 1 μg of total RNA was reverse-transcribed using 100 U (100 mol/min) of Moloney murine leukaemia virus RT (Promega, Charbonnières-les-Bains, France). Real-time quantitative PCR was performed on the 7000 Sequence Detection System (GeneTool, Silicone Valley, CA, USA) with SYBR green MESAGREEN Master Mix Plus (Eurogentec, Angers, France). The reaction contained 10 ng of reverse-transcribed total RNA, 500 nm forward and reverse primers and 5 × SYBR green mix. Primer sequences are available on request. All reactions were performed at least in duplicate and cyclophilin RNA amplification was used as a reference. Each couple of primers was tested in successive dilutions of complementary DNA to analyse and validate its efficiency.
The expression of sterol regulatory element-binding protein-1c (SREBP-1c), stearyl CoA desaturase 1 (SCD1), diacylglycerol O-acyltransferase 2, fatty acid synthase, PPARγ, carnitine palmitoyl transferase 1, microsomal TAG transfer protein, scavenger receptor class B type I (SR-B1), LPL and TNFα was measured in liver.
The expression of CD11b (present only in macrophage membrane), SCD1, PPARγ, fatty acid synthase, LPL and TNFα was measured in adipocytes and adipose tissue macrophages.
Statistics
Results were expressed as mean values and standard errors of the mean. Statistical analyses were performed using Statview software (SAS Institute Inc., Cary, NC, USA). Two-way repeated measures ANOVA followed by Fisher's protected least significant difference (PLSD) test were performed to estimate the effect of group and n-3 PUFA enrichment diet on obtained values. A simple regression was used to evaluate the correlation among variables. Differences were considered significant at P < 0·05.
Results
Effects of dietary n-3 PUFA on body weight, body fat percentage, muscle lipid content and glucose tolerance
At the end of 20 weeks, the HF group had a higher BW than the Control group (P < 0·05), and the BW of the HFn-3 group was lower than that of the HF group (P < 0·05) and did not differ from the Control group (Table 2). A higher percentage of fat body mass was observed in the HF group compared to the Control group (P = 0·048), whereas fat percentage tended to be lower in the HFn-3 group compared to the HF group (P = 0·07) and did not differ from the Control group. Muscle TAG content was similar in the Control group (4·01 (sem 0·80) mg/g) and HF group (4·76 (sem 0·23) mg/g), but was significantly lower in the HFn-3 group (2·29 (sem 0·29) mg/g), with P = 0·008. No difference was observed in cholesterol content among groups.
Table 2 Effects of dietary n-3 PUFA on weight, percentage of fat mass, basal glycaemia, plasma lipid concentrations VLDL cholesterol (VLDLCH), LDL cholesterol (LDLCH) and HDL cholesterol (HDLCH)
(Mean values with their standard errors)

Control, control diet; HF, high-fat diet not enriched with n-3 PUFA; HFn-3, high-fat diet enriched with n-3 PUFA.
* Mean values were significantly different from those of the Control group (P < 0·05).
† Mean values were significantly different for the HFn-3 group from those of the HF group (P < 0·05).
Basal blood glucose was higher in the HF group than in the Control and HFn-3 groups (P < 0·05; Table 2). After intraperitoneal injection of glucose, the HF group showed an impairment of glucose tolerance assessed by glycaemia AUC compared to both Control (P < 0·0001) and HFn-3 (P < 0·005) groups. No significant difference was observed between the Control and HFn-3 groups (Fig. 1).

Fig. 1 Effect of different diets on glycaemia after an intra-peritoneal injection of glucose (1 g/kg body weight). Values were measured in control diet (Control, ![]() ), high-fat diet (HF,
), high-fat diet (HF, ![]() ) and high-fat diet enriched with n-3 PUFA (HFn-3,
) and high-fat diet enriched with n-3 PUFA (HFn-3, ![]() ) groups at t0, t10, t20, t30, t60 and t120 min. Area under the curve (AUC) values are given as means with their standard errors represent by vertical bars for a group of six to eight hamsters. * Mean values were significantly different from those of the Control group (P < 0·05). † Mean values were significantly different for the HFn-3 group from those of the HF group (P < 0·05).
