Introduction
Protected areas in the tropics are the last refuges of some endemic and threatened species of plants and animals (Gardner et al., Reference Gardner, Barlow, Chazdon, Ewers, Harvey, Peres and Sodhi2009). In the biodiversity hotspot of the Western Ghats in south-west India protected areas are under pressure from high human population density (Cincotta et al., Reference Cincotta, Wisenwski and Engleman2000), with enclaves inhabited by marginalized communities that traditionally depend on forest resources for their livelihoods (Anand et al., Reference Anand, Krishnaswamy, Kumar and Bali2010).
The Travancore tortoise Indotestudo travancorica is endemic to the Western Ghats. It inhabits evergreen, semi-evergreen, moist deciduous and bamboo forests and rubber and teak plantations (Deepak et al., Reference Deepak, Ramesh, Bhupathy, Vasudevan, Rhodin, Pritchard, van Dijk, Saumure, Buhlmann and Iverson2011). The species occurs in riparian patches and marshes and has a home range of 5.2–34 ha (Deepak et al., Reference Deepak, Ramesh, Bhupathy, Vasudevan, Rhodin, Pritchard, van Dijk, Saumure, Buhlmann and Iverson2011). Its diet consists of grasses, herbs, fruits, crabs, insects and molluscs, with occasional scavenging on dead animals (Deepak & Vasudevan, Reference Deepak and Vasudevan2012). It is categorized as Vulnerable on the IUCN Red List and listed in Appendix II of CITES (Asian Turtle Trade Working Group, 2000). It is also protected under Schedule IV of the Wildlife (Protection) Act of India (Deepak et al., Reference Deepak, Ramesh, Bhupathy, Vasudevan, Rhodin, Pritchard, van Dijk, Saumure, Buhlmann and Iverson2011). Ethnic communities (Kadar, Malai Pandaram, Kani, Malasar and Malaimalasar) and settlers opportunistically hunt the species throughout its range (Vijaya, Reference Vijaya1983; Frazier, Reference Frazier1989; Moll, Reference Moll, Swingland and Klemens1989; Deepak et al., Reference Deepak, Ramesh, Bhupathy, Vasudevan, Rhodin, Pritchard, van Dijk, Saumure, Buhlmann and Iverson2011). Other threats include forest fires and habitat loss and fragmentation (Bhupathy & Choudhury, Reference Bhupathy and Choudhury1995; Deepak et al., Reference Deepak, Ramesh, Bhupathy, Vasudevan, Rhodin, Pritchard, van Dijk, Saumure, Buhlmann and Iverson2011). Earlier assessments of the Travancore tortoise reported low encounter rates in Anamalai and Parambikulam Tiger Reserves (Bhupathy & Choudhury, Reference Bhupathy and Choudhury1995; Ramesh, Reference Ramesh2008). These assessments were of short duration and did not evaluate the status of tortoise populations in protected areas. Here we estimate occupancy of the tortoise, identify factors that influence occupancy and detection probability, highlight threats in two protected areas in south India and recommend options for management.
Study area
Our study area included parts of Anamalai (958.6 km2) and Parambikulam (285 km2) Tiger Reserves (Fig. 1), which are Category Ib protected areas (Dudley, Reference Dudley2008). There are four dominant forest types in the area: (1) southern tropical wet evergreen forest, comprising Dipterocarpus indicus, Dipterocarpus bourdilloni, Strombosia ceylanica; (2) bamboo brakes with mixed deciduous trees, comprising Grewia tiliifolia, Terminalia tomentosa, Lagerstroemia lanceolata and Cassia fistula; (3) moist deciduous forest, comprising T. tomentosa, Terminalia bellerica, Terminalia paniculata, L. lanceolata, Schleichera oleosa, Pterocarpus marsupium, Anogeissus latifolia and Dillenia pentagyna; and (4) teak Tectona grandis plantations (Champion & Seth, Reference Champion and Seth1968; Pascal et al., Reference Pascal, Ramesh and De Franceschi2004). Abandoned teak monoculture plantations cover 2.8% of Anamalai and 31.9% of Parambikulam Tiger Reserves (Wilson, Reference Wilson1973). The original habitat has been modified for silviculture (Harikrishnan et al., Reference Harikrishnan, Vasudevan, Udhayan and Mathur2012) and hydro-power projects (Sekar & Ganesan, Reference Sekar and Ganesan2003) and features man-made reservoirs, perennial tributaries, seasonal tributaries and streams. Grass marshes, known locally as vayals, are interspersed through all the major habitat types. The Reserves lie at altitudes of 200–2,500 m (Fig. 1a); we conducted sampling in forest areas at < 1,000 m. Mean annual precipitation during 2006–2009 in the town of Topslip was 1,711 mm, with seasonal peaks during the south-west monsoon (June–August) and the north-east monsoon (September–November). Diverse indigenous communities (Sekar & Ganesan, Reference Sekar and Ganesan2003; Chandi, Reference Chandi2008), mostly hunter–gatherers, inhabit enclaves within the Reserves (Supplementary Table S2).

