Introduction
Infection with hepatitis B and hepatitis C (HBV/HCV) is a major public health problem.Reference Cooke, Andrieux-Meyer and Applegate1 Approximately two billion people worldwide have been infected with HBV. Overall, 360 million have chronic infection, and 600,000 die each year from HBV-related liver disease.Reference Cooke, Andrieux-Meyer and Applegate1,Reference Shepard, Simard, Finelli, Fiore and Bell2 Most chronic HBV infections are acquired in infancy or early childhood, primarily through mother-to-child transmission.Reference Cooke, Andrieux-Meyer and Applegate1,Reference Tang, Covert, Wilson and Kottilil3 Exposure later in life can also lead to chronic infection but more frequently results in viral clearance and immunity.Reference Shepard, Simard, Finelli, Fiore and Bell2
The estimated global prevalence of hepatitis C (HCV) is 1.5–2%, affecting up to 71 million people.Reference Thomas4 The prevalence of HCV acquired by vertical transmission is 0.2–0.4% in the USA and Europe.Reference Dibba, Cholankeril and Li5 Perinatal transmission of HBV/HCV from an infected mother to her infant may occur via the following three possible routes: (1) transplacental transmission of virus in uteroReference Thomas4–Reference Wiseman, Fraser and Holden7; (2) perinatal transmission during delivery and (3) postnatal transmission.Reference Chaudhary8,Reference Le Campion, Larouche, Fauteux-Daniel and Soudeyns9
Maternal HBV/HCV has been found to be associated with adverse pregnancy and delivery outcomes, including premature deliveries, premature rupture of membranes, placental abruption, caesarean deliveries, perinatal mortality, congenital malformations and low birthweight,Reference Money, Boucoiran and Wagner10,Reference Safir, Levy, Sikuler and Sheiner11 as well as later effects on offsprings’ health during childhood and adulthood, including long-term gastrointestinal morbidity.Reference Yoles, Sheiner, Abu-Freha and Wainstock12, higher prevalence of respiratory*,Reference Govrin-Yehudain, Wainstock, Abu-Freha and Sheiner13 endocrine Reference Abu Freha, Wainstock, Menachem and Sheiner14 and infectious hosppitalisations.Reference Abu Freha, Wainstock, Poupko, Yonat Shemer, Sergienko and Sheiner15 However, to the best of our knowledge, the effect on neurological morbidity has not yet been investigated.
Therefore, we sought to examine the association between maternal HBV/HCV status during pregnancy and offspring neurological hospitalisations during childhood.
Method
Study design
A population-based cohort analysis was performed, which included all singleton infants born between the years 1991 and 2014 and discharged alive from Soroka University Medical Centre (SUMC). SUMC is the only tertiary hospital in Israel’s southern region and includes the largest birth centre in Israel. In Israel, all prenatal care and tests are fully covered by a national health law, allowing free access to prenatal care; therefore, this is a non-selective population-based study. The study protocol was approved by the SUMC institutional review board, and informed consent was exempt.
The independent variable was defined as maternal HBV/HCV status verified through medical records, which were summarised in an electronic chart. The perinatal data set consisted of information recorded directly following delivery by the midwife and obstetrician. This perinatal data set was cross-linked and matched with the paediatric hospitalisation data set based on personal identifying numbers of both the mother and offspring.
The outcome variable was defined as the first paediatric hospitalisation of the offspring with any neurological diagnosis. The paediatric hospitalisation data set included ICD-9 codes for the diagnoses as well as demographic information. All neurological diagnoses mentioned in the paediatric admission/discharge database were identified and grouped. A list of the grouped diagnoses and ICD-9 codes is presented in Supplementary Table 1. The follow-up time in the study was calculated from birth to an event, death (if occurred in the hospital), end of the study period or age 18 (censored). If more than one neurological diagnosis existed in the record of first hospitalisation, all diagnoses were counted and compared between the study groups. Since neurodevelopmental impairment maybe higher in newborns with congenital malformations or multiple gestations, these newborns were excluded from the study. The study protocol was approved by the SUMC institutional review board, and informed consent was exempt.
Statistical analysis
The initial analysis compared the background, pregnancy and perinatal characteristics of the two study groups using chi-square (or Fisher’s exact test in case less than 5 cases per cell) and t-tests based on variable characteristics and normal distribution. All analyses were two-sided with p < 0.05 considered statistically significant.
The background and perinatal characteristics included maternal age, parity, pregnancy complications (such as pregestational or gestational diabetes [GDM]), hypertensive disorders of pregnancy (including chronic hypertension, gestational hypertension and preeclampsia) and gestational age at delivery.