) groups at t0, t10, t20, t30, t60 and t120 min. Area under the curve (AUC) values are given as means with their standard errors represent by vertical bars for a group of six to eight hamsters. * Mean values were significantly different from those of the Control group (P < 0·05). † Mean values were significantly different for the HFn-3 group from those of the HF group (P < 0·05).
Effects of dietary n-3 PUFA on plasma lipids
The effect of diets on plasma lipids is shown in Table 2. Plasma TAG and cholesterol were higher in the HF group (P < 0·001) compared to the Control group, whereas the HFn-3 group showed a lower concentration of TAG (P < 0·01) and cholesterol (P < 0·001) compared to the HF group. TAG concentration was higher in the HFn-3 group compared to the Control group. No difference was observed in plasma NEFA concentrations.
Data from fast protein liquid chromatography are shown in Table 2. Cholesterol level was higher in VLDL and LDL in both HF and HFn-3 groups compared to the Control group (P < 0·005), whereas it was lower in HDL of the HFn-3 group compared to both Control and HF groups (P < 0·0005). Diet enriched with n-3 PUFA significantly decreased VLDL TAG. No change was observed in LDL and HDL TAG content among groups.
Effect of dietary n-3 PUFA on endogenous TAG secretion and liver lipid content
In vivo TAG secretion rate was measured after administering an intravenous injection of tyloxapol (Fig. 2). The results show that plasma TAG was higher in the HF group compared to Control and HFn-3 groups at t30 (P < 0·05) and t120 (P < 0·01) after tyloxapol injection. No significant difference was observed at t180 between the Control and HF groups (P = 0·11), whereas plasma TAG in HFn-3 group was lower than that in the HF group (P = 0·002) and tended to be lower compared to the Control group (P = 0·06). The HFn-3 group presented a lower TAG secretion rate (1·19 (sem 0·14) mmol/l per h) compared to the HF group (1·84 (sem0·22) mmol/l per h; P = 0·006) and tended to decrease compared to the Control group (1·46 (sem0·20) mmol/l per h; P = 0·098). Liver TAG content was higher in the HFn-3 group compared to the Control group (P < 0·05), but lower compared to the HF group (P < 0·05). No difference was observed in hepatic cholesterol content among the three groups.

Fig. 2 (a) Effect of dietary fats on hepatic TAG secretion. Hamsters were fed with different types of diets for 20 weeks. After 18 h of food deprivation, retro-orbital blood sample was drawn and tyloxapol was injected. Thereafter, blood samples were collected at fixed time intervals (30 min, 2 h and 3 h) and plasma TAG level was determined. Values are means, with their standard errors represented by vertical bars (n 6). (b) TAG content in liver given in mg/g of liver. * Mean values were significantly different from those of the control diet (Control, ![]() ; P < 0·05) group. † Mean values were significantly different for the high-fat diet enriched with n-3 PUFA (HFn-3,
; P < 0·05) group. † Mean values were significantly different for the high-fat diet enriched with n-3 PUFA (HFn-3, ![]() ) group from those of the high-fat diet (HF,
) group from those of the high-fat diet (HF, ![]() ; P < 0·05) group.
; P < 0·05) group.
Post-prandial lipaemia
AUC of plasma TAG after olive oil oral administration was higher in the HF group compared to the Control group (P < 0·05; Fig. 3) and was not significantly different from that of the HFn-3 group (P = 0·11). Nevertheless, plasma TAG concentration was significantly lower in the HFn-3 group at 6, 8 and 10 h after oral administration compared to the HF group, although it did not differ from the Control group at these time periods.
