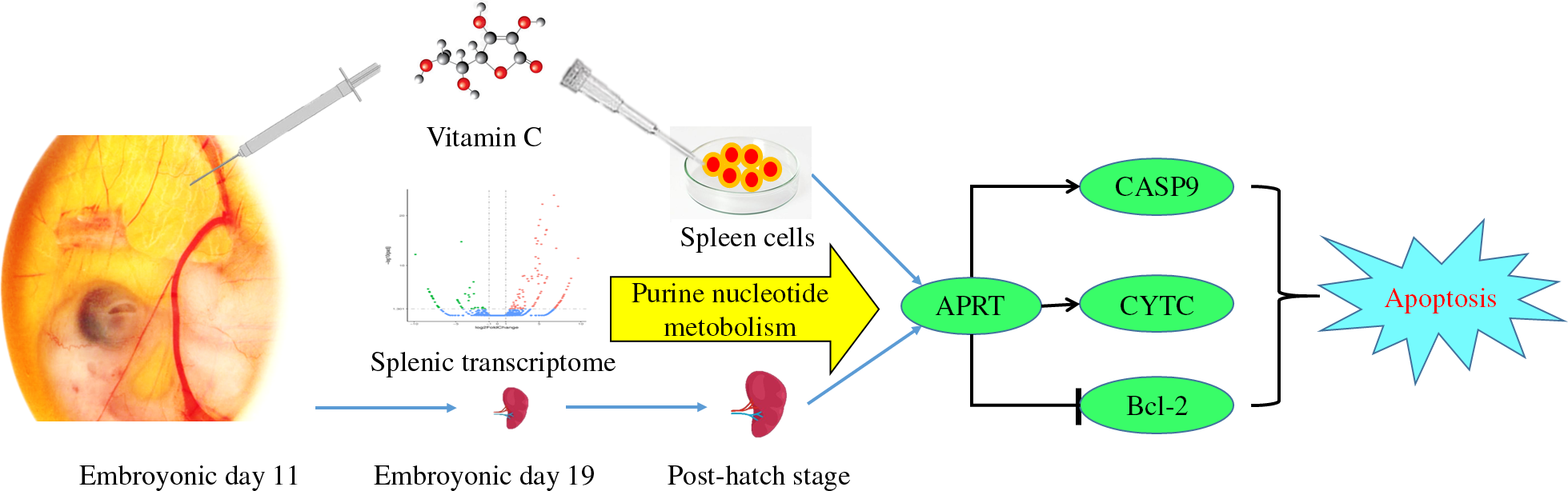
The metabolic disorder diseases such as obesity and diabetes are growing concern worldwide along with the elevation of the incidence rate in the young. This change may be concerned with the modern life style, such as high-energy diets and reductions in physical activity. More and more recent studies discovered the nutrition condition of earlier life, such as maternal nutrition and lifestyle, and nutrition of the infant period, which has a long-term influence on the health of its later period(Reference Dominguez-Salas, Moore and Baker1–Reference Stephenson, Heslehurst and Hall3). Accordingly, the development of nutrient regulatory strategies in earlier stages of life is crucial. Interestingly, the in ovo feeding (IOF) approach has been used for many years as a useful, convenient and cost-effective in vivo method to investigate the early nutrition.
The in ovo injection technology was originally designed to induce immune response by vaccination delivery in broiler hatcheries. Later, with increasing focus on the delayed intake of water and nutrients to chicks during the transition between the hatchery and the broiler farm, the use of in ovo injection for efficacy evaluation of the various nutrients in improving embryonic development, hatchability, post-hatch performance and immune function is gradually extended, such as amino acids, carbohydrates, vitamins, probiotics and plant extracts(Reference Yu, Gao and Zhao4–Reference Hou, Kolba and Glahn8). In addition, with the shortening of the market cycle of broilers, the proportion of the incubation period in the whole life cycle is increased correspondingly. Therefore, the nutritional state of the embryonic period is more important for regulating the development of tissues and organs in the whole growth stage in broilers. The IOF method may easily implement nutrition regulation in the embryonic period.
Vitamin C is widely regarded as an enhancer of immune function(Reference Carr and Maggini9), which included improved antioxidant capacity(Reference Linster and Schaftingen10) and resistance to inflammatory reactions(Reference Molina, Morandi and Bolin11). Moreover, a recent study identified that vitamin C could promote T-cell maturation in vitro by enhancing the conversion of double-negative T-cells to double-positive T-cells(Reference Manning, Mitchell and Appadurai12). Vitamin C can be synthesised endogenously in poultry, but the synthesis ability of chicks is weak, and it is easy to cause vitamin C deficiency under stress stimulation(Reference Pardue and Thaxton13). Previous studies have shown that the immune activity and anti-stress ability of broilers can be effectively improved in response to IOF of vitamin C(Reference El-Senousey, Chen and Wang14,Reference Zhang, Elliott and Durojaye15) . Also, our laboratory had reported that vitamin C supplementation in the embryonic period was able to improve plasma antibody levels and regulated immune-related gene expression, thus improved the immune status of broilers(Reference Zhu, Li and Duan6,Reference Zhu, Li and Sun16) .