Fig. 1 (a) Anamalai and Parambikulam Tiger Reserves, in the Western Ghats of south India. The rectangle on the inset shows the location of the main map in India. (b) The study area, comprising 395.5 km2 of available habitat for the Travancore tortoise Indotestudo travancorica in the Reserves.
Methods
Presence–absence surveys
We carried out surveys during the monsoon (June–November), when the tortoises were reportedly active. After familiarizing ourselves with the tortoise and its spoor we searched for tortoises during 16.00–19.00 on survey days. We followed a stratified random sampling design, marking and surveying 25 2-km trails in four forest types (three in moist deciduous, five in evergreen, eight in bamboo mixed and nine in teak plantation forest). The number of trails in each forest type varied according to the percentage cover in the study area. The starting points were determined randomly (Supplementary Table S1). Each trail was confined to a single forest type and was marked permanently. Trails were 0.5–2.0 m wide, depending on usage by wildlife and humans, and typically traversed multiple microhabitats such as grass marshes, rocky outcrops and streams. Some trails were straight and some were curved; none were circular and there was no overlap between trails. Two researchers searched up to 7 m on either side of the trail, one walking behind the other at a distance of < 5 m. Each trail was surveyed several times each year. All surveys were carried out in the same manner in different habitat types.
Indirect evidence included trails of flattened grass or shrubs (with or without footprints), triangular bite marks on grasses and herbs, and shells of dead tortoises (Supplementary Plate S1). The state of tortoise tracks varied depending on the condition of the ground vegetation. They were prominent in tall grasses during monsoon and post-monsoon seasons and were 15–25 cm wide. Surveys were ranked 1 if there was more than one record of indirect evidence or one or more direct sightings of Travancore tortoise and 0 if no sightings or indirect evidence were recorded. Tracks were erased or marked and were not counted during the subsequent survey on the same trail. The aquatic Indian black pond turtle Melanochelys trijuga co-occurred with Travancore tortoise and their tracks could not be differentiated. We minimized error in identification by requiring more than one piece of evidence at any location to record the presence of the Travancore tortoise. All detections were made by sight; we did not use dogs or auditory cues to detect the tortoise.
We sampled during monsoon and post-monsoon seasons. We conducted five surveys on each trail during 2006, three during 2007, seven during 2008 and three during 2009. Of 450 surveys on 25 trails 342 were completed successfully and the remainder were abandoned because of constraints during sampling. VD was involved in 253 of the successful surveys. We carried out 67 surveys on five evergreen forest trails, 133 on eight bamboo mixed-forest trails, 103 on nine teak forest trails and 39 on three moist deciduous forest trails. The time between consecutive surveys on the same trail was 3–16 days. Two field personnel were trained to detect the Travancore tortoise and its signs during an initial set of 23 surveys, which were excluded from analyses.