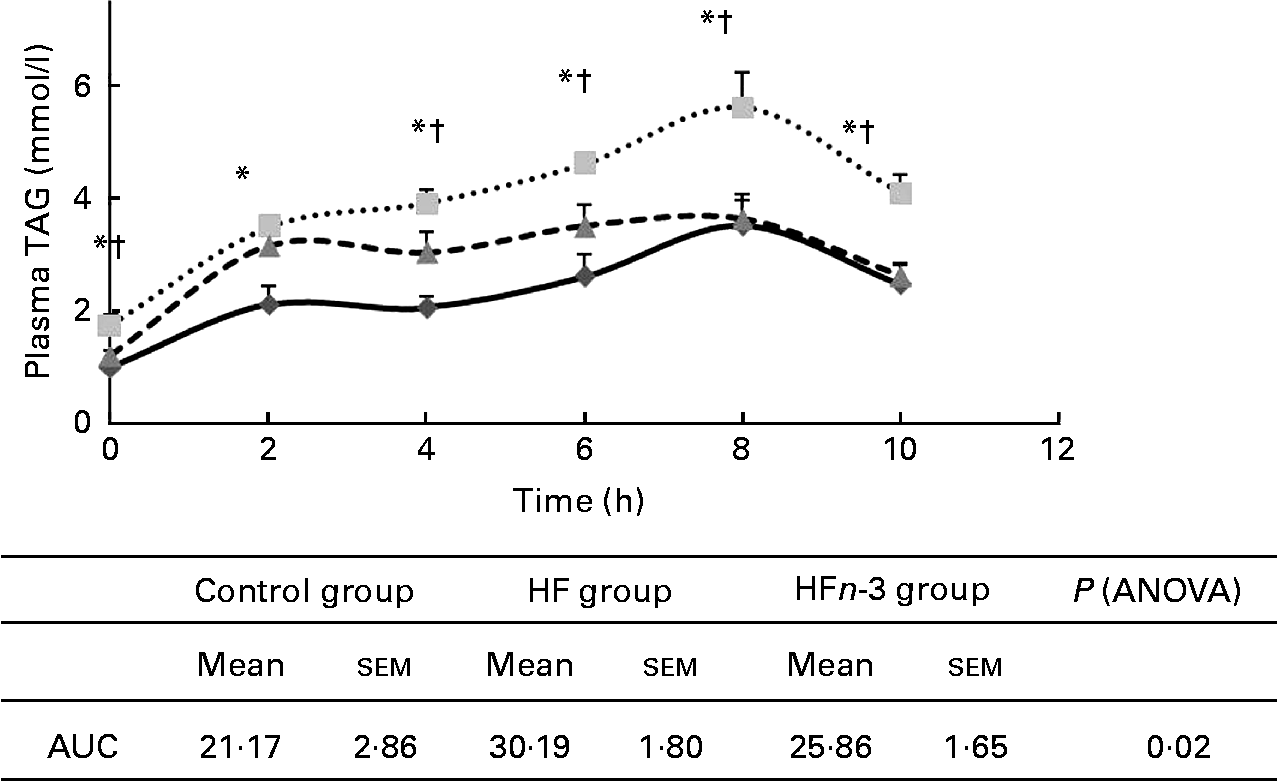
Fig. 3 Effect of different diets on TAG after gavages with olive oil and area under the curve (AUC) measurement. Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different for the high-fat diet (HF, ![]() ) group from those of the control diet (Control,
) group from those of the control diet (Control, ![]() ; P < 0·05) group. † Mean values were significantly different for the HF group from those of the high-fat diet enriched with n-3 PUFA (HFn-3,
; P < 0·05) group. † Mean values were significantly different for the HF group from those of the high-fat diet enriched with n-3 PUFA (HFn-3, ![]() ; P < 0·05) group.
; P < 0·05) group.
Lipid peroxidation
The measurement of MDA in plasma of the different groups showed no difference between the Control (11·59 (sem 1·19) μmol/l) and HF (13·75 (sem1·59) μmol/l) group. MDA concentration in the HFn-3 group (27·64 (sem2·21) μmol/l) was higher compared to both the Control and HF groups (P < 0·001).