The development of tissue structure and lymphocyte of spleen was closely related to immune function in chicken(Reference Mast and Goddeeris17). As the largest peripheral immune organ, spleen is composed of a large number of lymphocytes and macrophages(Reference Mebius and Kraal18). The later period of embryonic development and early hatching are the key stages for splenic tissue construction in chickens, accompanied by the renewal and reconstruction of spleen cell composition and the development of characteristic tissue structure. The hematopoietic function of the spleen gradually degenerated(Reference Yassine, Fedecka-Bruner and Dieterlen-Lièvre19), and lymphocytes began to accumulate, turning from hematopoietic organs to immune organs in the later period of embryonic development in chickens. With the rapid development of the splenic tissue structure and lymphocyte composition after hatching, the immune function also matured. At this time, the change of nutritional status of chicken embryo could regulate the occurrence and maturation of spleen by affecting various metabolic pathways and biological processes. Therefore, with the splenic metabolism and growth as the points of focus, exploring the effects of IOF of vitamin C on the splenic metabolic pathway and development process can provide a basis for the realisation of the ultimate regulation of immune function.
Thus, we hypothesised that in ovo injection of vitamin C could regulate the development of the spleen during the pre- and post-hatch period in broilers. By using a chicken model that was fed vitamin C during the embryonic period and without in commercial diets at the post-hatch stage, we addressed the altered splenic metabolism and growth in response to IOF of vitamin C and we further studied the underlying mechanisms.
Materials and methods
This experiment was approved by the Institutional Animal Care and Use Committee of Northwest A&F University (permit number: NWAFAC 1008), and all procedures were performed in accordance with ethical guidelines for research in animal science.
Embryo egg incubation experiment
In the study, 360 fertile eggs from Arbor Acres broiler breeders with similar weight (63·4 (sd 0·17) g) were individually and randomly assigned to a normal saline group (CON) and vitamin C group (VC) and incubated according to the normal hatching procedure after sterilisation. The eggs were injected on embryonic day (E) 11(Reference Zhu, Li and Duan6). The experimental treatment is as follows: (1) the CON group was injected with normal saline, and the injection volume was 0·1 ml; (2) the VC group was injected with the same volume of vitamin C (Sigma-Aldrich Inc.) solution with a concentration of 30 mg/ml(Reference Zhu, Li and Duan6). The injection procedure is as follows: remove the embryo-free eggs and dead sperm eggs, and determine the position of the yolk sac after candling; sterilise the air chamber with 75 % alcohol, drill a hole with a diameter of about 1 mm, and then sterilise the surrounding area with 75 % alcohol again; the nutrients were slowly injected into the yolk sac of the embryo egg with 1 ml syringe. After the injection, seal the hole with melted paraffin and continue to incubate.
Broiler feeding experiment
Chicks were hatched from CON and VC groups in the incubation experiment, and sixty healthy and normally developing chicks (close to average body weight and not distinguishing sex) from each group were randomly divided into six replicates with ten broilers per replicate for feeding until day 42. Experimental diets met the energy and nutrient requirements of the birds outlined by NRC 1994 (Table 1). The temperature inside the poultry house was maintained at 35°C during the first 3 d, between 28 and 30°C during the subsequent couple of weeks and about 25°C in the last 3 weeks of the study. Constant light for 24 h was provided throughout the experimental period. All birds had free access to feed and water. Vitamin C was not added throughout the feeding stage. The number of hatched chicks was counted, and the hatchability was calculated at the end of the incubation.
Table 1. Ingredients and composition of the control experimental diets

DDGS, distillers dried grains with solubles.
* The premix provided per kg of diets: vitamin A 2·76 mg, vitamin D 75 μg, vitamin E 38 mg, vitamin K3 3 mg, vitamin B1 3 mg, vitamin B2 10 mg, vitamin B6 5 mg, vitamin B12 0·04 mg, niacinamide 40 mg, calcium pantothenate 16 mg, folic acid 2 mg, biotin 0·3 mg, Fe 66 mg, Cu 15 mg, Mn 95·4 mg, Zn 96·6 mg, iodine 0·38 mg and Se 0·41 mg.
† The nutrient levels were calculated.
Sample collection
On E 19, sixty well-developed chick embryos were selected from CON and VC groups and unbroken spleens were collected. The spleens were placed in a cryopreservation tube without RNA enzymes and frozen in liquid N2 immediately. All samples were stored at −80°C for further RNA extract for mRNA sequencing. Furthermore, on day 21 and day 42, one healthy bird was selected from each replicate whose weight was close to the average level of the replicate and euthanised by an intraperitoneal injection of sodium pentobarbitone (30 mg/kg body weight). The tissue of immune organ was collected, stored in liquid N2 and then transferred to −80°C until subsequent analysis for vitamin C content and mRNA expression.
RNA isolation for RNA sequencing
Because of the small sample of spleen on E 19, twenty RNA samples were merged into one and three replicates were set for each group. Total RNA from spleen sample was extracted with TRIzol Reagent protocol (Invitrogen). The concentration and quantity of total RNA were analysed by a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific Inc.), and the integrity of total RNA was determined by Agilent 2100. RNA sample will be used for further analysis when optical density (OD) 260/280 > 1·8, OD 260/230 > 2·0 and the RNA integrity number > 7·0.