Individual diagnoses were grouped, that is, movement disorders or developmental disorders (Supplementary Table 1) and the analysis was conducted on the group level.
Incidence rates of neurological-related hospitalisations were calculated and compared between study groups. Kaplan–Meier survival curves were constructed, and the cumulative neurological-related hospitalisation incidence was compared between the groups using the Cox–Mantel log-rank test.
A Cox multivariable survival analysis was conducted, after verifying its assumptions were met, to study the association between either HCV or HBV and neurological-related hospitalisations while adjusting for confounding variables. In addition to variables with clinical importance, variables significantly different between the study groups, which were also associated with morbidity risk (but were not an intermediate variable between exposure and outcome) and suspected as confounders, were considered in the multivariable models. These variables included maternal and gestational age (continuous), and pregnancy complications (dichotomised), year of birth (to address possible cohort effect) and ethnicity (as a measure of socio-economic status). The final model was selected based on best fit and minimum log-likelihood.
Results
During the study period, 242,342 newborns met the inclusion criteria. In total, 777 (0.3%) newborns were born to mothers with either HCV or HBV (HBV/HCV+), and 241,571 (99.7%) newborns were born to non-HBV/non-HCV mothers. Table 1 presents background characteristics of the study groups. HBV/HCV mothers were older than non-HBV/non-HCV mothers, and they were more likely to have hypertensive disorders, premature deliveries, low birthweight and caesarean delivery.
Table 1. Obstetrical and perinatal characteristics by maternal HBC/HCV status

During the follow-up period (median = 10.51 years; range = 0–18 years), 7543 neurological-related hospitalisations were documented (3.1%). The incidence rates by study group of selected neurological morbidities at hospitalisations are presented in Table 2. Compared to the offspring of non-HBV/non-HCV mothers, a higher incidence of neurological-related hospitalisations was documented among offspring in the HBV/HCV group (4.5 vs. 3.1%, HR = 1.92, 1.37–2.67). Specifically, hospitalisations for movement and developmental disorders were more likely to occur in the offspring of HBV/HCV mothers (3.6 vs. 1.8%, HR = 2.35, 1.62–3.40 and 0.4 vs. 0.1%, HR = 4.91, 1.57-15.33), respectively.
Table 2. Main neurological hospitalisations

Fig. 1 presents the Kaplan–Meier log of survival for total neurological hospitalisations by study group. Cumulative survival among non-HBV/non-HCV offspring was significantly higher than that of HBV/HCV offspring (log-rank p < 0.001).

Fig. 1. Kaplan–Meier log of survival for total neurological hospitalisations by study group (n at risk = 242,342).
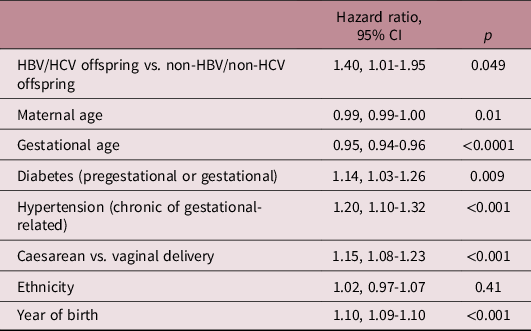
Table 3 shows the results of the Cox multivariable model comparing the risk for hospitalisation with neurological morbidities between study groups. The model adjusted for maternal age, gestational age, diabetes (gestational or pregestational), hypertension (gestational or chronic), delivery mode (caesarean vs. vaginal delivery), ethnicity and year of birth. Compared to non-HBV/non-HCV offspring, HBV/HCV offspring were at increased risk for neurological-related hospitalisations (adjusted HR = 1.40, 1.01–1.95).
Table 3. Cox multivariable model for the association between HBV/HCV mothers and offspring neurological hospitalizations
Discussion
In the current study, maternal HBV/HCV was associated with a significantly increased risk for offspring neurological-related hospitalisations, mainly for movement disorders (tremor, chorea and other extrapyramidal movement disorders) and developmental disorders (mild cognitive impairment, unspecified mental retardation and unspecified delay in development; Supplement A). To the best of our knowledge, this association has not yet been described. Maternal HBV/HCV was previously found to be associated also with offspring endocrine, respiratory and infectious morbidity.Reference Govrin-Yehudain, Wainstock, Abu-Freha and Sheiner13–Reference Abu Freha, Wainstock, Poupko, Yonat Shemer, Sergienko and Sheiner15 Thus, offspring of mothers having a chronic infectious disease (HBV/HCV) are prone to higher risk of non-communicable diseases, confirming again the concept of fetal origin of lifespan diseases. Because HBV/HCV mothers had higher premature birth rates, one may speculate that this may explain higher rates of neurological hospitalisations in offspring. However, this association remained significant even after adjusting for mode and week and of delivery as well as maternal complications (Table 3).