Endothelial function
The contraction produced in response to PE was the same in all the three groups, whereas carbachol added to pre-constricted rings with 10− 6 m of PE showed significant difference of relaxation between the HFn-3 group compared to both the Control and HF groups at 10− 6, 10− 5 and 10− 4 m-carbachol with P < 0·01. Vessels of HFn-3 hamsters were more sensitive to carbachol (10− 6 m)-induced relaxation (47·5 (sem 5·0) %) compared to the Control (22·13 (sem 5·7) %; P = 0·01) and HF groups (7·28 (sem 4·85) %; P < 0·0005). Control and HF groups did not differ significantly (P = 0·09). At 105 and 104 m of carbachol, HFn-3 hamsters vessel relaxation remained higher (P < 0·005), whereas no difference was observed between the Control and HF groups (Fig. 4).

Fig. 4 Dose–response curves to (a) phenylephrine (Phe) and (b) carbachol (CCH) of control diet (Control, ![]() ), high-fat diet (HF,
), high-fat diet (HF, ![]() ) and high-fat diet enriched with n-3 PUFA (HFn-3,
) and high-fat diet enriched with n-3 PUFA (HFn-3, ![]() ) groups. Values are means with their standard errors represented by vertical bars (n 4). * Mean values were significantly different for the HFn -3 group from those of the HF and Control groups (P < 0·001).
) groups. Values are means with their standard errors represented by vertical bars (n 4). * Mean values were significantly different for the HFn -3 group from those of the HF and Control groups (P < 0·001).
Endothelium-dependant vasorelaxation correlated negatively with glucose intolerance (r − 0·591, P = 0·033) and with total cholesterol concentration (r − 0·84, P = 0·0003).
Gene expression in liver, adipocytes and macrophages
Hepatic SREBP-1c mRNA expression was strongly activated in the HF group compared to the Control group (P < 0·005), whereas it reduced in the HFn-3 group (P < 0·005). Diacylglycerol O-acyltransferase 2 and SCD1 expression was lower in the HFn-3 group compared to both the Control and HF groups (P < 0·05). There was no significant difference for fatty acid synthase (P = 0·08). PPARγ mRNA expression showed no significant increase with n-3 PUFA compared to the Control and HF groups. Carnitine palmitoyl transferase 1 gene expression was higher in the HFn-3 group (P < 0·05) compared to the HF group, whereas it did not differ from the Control group. Hepatic mRNA expression of LPL and TNFα genes was not affected by diet. SR-B1 gene expression was higher in the HFn-3 group compared to both the Control and HF groups (P < 0·05; Fig. 5).

Fig. 5 Relative gene expression of sterol regulatory element-binding protein 1c (SREBP-1c), stearyl CoA desaturase 1 (SCD1), diacylglycerol O-acyltransferase 2 (DGAT2), fatty acid synthase (FAS), PPARγ, scavenger receptor class B type 1 (SR-B1) and carnitine palmitoyl transferase 1 (CPT1) in hepatic tissue. PPARγ and TNFα in adipose tissue macrophages and adipocytes of control diet (C), high-fat diet (HF) and high-fat diet enriched with n-3 PUFA (HFn-3) groups. Values are means, with their standard errors represented by vertical bars (n 6).* Mean values were significantly different from those of the Control group (P < 0·05). † Mean values were significantly different from those of the HFn-3 group (P < 0·05).
In macrophages, the expression of PPARγ and TNFα was higher in the HF and HFn-3 groups compared to the Control group (P < 0·05). No difference was showed for fatty acid synthase, LPL and SCD1. In adipocyte, expression of these genes was not different among the three groups.
Discussion
In the present study, we investigated the preventive effect of n-3 PUFA on the development of metabolic syndrome-associated disturbances induced by a HFD in golden Syrian hamster.
We reported that n-3 PUFA enrichment ameliorated metabolic syndrome-associated cardiovascular risk by preventing BW gain, dyslipidaemia, high postprandial lipaemia and glucose intolerance. Despite the fact that HFD did not impair endothelial-dependent vasorelaxation in the HF group compared to the Control group, n-3 PUFA improved it in the HFn-3 group.