Covariates
We measured four trail-specific covariates and one survey-specific covariate. The trail-specific covariates were (1) the extent of grass marshes along the length of the trail, measured to the nearest cm, using a tape, (2) the number of water bodies (ponds or streams) within 10 m either side of the trail, (3) the level of human activity originating from enclaves within the protected areas, ranked 0–5, and (4) forest type. The level of human activity was calculated as the sum of individual scores assigned to each trail and was based on attributes recorded and indicators of opportunistic or targeted hunting of the Travancore tortoise: intensively used human trails; collection of tubers (Hemidesmus indicus and other edible species), which is practised in isolated enclaves where there is scarcity of grains; distance from the trail to human habitation (ranked 0 if > 1 km and 1 if < 1 km as there is increased probability of opportunistic hunting within a 1 km radius of the enclave); evidence of firewood collection (involves movement away from trails and considerable time spent in the forest, with potential for opportunistic and targeted hunting); and encountering domestic dogs, which could indicate targeted hunting. A score of 1 was assigned for each of these indicators of human activity; if none of these were encountered a score of 0 was assigned. Scores were summed to obtain a surrogate measure of human activity for each trail, and we included forest type as a categorical variable. The survey-specific covariate was the time spent on each trail during sampling.
Occupancy
We used multiple-season models, as described by MacKenzie et al. (Reference MacKenzie, Nichols, Hines, Knutson and Franklin2003), to estimate site occupancy (ψ) and detection probability (P) for the Travancore tortoise. We constructed models targeting different hypotheses on the influence of covariates on occupancy and detection probability (Table 1). We assumed that colonization and extinction probabilities were constant in all models, based on intensive monitoring of five tortoises over 4 years, using radio-tags, where both extinction and colonization probabilities were low in the study area (Vasudevan et al., Reference Vasudevan, Pandav and Deepak2010).
Table 1 Hypotheses used to identify factors that influence occupancy and detection probability of the Travancore tortoise Indotestudo travancorica in the study area in Anamalai and Parambikulam Tiger Reserves, south India (Fig. 1b).

Model evaluation was carried out using model set 1 (where detection probability (P) was explained using sampling year, forest type, number of water bodies, extent of grass marsh, and mean sampling effort for a trail in a year, maintaining occupancy (ψ) constant) and model set 2 (where occupancy (ψ) of the Travancore tortoise was explained using sampling year, forest type, number of water bodies, extent of grass marsh and surrogate measure of human activity originating from enclaves within the protected areas). In model set 2, detection probability (P) was modelled based on sampling year, forest type, number of water bodies, extent of grass marsh and mean sampling effort per trail in a year. Some models yielded unrealistic standard errors because estimates approached parameter boundary values (ψ = 0 or 1). These were removed before model averaging was carried out. We used 17 different models to test the suite of hypotheses.
Model selection and goodness of fit
We sorted the best-fit models according to delta Akaike Information Criterion (ΔAIC) values and used Akaike weights (w i ) to identify the model that best explained the data. ΔAIC and w i indicate the strength of evidence in support of a particular model in a set of models being evaluated (Burnham & Anderson, Reference Burnham and Anderson2002). Top models are those for which ΔAIC ≤ 2.0 relative to the best model (Burnham & Anderson, Reference Burnham and Anderson2002). Inferences are made based on the top models. There was no procedure available for testing the goodness of fit for multi-season occupancy models (MacKenzie & Bailey, Reference MacKenzie and Bailey2004). We calculated the relative importance of the variables, based on their Akaike weights, following Burnham & Anderson (Reference Burnham and Anderson2002). This was done by summing the AIC model weights across all the models that included the variable of interest.
Geographical information system (GIS) layers
We measured the distances from the trails to the human settlements, using ArcMap v. 9.2 (ESRI, Redlands, USA). We used the ASTER global digital elevation model layer with 30 m resolution (ASTER, 2011) and IIRS WG 56 m resolution layers (IIRS, 2002) to extract information on elevation and grass marsh. We calculated the area of different elevation zones by multiplying the total number of cells in each zone by the cell area. We used PRESENCE v. 3.1 (MacKenzie et al., Reference MacKenzie, Nichols, Hines, Knutson and Franklin2003) to estimate occupancy.