RNA isolation and complementary DNA synthesis for mRNA expression
The extraction procedure of RNA from day 21 and day 42 spleen sample was the same as above. Total RNA concentration was determined by a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific Inc.). Then, the concentration of RNA was uniformly adjusted to 500 ng/μl with diethyl pyrocarbonate water and used to complete complementary DNA (cDNA) synthesis by a PrimeScript® RT reagent kit (Takara). All cDNA samples were stored at −20°C.
mRNA sequencing and bioinformatics analysis
After the RNA samples for sequencing analysis reached the standard, the library was constructed. The specific process is as follows: (1) eukaryotic mRNA was enriched by magnetic beads with Oligo (dT); (2) fragmentation buffer was added to randomly interrupt the mRNA; (3) using mRNA as a template, the first cDNA strand was synthesised with random hexamers and then buffer, deoxy-ribonucleoside triphosphate, RNase H and DNA polymerase I were added to synthesise the second cDNA strand. AMPure XP beads were used to purify cDNA; (4) the purified double-stranded cDNA was repaired at the end, a tail was added and the sequencing connector was connected. Then, AMPure XP beads were used to select the size of cDNA segments; (5) finally, cDNA library was obtained by PCR enrichment. After the library inspection reached the standard, high-throughput sequencing was performed with HiSeq4000 and the reading length of sequencing was PE150. RNA sample detection, library construction and computer sequencing were all entrusted to Breeding Biotechnologies Company.
The differentially expressed genes (DEG) were identified based on fragments per kilobases per million reads using RSEM 1.2.31(Reference Ogata, Goto and Sato20). The resulting P values were adjusted using the Benjamini and Hochberg’s approach for controlling the false discovery rate. Genes with an adjusted P value < 0·05 found by DESeq were assigned as differentially expressed. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis for the DEG was performed by using KOBAS software(Reference Livak and Schmittgen21). Gene Ontology (GO) enrichment analysis for the screened DEG was carried out using the GOseq platform(Reference Krylov, Wolf and Rogozin22).
Vitamin C quantification
Vitamin C can reduce Fe3+ to Fe2+ due to its strong reducibility. Then, the content of vitamin C in plasma and tissues can be determined by the chromogenic reaction of Fe2+ and phenanthroline. The detailed determination procedure refers to the kit (Nanjing Jiancheng Biological Engineering Co. Ltd).
Real-time quantitative PCR
The mRNA levels of the following genes in the spleen were analysed by real-time quantitative PCR: adenosine deaminase (ADA), adenine phosphoribosyltransferase (APRT), serine/threonine kinase 1 (AKT-1), proliferating cell nuclear antigen (PCNA), B-cell lymphoma 2 (Bcl-2), cysteinyl aspartate specific proteinase 3 (CASP3), cysteinyl aspartate specific proteinase 9 (CASP9) and cytochrome C (CYTC). The primer sequences for all the genes are shown in Table 2. Quantification was carried out on the IQ5 (Bio-Rad) by using a SYBR Premix Ex Taq kit (TaKaRa). The cycling protocol was: 95°C for 5 min, then 40 cycles of 95°C for 10 s, 60°C for 30 s and 72°C for 30 s, finally at 72°C for 5 min. Each sample was examined in triplicate. Gene expression was calculated by the 2−ΔΔCt method.
Table 2. Primers used in real-time quantitative PCR
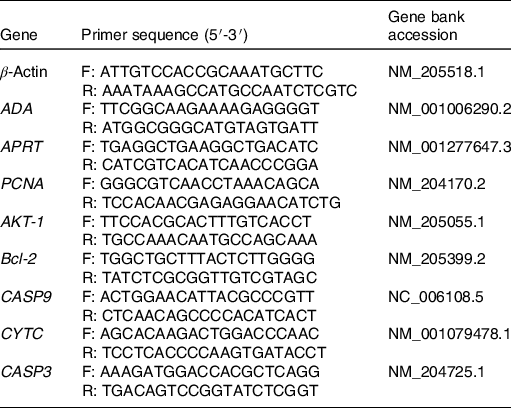
F, forward; R, reverse; ADA, adenosine deaminase; APRT, adenine phosphoribosyltransferase; PCNA, proliferating cell nuclear antigen; AKT-1, serine/threonine kinase 1; Bcl-2, B-cell lymphoma 2; CASP9, cysteinyl aspartate specific proteinase 9; CYTC, cytochrome C; CASP3, cysteinyl aspartate specific proteinase 3.
Primary spleen cells extraction
Spleen samples were collected from healthy chickens aged 21 d and washed with PBS solution containing penicillin and streptomycin for 2–3 times. Then, the samples were ground gently in a sterile petri dish with an appropriate amount of PBS and collected the filtrate. The cell filtrate was added to the lymphocyte isolation liquid (Solarbio), centrifuging at 2500 rpm for 20 min at 4°C. Then, cell suspensions in the middle layer were collected and washed with PBS twice. The cells were resuspended in the RPMI 1640 medium (HyClone) and adjusted concentration to 1 × 107/ml. A control group (CON) and four vitamin C concentration treatment groups were set for the experiment. The final concentration of vitamin C was 250 mg/l (VC-1), 500 mg/l (VC-2), 1000 mg/l (VC-3) and 2000 mg/l (VC-4) and cultured for 24 and 48 h. There are six replicates for each group.