We showed that HFD induced obesity (as BW gain and increase in fat mass percentage) in the HF group compared to the Control group, but n-3 PUFA enrichment attenuated BW gain and reduced fat mass percentage in the HFn-3 group compared to the HF group. Data from studies on the effect of n-3 PUFA on BW reduction are inconsistent. In human studies, the effect on BW was only effective when n-3 PUFA were combined with a BW loss regimen(Reference Mori, Bao and Burke29, Reference McCombie, Browning and Titman30). Some studies described a limited extension of adipose tissue in rats(Reference Storlien, Kraegen and Chisholm31, Reference Hassanali, Ametaj and Field32), mice(Reference Okuno, Kajiwara and Imai33, Reference Rossmeisl, Jelenik and Jilkova34) and salmon(Reference Todorcevic, Kjaer and Djakovic35) explained by different mechanisms, whereas in other studies no effect was observed(Reference Burghardt, Kemmerer and Buck36) or observed only in mice fed with high-fat/high-sucrose diet(Reference Sato, Kawano and Notsu37). In our study, we showed a lower hepatic SCD1 gene expression in the HFn-3 group. The inhibition of SCD1 is described to protect from HFD-induced obesity related to an up-regulation of genes involved in β-oxidation and a down-regulation of lipogenesis gene expression, as shown in mice SCD− / − (Reference Ntambi, Miyazaki and Stoehr38). However, another study in mice reported a decrease in SCD1, but with no consequence on BW(Reference Kajikawa, Harada and Kawashima39).
Impaired glucose tolerance is widely described as a cardiovascular risk factor(Reference Balkau, Charles and Drivsholm10). In our study, the effect of EPA and DHA enrichment on glucose tolerance, as assessed by fasting glycaemia and AUC, was impaired in the HF group and prevented by n-3 PUFA. The same result was reported in rodents(Reference Samane, Christon and Dombrowski40, Reference Kalupahana, Claycombe and Newman41) and human subjects, and was related to an increasing membrane fluidity and GLUT4 transport(Reference Manco, Calvani and Mingrone42) or affecting insulin receptor and insulin receptor substrate expression and protein abundance(Reference Lombardo and Chicco43). The prevention of BW gain associated with low muscle lipid content in the HFn-3 group can also explain the improvement of glucose tolerance measured in this group. Indeed, it was reported that BW loss induced by energy restriction decreased muscle lipid content in obese individuals with and without type 2 diabetes(Reference Goodpaster, Theriault and Watkins44), in conjunction with enhancement of insulin sensitivity(Reference Goodpaster, Kelley and Wing45).
Obesity is associated with an increase in the recruitment of adipose tissue macrophages(Reference Weisberg, McCann and Desai46, Reference Cancello, Tordjman and Poitou47). Interventions that inhibit the recruitment of macrophages(Reference Kanda, Tateya and Tamori48, Reference Weisberg, Hunter and Huber49) or decrease the pro-inflammatory activity(Reference Arkan, Hevener and Greten50, Reference Odegaard, Ricardo-Gonzalez and Red Eagle51) of macrophages improve the insulin sensibility in obesity. In our study, the expression of genes relative to lipid metabolism and TNFα in adipocytes and in adipose macrophages was measured. The level of TNFα gene expression did not differ among groups when measured in adipocytes. However, we observed an increase in the level of gene expression of TNFα and PPARγ in macrophages of both HF and HFn-3 groups, with no effect of n-3 PUFA against inflammatory and pro-adipogenic activity of macrophages. In other studies, n-3 PUFA were described to have a protective effect against adipose tissue inflammation induced by a HFD in obese diabetic mice(Reference Todoric, Loffler and Huber52) and against TNFα production(Reference Hao, Wong and Liu53).