Results
We carried out 342 surveys on 25 trails during 2006–2009, which amounted to 684 km in total. We recorded 45 tortoises: 24 females, 16 males, three hatchlings and two juveniles. Of these, two males and one female were sighted more than once. Six of the 45 tortoises were sighted outside the trails in the study area and were not included in the analyses. Tortoise presence on the trails was confirmed by sightings of 39 tortoises and 61 instances of indirect evidence of tortoises (Fig. 2). There was considerable interannual variation in both direct and indirect evidence of tortoises in the study area (Fig. 2).

Fig. 2 Number of direct sightings of tortoises and recordings of indirect evidence during 2006–2009.
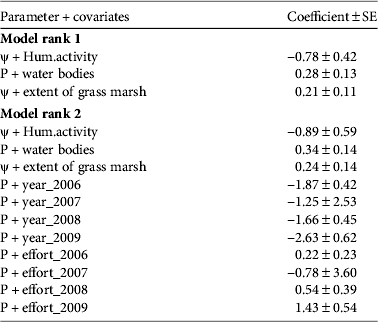
We analysed 15 models to test different hypotheses concerning detection probability. The probability of detecting a tortoise on a trail in the study area was 0.14–0.45. The top-ranking model that explained the variation in detection probability was influenced by the variables year, effort, number of water bodies and extent of grass marshes (Tables 2 & 3). A competing model with ΔAIC ≤ 2.0 had number of water bodies and extent of grass marshes as the influencing variables (Tables 3 & 4). Number of water bodies had the most significant influence on detection probability, accounting for 91% of the AIC model weights. It was followed by extent of grass marsh (83%), sampling year (62%) and sampling effort (61%). Contrary to expectation, forest type did not influence detection probability.
Table 2 Top-ranked models of relationships between covariates and detection probability of the Travancore tortoise, based on surveys carried out during 2006–2009 in the study area (Fig. 1b).

1 No. of water bodies
2 Extent of grass marshes
Table 3 Top-ranked models of the effect of covariates on occupancy and detection probability of the Travancore tortoise in the study area (Fig. 1b).

1 Index of human activity
2 No. of water bodies
3 Extent of grass marshes
Two top-ranked models had ΔAIC ≤ 2.0 and were used to draw inferences on occupancy (Table 3). The coefficients for the covariates in these models revealed that detection probability was positively influenced by the extent of grass marshes (Table 4). Water bodies and grass marsh accounted for 88% of AIC weight in the models that accounted for heterogeneous detection probabilities (Table 3). The naïve occupancy in the study area was 0.84 and the estimate was 0.41–0.97 for the first model and 0.50–0.99 for the second. The estimates of occupancy were the same for both models (Fig. 3a,b). The surrogate measure of human activity originating from enclaves within the protected areas was the most important variable to influence occupancy negatively (Fig. 3a,b) and it accounted for 73% of the AIC model weights. It was followed by number of water bodies, which accounted for 20% of the AIC model weights (Table 3). Excluding the eastern slopes with < 500 mm annual rainfall and the areas higher than 1,000 m we estimate that 25.4% (367 km2) of the protected areas constitute suitable habitat for the tortoise. Of this, 28.5 km2 is submerged by reservoirs and there are 12 human settlements, with a total population of 2,066 (Supplementary Table S3; Fig. 1b). Our data suggest that the Travancore tortoise occupies almost the entire area of suitable habitat within the protected areas.

Fig. 3 Estimates of occupancy of the Travancore tortoise, showing a decreasing trend with increasing index of human activity. (a) and (b) correspond to the first- and second-ranked models, respectively, in Table 3.
Discussion
Factors influencing detection probability and occupancy
Surveys carried out in 1991 detected one Travancore tortoise for every 6.7 hours of survey effort (Bhupathy & Choudhury, Reference Bhupathy and Choudhury1995). Surveys in the same area during 2002–2003 detected one tortoise for every 3.4 hours of search (Ramesh, Reference Ramesh2008). The encounter rates from these studies cannot be directly compared to our study because they were confined to streams and grass marshes, whereas we surveyed the entire area, traversing different habitat types. The Travancore tortoise is a cryptic species and its presence can be overlooked. Previous studies underestimated the population of Travancore tortoise in the study area, whereas our surveys included both direct and indirect evidence to confirm presence (spoors were clearly visible in wet areas and in patches of grass). We advocate the use of this survey protocol for monitoring the tortoise population in the Western Ghats.