3-(4,5)-Dimethylthiahiazo (-z-y1)-3,5-di- phenytetrazoliumromide (MTT) assay
At 4 h before the end of the culture, the cells of the CON group and VC group were taken out and washed twice with PBS. After centrifugation at 1300 rpm for 3 min, the cells were resuspended in the RPMI-1640 medium without vitamin C. Then, cells from each treatment group were inoculated into ninety-six-well plates at a cell density of 1 × 106/ml; 20 μl MTT (5 g/l) was added to each well, and culture was continued for 4 h. Patting the plate around to mix the solution evenly. At the end of treatment, the culture solution was removed, 180 μl of dimethyl sulfoxide (Sigma-Aldrich Inc.) was added to each hole. The absorbance was detected with a Multifunction microplate reader (Biotek) after shook the plate gently for 10 min.
Apoptosis measurements
To determine the cell apoptosis rate induced by vitamin C, cells were stimulated with 1000 mg/l vitamin C for 48 h. The annexin V-FITC/PI (fluorescein isothiocyanate/propidium iodide) (KeyGEN Biotechnology Co. Ltd) staining was carried out as follows: Cells from CON and VC groups were gathered by centrifuging at 2000 rpm for 5 min, washed with PBS and recentrifuged. The cell pellets were resuspended in 500 μl binding buffer. Following, 5 μl annexin V-FITC was added to the cell suspension and mixed; 5 μl propidium iodide was added and mixed again. The solution was incubated for 5–15 min at 4°C in the dark. The measurement was performed on a BD FACSAria™ III flow cytometer (BD) within 1 h.
Statistical analysis
The statistical analysis of the experimental results was performed by one-way ANOVA using SPSS 21.0. The noteworthy differences between treatments were identified by Duncan’s multiple comparisons test. Data are presented as mean values with their standard errors, and differences with P < 0·05 were considered statistically significant.
Results
The hatchability of CON and VC groups was 91·6 and 93·3 %, respectively, >90 % (Table 3). It had shown the incubation was well and the in ovo injection technology was all right.
Table 3. Effect of in ovo feeding of vitamin C on the hatchability of fertilised eggs

CON, 0·1 ml saline injection at embryonic day 11; VC, 0·1 ml vitamin C injection of 30 mg/ml at embryonic day 11.
Function annotation of differentially expressed mRNA
In order to reveal the impact of vitamin C on spleen development in broilers, we filtrated the potential regulated manner by mRNA sequencing. Based on the criteria of fold change ≥2 and false discovery rate < 0·01, we get 141 DEG in the VC group relative to the CON group; among them, 101 genes were up-regulated and forty-one genes were down-regulated. According to the GO and KEGG enrichment analyses based on DEG, we get the relevant function of these genes. The GO term annotation has significantly enriched several biological processes mainly involved in P metabolism, nucleotide metabolism and biosynthesis, nucleotide phosphate metabolism and biosynthesis, purine nucleotide metabolism, gametogenesis and multicellular biological reproduction, etc. The top twenty-five most significant GO term annotation was listed (Fig. 1). Meanwhile, KEGG analysis has identified eight significantly enriched KEGG pathways which involved in purine metabolic pathway, fatty acid metabolism and degradation pathways, propionate metabolic pathway and vascular smooth muscle contraction pathway, etc. (Table 4). Among them, the most significant level of pathway enrichment occurred in purine metabolic pathway. Combined with the result of GO and KEGG analysis, we obtained the information that IOF of vitamin C affected mainly the splenic purine nucleotide metabolism pathway on E 19.

Fig. 1. Distribution of the Gene Ontology (GO) categories assigned to the embryonic day 19 splenic transcriptome. The treatments were as follows: CON = 0·1 ml saline injection at embryonic day 11; VC = 0·1 ml vitamin C injection of 30 mg/ml on embryonic day 11. n 3 per treatment group.
Table 4. Enrichment analysis of the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway of differentially expressed genes (n 3 per treatment group)

CON, 0·1 ml saline injection at embryonic day 11; VC, 0·1 ml vitamin C injection of 30 mg/ml on embryonic day 11.
Vitamin C content on day 21 and day 42
We have obtained the primary understanding that purine nucleotide metabolism might be a potential way for IOF of vitamin C to regulate spleen development at the embryonic period. Next, we continue to detect the regulatory role of IOF of vitamin C in broilers in adulthood. We measured the content of vitamin C in the immune organs on day 21 and day 42, first. The result showed that the splenic vitamin C content was significantly increased in the VC group on day 21 (P < 0·001), whereas had no significant effect on vitamin C content of day 42 spleen, also in thymus and bursa (Table 5). These results suggested that IOF of vitamin C could specifically increase the splenic vitamin C content in the adult period of broilers, and the effect can be continued to day 21 at least.
Table 5. Effect of in ovo feeding of vitamin C on vitamin C content in immune organs (n 6 per treatment group)
(Mean values and standard errors)

CON, 0·1 ml saline injection at embryonic day 11; VC, 0·1 ml vitamin C injection of 30 mg/ml at embryonic day 11.