In this study, the HFD led to a trend to increase HDLCH when compared to the control diet. This result is in accordance with what we and others have reported in hamsters fed with a HFD or cholesterol-enriched diet, with increases in TAG, total cholesterol and HDL CH(Reference Kanashiro, Andrade and Kabeya54–Reference Treguier, Briand and Boubacar56). We showed that EPA and DHA decrease fasting and postprandial plasma TAG and HDLCH. The decrease of total cholesterol has been reported in other studies in hamsters(Reference Lin, Lu and Huang57, Reference Mast, Shafaati and Zaman58) and mice(Reference le Morvan, Dumon and Palos-Pinto59) to be associated with a decrease in HDLCH. It was reported that this decrease was associated with an overexpression of SR-B1(Reference le Morvan, Dumon and Palos-Pinto59, Reference Spady, Kearney and Hobbs60), whereas a study in obese rats reported an increase in SR-B1 activity, but with no change in HDLCH level(Reference Sheril, Jeyakumar and Jayashree61). Most studies of reverse cholesterol transport (RCT) are conducted in mice or rats. These species do not express cholesteryl ester transfer protein, a protein that transfers cholesteryl ester from HDL to VLDL/LDL, followed by a hepatic uptake of these lipoproteins. As this pathway is the major route of RCT in human subjects, a cholesteryl ester transfer protein-expressing species, such as the hamster, represents a convenient preclinical model for investigating novel therapies for the treatment of dyslipidaemia in human subjects. It has been revealed that the overexpression of hepatic SR-BI increases RCT and has an anti-atherosclerotic role, despite low HDLCH concentrations(Reference Arai, Wang and Bezouevski62–Reference Nishimoto, Pellizzon and Aihara64). We recently reported in hamsters fed with a cholesterol-enriched diet a high HDLCH and low SR-B1 gene expression associated with a decrease in RCT efficiency(Reference Treguier, Briand and Boubacar56). In the present study, we found an increase of SR-B1 expression in liver. These data suggested that n-3 PUFA stimulates one step in the rate of cholesterol ester transport from peripheral tissues to the liver, probably by increasing the amount of SR-B1. The level of LDL and VLDL cholesterol did not change with n-3 PUFA enrichment, whereas some studies described an increase in LDL-cholesterol in fish oil-fed hamsters(Reference de Silva, Agarwal-Mawal and Davis65) and in type 2 diabetic patients(Reference Kissebah, Alfarsi and Evans66).
The hypotriacylglycerolaemic effect of n-3 PUFA shown in several studies in both rodents(Reference Rustan, Christiansen and Drevon4, Reference Flachs, Mohamed-Ali and Horakova5) and human subjects(Reference Woodman, Mori and Burke6, Reference Balk, Lichtenstein and Chung7) was confirmed in this study. We also showed a lower liver TAG content with n-3 PUFA related to a decrease in VLDL secretion. This decrease may be explained by both a decrease in lipid synthesis and catabolism. We measured the gene expression of SREBP-1c, the main transcriptional factor controlling lipid synthesis in the liver. We observed an increase in SREBP-1c expression in the presence of the HFD and a decrease in the presence of n-3 PUFA. We noted that n-3 PUFA reduced diacylglycerol O-acyltransferase 2, an enzyme that catalyses the final step of TAG synthesis. Furthermore, CPT-1, an enzyme involved in the transport of fatty acids into the mitochondria, was higher in the HFn-3 compared to the HF group and did not differ from the Control group. This result suggested an active mitochondrial β-oxidation. The same results were observed in mice fed with a DHA-supplemented diet(Reference Sun, Wei and Li67). This was not observed in rats fed with EPA, in which no effect was observed in SREBP-1c expression(Reference Perez-Echarri, Perez-Matute and Marcos-Gomez68). Unexpectedly, the effect of n-3 PUFA on lipogenesis and hepatic lipid accumulation was not related to the activation of PPARγ, but seems to result from the inhibition of SREBP-1c expression. The same result was obtained recently in mice fed with EPA and was explained by the inhibition of maturation of SREBP-1c by n-3 fatty acids(Reference Tanaka, Zhang and Sugiyama69).