Several theories may explain these findings, including the microbiome theory, which suggest that HBV alters the normal gut microbiota.Reference Wei, Yan and Zou16,Reference Zhang, Lun and Tsui17 Immune alterations, such as viral infections during pregnancy, have been shown to alter maternal intestinal microbiome, resulting in potentially long-lasting consequences for offspring.Reference Zhang, Lun and Tsui17 Moreover, among patients with chronic HBV infection, the composition of gastrointestinal fungi has been found to be positively correlated with disease progression.Reference Chen, Chen and Guo18 Alterations in maternal microbiota have been found to affect various offspring health conditions, such as fetal microbiome,Reference Bhagavata Srinivasan, Raipuria, Bahari, Kaakoush and Morris19 glucose and lipid metabolism.Reference Zhou, Xiao and Zhang20 In animal models, an aberrant maternal microbiome has been shown to affect fetal brain development, and neurodevelopmental disorders have been suggested to determine life-long neurological outcomes.Reference Degroote, Hunting, Baccarelli and Takser21,Reference Hsiao, McBride and Hsien22 Moreover, HBV/HCV women had a higher rate of caesarean delivery, which was shown to alter fetal microbiome as compared to vaginal deliveries.Reference Salas Garcia, Yee, Gilbert and Dsouza23,Reference Shao, Forster and Tsaliki24 Thus, maternal microbiome changes in chronic HBV/HCV carriers contribute, at least in part, to the higher incidence of neurological diseases in offspring.
The association between maternal HBV/HCV and neurological disorders in offspring may also be explained by the immune theory. Several animal studies have shown that alteration in maternal immune response following viral infections results in increased incidence of various developmental, neurological and psychiatric disorders.Reference Shi, Tu and Patterson25–Reference Armario, Hernandez, Bluethmann and Hidalgo28 Injecting pregnant rodents with bacterial lipopolysaccharide, which induces strong innate immune responses in the absence of infection, results in behavioural and histological abnormalities in adult offspring similar to those observed in offspring of infected mothers.Reference Shi, Tu and Patterson25 Other studies in animal models have shown that alterations in maternal immune response result in increased incidence of various developmental, neurological and psychiatric disorders.Reference Patterson26,Reference Patterson27
HBV and HCV have been found to trigger epigenetic changes in hepatitis carriers,Reference Vivekanandan, Daniel, Kannangai, Martinez-Murillo and Torbenson29–Reference Hong, Kim and Guo31which may also explain the present findings. Cheng et al. found altered DNA methylation status in offspring of mothers with HBV compared to offspring of non-infected mothers, and these authors concluded that prenatal HBV exposure, even without malformations or premature birth, may alter the epigenome profile in newborns.Reference Cheng, Zhao and Huang32
Our study has several strengths. This is a long follow-up study on a large cohort of a single tertiary hospital in this region. Thus, offspring born in this centre are admitted to the same hospital, which enables accessibility to data regarding offspring hospitalisation events.
Our study has several limitations. The study data were obtained from hospital admission charts; therefore, we expect an underestimation of the outcome because only the more severe cases of neurological morbidities are hospitalised. This misclassification, however, would most likely be non-differential and occur independently of maternal HBV/HCV status, and it would shift our findings towards the null hypothesis. In addition, data on antiviral treatment of HBV/HCV mothers were unavailable, but most parturients do not require therapy. Antiviral therapies are listed as category B or C under the FDA pregnancy categories.Reference Wong, Seto, Wong, Yuen and Chan33,Reference Bzowej34 Therefore, it is unlikely that maternal antiviral treatment accounted for our findings.
Studies should further investigate whether changes in the microbiome of HBV/HCV mothers, immune system modulation or epigenetic changes lead to increased susceptibility to neurological hospitalisations as found in the current study. Future studies should also include data on antiviral treatments and consider their effect on offspring health.
Conclusions
Maternal HBV/HCV during pregnancy is associated with a significantly increased risk for offspring neurological hospitalisations. These findings may be explained, at least in part, by microbiome, immune or epigenetic changes in the offspring of mothers with HBV/HCV, but the exact mechanism merits further investigation.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S2040174420001397
Financial support
No financial support was received for the study.
Conflicts of interest
None of the authors have any commercial association or other conflicts of interest to declare.
Ethical standards
The study protocol was approved by the SUMC institutional review board, and informed consent was exempt.