Gene expression analysis related to purine nucleotide metabolism
Following, mRNA expression related to purine nucleotide metabolism was measured in the spleen which was filtrated from E 19 splenic mRNA sequencing, including ADA and APRT. The splenic expression of ADA was significantly decreased in the VC group on day 42 (P < 0·05) and had no significant effect on the expression of APRT between CON and VC groups on day 21 and day 42 (P > 0·05, Fig. 2). The present results evidenced that the regulatory effect of IOF of vitamin C on purine nucleotide metabolic pathways can last from the embryonic period to the late growth period and had an impact on the adenylate metabolism pathway by down-regulating ADA gene expression.

Fig. 2. Effect of in ovo feeding of vitamin C on splenic expression of adenosine deaminase (ADA) and adenine phosphoribosyltransferase (APRT) of broilers. (a) Day 21; (b) day 42. The treatments were as follows: CON = 0·1 ml saline injection at embryonic day 11; VC = 0·1 ml vitamin C injection of 30 mg/ml at embryonic day 11. Values are means with their standard errors (n 6). * 0·01 < P ≤ 0·05 v. CON. ![]() , CON;
, CON; ![]() , VC.
, VC.
Gene expression analysis related to cell proliferation and apoptosis
In order to explore the specific regulation of IOF of vitamin C on splenic development, genes associated with cell proliferation and apoptosis were detected. On day 21, the splenic expression of AKT-1 and PCNA was significantly down-regulated and CASP9 was up-regulated in the VC group (P < 0·05), and the expression of Bcl-2 and CASP3 had no significant effect (P > 0·05). On day 42, the VC group significantly up-regulated the splenic expression of CASP9, whereas the expression of Bcl-2 was down-regulated (P < 0·05); there was no significant difference in the expression of AKT-1, PCNA and CASP3 (P > 0·05, Fig. 3). On the whole, these results suggested that vitamin C could inhibit the expression of genes related to cell proliferation and promote the expression of genes related to apoptosis, which may associate with splenic maturation.

Fig. 3. Effects of in ovo feeding of vitamin C on the relative mRNA expression of cell proliferation-related and apoptosis-regulatory genes in spleen of broilers. (a) Proliferation-related genes on day 21; (b) proliferation-related genes on day 42; (c) apoptosis-regulatory genes on day 21 and (d) apoptosis-regulatory genes on day 42. AKT-1, serine/threonine kinase 1; PCNA, proliferating cell nuclear antigen, Bcl-2, B-cell lymphoma 2; CASP9, cysteinyl aspartate specific proteinase 9; CASP3, cysteinyl aspartate specific proteinase 3. The treatments were as follows: CON = 0·1 ml saline injection at embryonic day 11; VC = 0·1 ml vitamin C injection of 30 mg/ml at embryonic day 11. Values are means with their standard errors (n 6). * 0·01 < P ≤ 0·05 v. CON. ![]() , CON;
, CON; ![]() , VC.
, VC.
Effects of vitamin C on spleen cell proliferation
Further, to verify the role of vitamin C on spleen maturation, we performed experiments on primary spleen cells. The proliferation of spleen cells stimulated by different doses and times of vitamin C is shown in Fig. 4. After 24 h of treatment, the treatment concentration of the VC-2 group (500 mg/l) was taken as the inflection point; with the increase of vitamin C concentration, the regulatory effect of vitamin C on the proliferation of spleen cells showed a tendency of first enhancement and then inhibition. After 48 h of treatment, the proliferation of spleen cells was gradually inhibited with the increase of vitamin C concentration. Together, we concluded that the effect of vitamin C on the proliferation of spleen cells is related to the dose and time of vitamin C stimulation, in which low concentration but prolonged and high concentration of vitamin C performed inhibitory effects on spleen cell proliferation.

Fig. 4. Effects of vitamin C at different concentrations on spleen cell proliferation: (a) cultured for 24 h; (b) cultured for 48 h. A control group (CON) and four vitamin C concentration treatment groups were set for the experiment. The final concentration of vitamin C was 250, 500, 1000 and 2000 mg/l. Values are means with their standard errors (n 6). * 0·01 < P ≤ 0·05, ** 0·001 < P ≤ 0·01, *** P ≤ 0·001 v. CON.
Gene expression analysis in vitro
Ulteriorly, we measured the relative expression of ADA, APRT, CASP9, CYTC and CASP3 to explore whether vitamin C regulates splenic development through purine nucleotide metabolism and cell apoptosis. mRNA analyses indicated that the relative expression of APRT, CASP9 and CYTC was significantly increased in the VC-3 (1000 mg/l) and VC-4 (2000 mg/l) groups compared with other groups (P < 0·05), whereas the expression of CASP3 was significantly decreased (P < 0·05). The tendency of gene expression induced by vitamin C treatment was consistent with 24 h (Fig. 5(a)) and 48 h (Fig. 5(b)). But, the change amplitude of gene expression increased as the stimulation time increased, that is, the regulation effect of vitamin C increased accordingly. Once again, these results showed that vitamin C had an impact on the adenylate metabolism pathway by up-regulating APRT gene expression and promoted the apoptosis of spleen cell in a time- and concentration-dependent manner, in which vitamin C concentration of 1000 mg/l and above could promote the occurrence of apoptosis.