The decrease of VLDL secretion by the liver was also reported in other studies. In vitro, HepG2 cells treated with DHA resulted in overall reduced apoB100 secretion(Reference Wu, Shang and Jiang70). In rats, DHA decreased VLDL secretion(Reference Ikeda, Kumamaru and Nakatani71, Reference Zheng, Avella and Botham72). Diabetic patients treated with EPA and DHA showed a decrease in VLDL apoB100 concentration related to a decrease in VLDL production(Reference Ouguerram, Maugeais and Gardette73). Recently, another mechanism implying lipid peroxidation and oxidative stress on ApoB100 degradation via a post-endoplasmic reticulum presecretory proteolysis, was described to be relevant to the hypotriacylglycerolaemic effect of dietary n-3 fatty acids(Reference Pan, Cederbaum and Zhang74, Reference Krauss75). Consistent with these data, we showed an increase in the level of MDA in the HFn-3 group compared to the Control and HF groups.
In this study, although the postprandial lipaemia magnitude, as calculated by the AUC, was similar between the HF and HFn-3 groups, the triacylglycerolaemia at 6, 8 and 10 h of kinetic were lower in the HFn-3 group. Postprandial TAG concentration reflects production and clearance of chylomicrons. Harris et al. (Reference Harris, Lu and Rambjor9) suggested that n-3 PUFA accelerate chylomicron lipid clearance by facilitating LPL-mediated lipolysis. Similarly, a study in mice also showed an enhancement of blood clearance of TAG-rich particles(Reference Qi, Fan and Jiang76). As no difference was observed in LPL gene expression in our study, we suggested that n-3 PUFA lowered postprandial lipaemia by decreasing the release of TAG-rich lipoproteins.
In our experimental conditions, the evaluation of endothelial function did not show a difference between the Control and HF groups despite the difference in glucose tolerance and lipid plasma concentrations. However, in the presence of n-3 PUFA, we observed better endothelium-dependent vascular response in accordance with previously reported data(Reference Conde, Cyrino and Bottino77). The endothelium relaxation was negatively correlated with the impairment of glucose tolerance and plasma cholesterol concentration. This improvement can be explained by the incorporation of n-3 PUFA into cell membranes, thus affecting function of cell and tissues with subsequent impact on the production of various vasoactive eicosanoids and other mediators(Reference Din, Newby and Flapan78).
In conclusion, we demonstrated that, although no effect was observed on inflammation (TNFα in macrophages), n-3 PUFA have a preventive effect on the development of BW gain, glucose intolerance and dyslipidaemia induced by a HFD with decrease of hepatic secretion of VLDL TAG by reducing the expression of genes involved in lipogenesis, especially SREBP-1c and diacylglycerol O-acyltransferase 2. These disturbances induced by the HFD were not associated with endothelial function impairment in the HF group, but we showed a beneficial effect on this function with n-3 PUFA enrichment. Finally, we showed, under n-3 PUFA supplementation, a decrease in HDLCH concentration related to an increase in SR-B1 gene expression, suggesting an improvement of RCT. This later effect needs more investigation to elucidate the effect of n-3 PUFA on HDL turnover and RCT.
Acknowledgements
The authors thank Nathalie Vaillant for her technical assistance. The present study was supported by CRNH (Centre de Recherche en Nutrition Humaine, Nantes, France) and research programme NUPEM supported by Region Pays de la Loire. The n-3 PUFA were provided by Pierre Fabre Santé. F. K. C. conducted the experiment, data analysis and wrote the manuscript; A. A. provided technical assistance in the animal experient; X. P. carried out the measurement of gene expression; A. M. carried out the diet analysis; G. L. advised on the evaluation of endothelial function; M. K. provided significant advice and consultation; P. N. helped to draft the manuscript and provided significant advice; K. O. conceived the study, and participated in the design, coordination and the writing of the manuscript. All authors have contributed to the preparation of the manuscript and agreed with the content of the submitted manuscript. The authors declare no conflict of interest.