Grass is an important part of the tortoise's diet (Vijaya, Reference Vijaya1983; Ramesh & Parthasarathy, Reference Ramesh and Parthasarathy2006; Deepak et al., Reference Deepak, Ramesh, Bhupathy, Vasudevan, Rhodin, Pritchard, van Dijk, Saumure, Buhlmann and Iverson2011), and therefore open areas with grass cover could constitute foraging sites. We identified water bodies and grass marshes as important variables that positively influenced detection probability (Tables 2 & 3). Others included the intensity of the search and the year of sampling (Tables 2 & 3). Although grass marshes constituted only 1% (37 km2) of the potential habitat available, they are an important provider of forage and could play a significant role in conservation of the tortoise.
On nine of the 25 trails sampled we observed local people accompanied by domestic dogs and on one occasion a dog had captured a tortoise. Their olfactory sensitivity enables dogs to detect tortoises on the forest floor (Nussear et al., Reference Nussear, Esque, Heaton, Cablk, Drake and Valentin2008) and makes furtive hunting possible. The Travancore tortoise is an easy target for furtive hunters because it can be hunted without leaving any evidence or incurring cost. Poor detection during our surveys could have resulted from reduced activity of tortoises in the study area, where hunting has taken place for a long time, and from low recolonization or high mortality rates among colonizing individuals. Luiselli (Reference Luiselli2003) found that Afrotropical tortoises altered their behaviour in response to hunting pressure, significantly reducing their activity.
Delayed maturation, low fecundity and poor juvenile survival rates are common in tortoises (Hailey et al., Reference Hailey, Wright and Steer1988) and populations are particularly sensitive to losses of reproducing adults (Lambert, Reference Lambert1982; Hailey et al., Reference Hailey, Wright and Steer1988). It takes c. 11 years for the Travancore tortoise to reach 163 mm in length (straight carapace length) and Whitaker (Reference Whitaker and Wahl2012) found that a female of 192 mm had oviducal eggs and two males of 150 and 159 mm had well-developed testes. As hunting selectively removes large breeding individuals it could threaten the persistence of the Travancore tortoise in the Western Ghats.
Conservation implications
The Travancore tortoise has been reported from 15 protected areas and seven Reserve Forests (Vasudevan et al., Reference Vasudevan, Pandav and Deepak2010; Supplementary Table S3). Grass marshes, or vayals, represent a small fraction of the protected areas but constitute an important foraging ground for this species, and therefore they should be a focal point for monitoring and protection measures. Furtive hunting linked to human enclaves within protected areas is a threat to this tortoise. If the pressure from the enclaves is to be removed, acquisition of land occupied by people and integrating it into protected areas must be considered as an option in the long term (Ali & Pai, Reference Ali and Pai2001). More immediately, protected area management should focus on strengthening capacity for monitoring populations of cryptic and threatened species and involving local people in actions to protect the species and its habitat.
We reported our findings to the relevant officials in the protected areas, and engaged with policy-makers, and subsequently the government offered support for the preparation of a Conservation Action Plan for the species.
Acknowledgements
We thank A. Silamban, S. Rajamani, R. Karuppamy and S. Sengupta for their support in field; the Wildlife Institute of India for grant-in-aid funding; Kerala and Tamil Nadu State Forest Departments for logistical support; N. Whitaker for sharing information; Jumbo Foundation for support to VD; and B. Noon, B. Pandav, P.K. Mathur and A. Harihar for their comments and suggestions.
Biographical sketches
V. Deepak's interests are the ecology and systematics of amphibians and reptiles. He has studied the population ecology of several endemic species in the Western Ghats. Karthikeyan Vasudevan's research focuses on the ecology and conservation of tropical reptiles and amphibians. He is involved in field surveys to document herpetofaunal diversity and conducting research into species ecology in different parts of India.