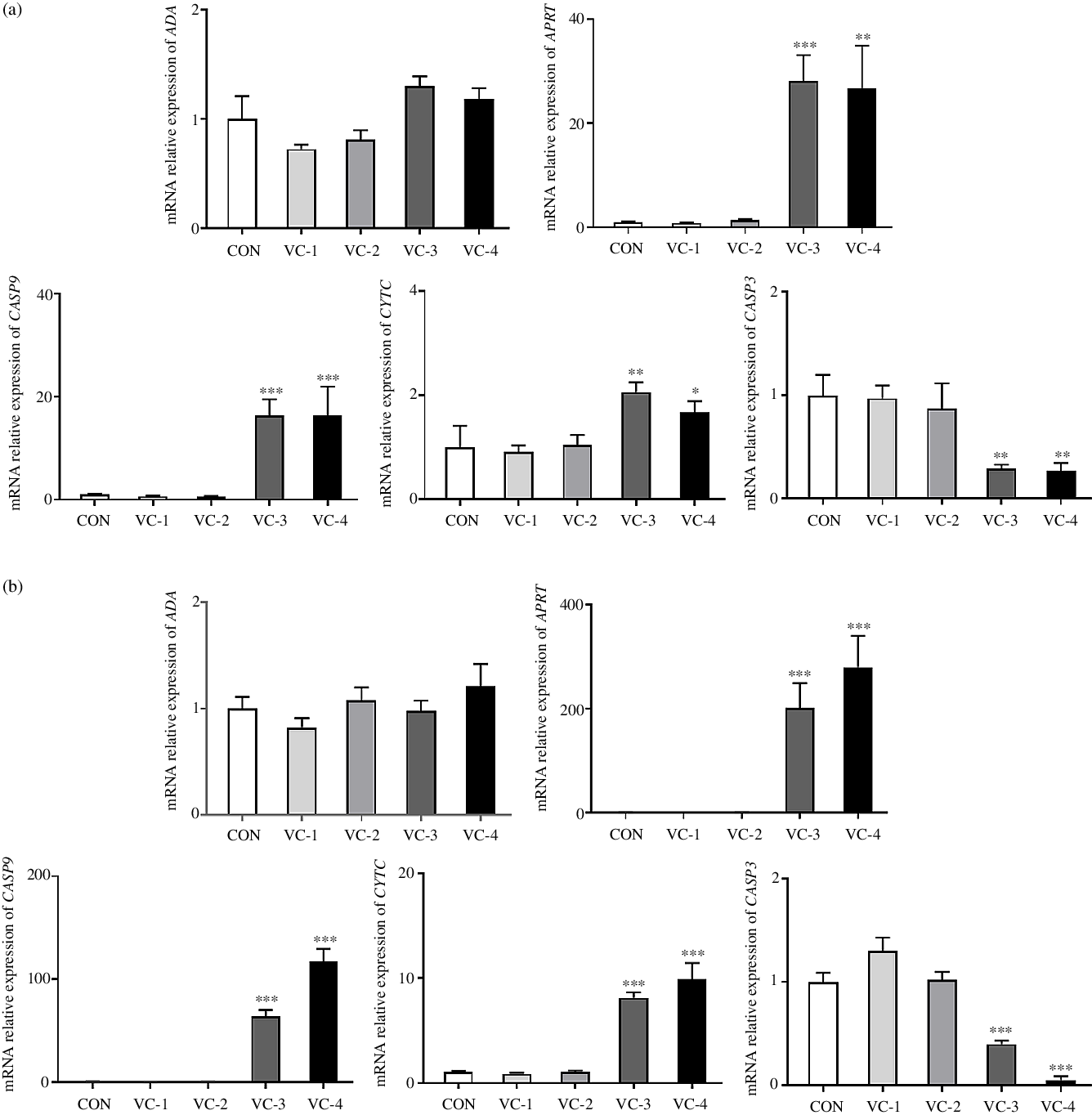
Fig. 5. Effects of vitamin C on the expression of genes related to purine nucleotide metabolism and apoptosis: (a) cultured for 24 h; (b) cultured for 48 h. ADA, adenosine deaminase; APRT, adenine phosphoribosyltransferase; CASP9, cysteinyl aspartate specific proteinase 9; CYTC, cytochrome C; CASP3, cysteinyl aspartate specific proteinase 3. A control group (CON) and four vitamin C concentration treatment groups were set for the experiment. The final concentration of vitamin C was 250 mg/l (VC-1), 500 mg/l (VC-2), 1000 mg/l (VC-3) and 2000 mg/l (VC-4). Values are means with their standard errors (n 6). * 0·01 < P ≤ 0·05, ** 0·001 < P ≤ 0·01, *** P ≤ 0·001 v. CON.
Apoptosis level of spleen cell induced by vitamin C
Since the trend of CASP3 expression is contrary to the induction of apoptosis, in order to confirm clearly the promotion effect of vitamin C, we detected the cell apoptosis rate by annexin V-FITC/PI staining and shown in Fig. 6. The number of early apoptotic cells (Q2) and late apoptotic cells (Q3) was significantly increased due to vitamin C stimulation (P < 0·05). That is, vitamin C increased the apoptosis rate of spleen cells.

Fig. 6. Effect of vitamin C (1000 mg/l for 24 h; VC) on the apoptosis rate of spleen cells was detected by annexin V-FITC/PI (fluorescein isothiocyanate/propidium iodide) staining. Values are means with their standard errors (n 6). *** P ≤ 0·001 v. control (CON).
Discussion
The RNA composition of cells or tissues will change with the growth stage, growth environment and physiological state. Therefore, the transcriptome has a highly dynamic and variable characteristic, which can reflect the transcription characteristics of cell or tissue under a specific physiological state or growth stage(Reference Wang, Gerstein and Snyder23). Transcriptomics is based on gene expression profiles, using GO and KEGG databases to perform functional annotation and enrichment analysis. It has been well established that IOF of vitamin C could improve production traits, muscle development and immune function in poultry(Reference El-Senousey, Chen and Wang14,Reference Foye, Uni and Ferket24) , but the potential regulatory mechanisms were uncleared. Based on the functional analysis of GO and KEGG of DEG induced by IOF of vitamin C, our data found that the metabolic pathways and biological processes were extensively regulated during the embryonic period of the spleen growth, such as P metabolism, nucleotide metabolism and biosynthesis, purine nucleotide metabolism, purine-containing compound metabolism and reproduction of multicellular organisms, etc. Meanwhile, purine metabolism pathway, fatty acid metabolism pathway, propionate metabolism pathway and others were also observed by KEGG enrichment analysis. Among them, the most significant enrichment was in the purine metabolic pathway. Prior studies have reported that vitamin C deficiency led to increased metabolites of purine nucleotide cycles, including IMP, adenylosuccinate and AMP(Reference Kirkwood, Lebold and Miranda25). Another study reported that vitamin C could regulate the energy charge ratio of adenosine in chondrocytes, showing that vitamin C has an effect on relevant metabolites of adenosine circulation(Reference Shapiro, Leboy and Tokuoka26). These studies preliminarily indicated the association between vitamin C and purine nucleotide metabolism. Our study suggested that purine nucleotide metabolism might be a potential way for vitamin C to regulate spleen development. And screening out a variety of catalytic enzyme systems involved in purine nucleotide cycle, such as ADA, APRT, adenosine kinase, adenylate cyclase and adenylate kinase, etc. Following, we further verified the splenic expression of ADA and APRT in the adult period. Compared with the CON group, there was no significant difference in the gene expression of ADA and APRT on day 21, but the expression of ADA was significantly down-regulated on day 42. Although the effect of IOF of vitamin C on splenic purine nucleotide metabolism is not continuous throughout the growing period of broilers, it could regulate the expression of related genes in the later growth period (day 42). Otherwise, the specific up-regulation of splenic vitamin C content on day 21 suggested that vitamin C could continue to play a role in spleen development after day 21, which could provide support for the down-regulation of the expression of ADA on day 42. In summary, our experiment demonstrated the effect of IOF of vitamin C on splenic purine nucleotide metabolism pathway, which can last from the embryonic period to the adult period in broilers, and screened out the key catalytic enzymes affecting the purine nucleotide cycle.
The technology of IOF has realised the nutritional support for the development of poultry during the embryo period, which is helpful to fully perform the potential of production in broilers. The nutritional advantages of IOF are representative in the early growth period after shelling(Reference Peebles27), but it is unclear whether it can be sustained in the later growth period. A previous study reported that the growth-promoting effect of IOF of β-hydroxy-β-methyl butyrate decreased gradually with the increasing age(Reference Ghanaatparast-Rashti, Mottaghitalab and Ahmadi28). In contrast, another study had shown that the weight advantage caused by IOF could be sustained to day 42 at least(Reference Ma, Zhang and Wang29). In this study, we found that IOF of vitamin C significantly increased the splenic vitamin C content on day 21, but there was no significant difference on day 42. The result showed that the effect of IOF of vitamin C could last to the middle and late growth period of broilers, which was helpful to prove the long-term advantage of IOF. In addition, our data suggested that IOF of vitamin C could specifically improve the vitamin C content in spleen compared with thymus and bursa, which may be related to that the spleen is an organ of vascular system, and blood circulation or exchange within the organisation is more frequent than in thymus and bursa. The specific increase of splenic vitamin C content supported the regulatory effect of vitamin C on spleen development.
Apoptosis plays an important role in maintaining the normal growth of multicellular organisms, homoeostasis, embryonic development and the maturation of the immune system(Reference Rathmell and Thompson30,Reference Legrand, Konstantinou and Goode31) . In our study, IOF of vitamin C significantly up-regulated the splenic expression of CASP9 on day 21 and day 42 and down-regulated the gene expression of Bcl-2 on day 42, whereas had no significant difference on the expression of CASP3. CASP9 is an apoptosis initiation factor, which activates downstream caspase under the stimulation of apoptosis-inducing factors, such as CASP3(Reference Würstle and Rehm32). CASP3 is an apoptosis-executing factor, which induces apoptosis by cleaving proteins essential for cell structure and life activities(Reference Herr33). Bcl-2 has the effect of anti-apoptosis(Reference Singh, Letai and Sarosiek34). To some extent, the result indicated that IOF of vitamin C could promote the apoptosis of spleen cells in the adult period in broilers, thus regulating the maturation of the spleen. Similarly, higher doses of vitamin C stimulation can also alter the expression of apoptosis-related genes in vitro and increase the apoptosis rate of spleen cells. Current studies suggested that the pro-apoptotic/anti-oxidative effects of vitamin C are related to the pro-oxidative/anti-oxidative effects in cells, which can induce apoptosis at high doses of vitamin C(Reference Perez-Cruz, Cárcamo and Golde35–Reference Chiu, Hu and Huang37). Nevertheless, the prior study showed that IOF of 3 mg vitamin C at E11 could increase the total antioxidant capacity of plasma at day 42 and had no significant effect on the levels of glutathione peroxidase, superoxide dismutase and malonaldehyde(Reference Zhu, Li and Duan6). Zhang et al. (Reference Zhang, Elliott and Durojaye15) reported that IOF of 12 mg ascorbic acid at E 17 could reduce the level of malonaldehyde in plasma and thus had a positive effect on the antioxidant capacity of broilers. That is to say, the time period and vitamin C dose of IOF of vitamin C in our study did not cause the pro-oxidative reaction in cells. Therefore, the mechanism of vitamin C-induced apoptosis still needs to be further verified. Subsequently, we explored the potential link between purine nucleotide metabolism and cell apoptosis.
Nucleotides are helpful for promoting the apoptosis of certain cells(Reference Single, Leist and Nicotera38,Reference Kluck, Esposti and Perkins39) . In the present study, the results showed that splenic expression of ADA was down-regulated at day 42 and the gene expression of APRT was up-regulated by supplementing with vitamin C in vitro. ADA catalysed the deamination of adenine nucleosides, which resulted in the accumulation of adenosine when the expression of ADA was down-regulated. Then, the phosphorylation reaction (adenosine +ATP→AMP + ADP) is promoted by the increased level of adenosine, so as to consume ATP in the cells(Reference Cardoso, Schetinger and Correia-de-Sá40). APRT catalysed the reaction of adenine with 5′-phosphoribosyl pyrophosphate to generate AMP. The up-regulation of APRT expression will lead to the accumulation of AMP, thereby driving the myokinase reaction (2ADP↔AMP+ATP) to the left, thereby reducing the energy level in the cells(Reference Arancio, Ranzoni and Delsignore41). Hardie et al.(Reference Hardie and Hawley42) showed that the concentration of AMP would increase exponentially when the level of cellular ATP was reduced, that is, ATP consumption was closely related to AMP accumulation. Meanwhile, apoptosis is closely related to intracellular energy level. High dose of vitamin C can cause an imbalance of intracellular energy metabolism and induce apoptosis, which has been widely studied in cancer cell lines. Yun et al.(Reference Yun, Mullarky and Lu36) found that vitamin C could inhibit the glycolytic pathway through glyceraldehyde-phosphate dehydrogenase, causing ATP depletion and inducing cell death in colorectal cancer. Uetaki et al. (Reference Uetaki, Tabata and Nakasuka43) reported that the cell death of breast cancer was also accompanied by changes in intracellular energy levels, which were manifested in decreased ATP levels and correspondingly increased ADP and AMP levels. The results of this study suggested that vitamin C may regulate energy metabolism by affecting the expression of ADA and APRT, which are key metabolic enzymes in the purine nucleotide cycle and have a tendency to reduce the level of ATP. This study suggested a potential mechanism for promoting apoptosis mediated by ADA and APRT by regulating cellular energy levels.
The pathophysiological process of tumorigenesis involves the imbalance of purine nucleotide metabolism. The expression of adenosine kinase and ADA was significantly up-regulated in II and III grade of glioma cells(Reference de Groot, Iyer and Zurolo44,Reference Huang, He and Chen45) , and Rocha et al.(Reference Rocha, Torres and Ojeda46) proposed that the growth and apoptosis of glioma cells may be affected by adenosine concentration. Our study proved the regulatory effect of vitamin C on purine nucleotide metabolism, which may provide a new idea for vitamin C to regulate the occurrence and development of specific tumours.
Vitamin C cannot be synthesised by humans due to lack of a key biosynthetic enzyme(Reference Nishikimi, Fukuyama and Minoshima47). Coincidentally, the synthesis ability of vitamin C in chicks is weak. However lucky is, chick embryo is a model of the earlier life nutrition well, and utilising the approach of IOF is much valuable for the study and application of vitamin C on the development of the child in utero and early in life.
In conclusion, our results indicated that IOF of vitamin C could regulate the splenic metabolic process in the embryonic stage, extensively, and it suggests that purine nucleotide metabolic pathway may be a potential way for vitamin C to regulate the growth of spleen. Simultaneously, the regulatory effects of IOF could continue to the middle and late period of development in broilers. To some extent, vitamin C promoted the apoptosis of spleen cells, thus regulating the maturation of spleen. Moreover, the apoptosis-promoted of vitamin C may associate with the purine nucleotide metabolic pathway mediated by APRT, and the intrinsic relationship between purine nucleotide metabolism and apoptosis should be further explored.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (grant number 2017YFD0502200) and the Program for Shaanxi Science and Technology (grant numbers 2017TSCXLNY-04-04, 2018ZDCXL-NY-0201 and 2018ZDXM-NY-051). These funders had no role in the design, analysis or writing of this article.
L. Q. Z. and X. Y. contributed in the data acquisition and statistical analysis, and drafting of the manuscript. L. Q. Z., J. W., Z. P. L. and H. Y. M. performed the experiments. L. Q. Z. and X. J. Y. designed the study. Y. F. Z., Z. Z. R. and X. J. Y. reviewed the manuscript. X. Y. is the corresponding author. All authors read and approved the final manuscript.
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of this article.