Conjugated linoleic acids (CLA) are a group of geometric and positional mixtures of linoleic acid with conjugated double-bond pairs. cis-9, trans-11 CLA and trans-10, cis-12 CLA are biologically active isomers(Reference Pariza, Park and Cook1). Previous studies have demonstrated that CLA can reduce obesity, improve immunity and have anticarcinogenic, antiatherosis, antidiabetes properties, indicating that CLA play a versatile role in regulating metabolic pathways. Most studies have demonstrated that dietary CLA modulates energy repartition in favour of body fat reduction in finishing pigs(Reference Ostrowska, Muralitharan and Cross2–Reference Jiang, Zhong and Zheng8). However, there have been reports that CLA did not affect or increased backfat thickness in pigs(Reference Ramsay, Evock-Clover and Steele9, Reference Gatlin, See and Larick10).
Interestingly, dietary CLA was found to increase intramuscular fat content(Reference Meadus, Maclnnis and Dugan4, Reference Sun, Zhu and Qiao7, Reference Jiang, Zhong and Zheng8, Reference Joo, Lee and Ha11, Reference Corino, Lo Fiego and Macchioni12), suggesting that effects of CLA on lipid metabolism varied with fat depots. However, other investigators reported either no change(Reference Tischendorf, Schöne and Kirchheim6) or even a decrease in intramuscular fat content(Reference Thiel-Cooper, Parrish and Sparks3, Reference Dugan, Aalhus and Schaefer13) in CLA-supplemented pigs. Thus, the biological effects of CLA on porcine subcutaneous and intramuscular adipose tissues as well as on skeletal muscle are probably complex. At present, mechanisms responsible for the actions of CLA are largely unknown.
Proteomics technology can simultaneously determine many proteins in tissues(Reference Wang, Li and Dangott14), including pig skeletal muscle(Reference Wang, Chen and Li15). This method is useful to identify proteins related to meat quality attributes(Reference Hwang, Park and Kim16–Reference Morzel, Terlouw and Chambon19), pathophysiology(Reference Milli, Cecconi and Campostrini20, Reference Sinha, Srivastava and Singh21) and nutrient metabolism(Reference Grider, Mouat and Scrimgeour22–Reference Wang, Ou and Yin24). Therefore, this approach was employed in the present study to determine proteome changes in Longissimus muscle of finishing pigs supplemented with 0 or 25 g CLA/kg diet, which was previously shown to differ in lipid content, according to CLA treatment.
Materials and methods
Animals and diets
The care and use of all animals in the present study were approved by the Institute of Animal Science in Guangdong Academy of Agricultural Sciences. Animal design, growth performance and carcass data of experimental pigs have been described in a joint experiment(Reference Jiang, Zhong and Zheng8). Briefly, seventy-two Duroc × Landrace × Large White gilts, free of the halothane gene, were assigned to three treatments with six pens in each treatment and four pigs in each pen. The initial and final body weights of pigs were approximately 60 and 100 kg, respectively (Table 2). All the pigs had free access to water and maize–soybean meal-based feed during this period. Treatments differed according to CLA supplementation in the diets. In the present experiment, only the two groups that were extremes for the content of CLA in the diet (i.e. 0 and 25 g/kg, respectively) were considered. The intermediate group supplemented with 12·5 g CLA/kg diet(Reference Jiang, Zhong and Zheng8) was abandoned to exclude the interaction between cis-9, trans-11 CLA, trans-10, cis-12 CLA and cis-9, cis-12 linoleic acid in the diets. The diets that met National Research Council-recommended nutrient requirements(25) and compositions are listed in Table 1. All the diets were supplemented with sunflower oil containing 667·3 mg cis-9, cis-12 linoleic acid/g fatty acids (supplied by Zhuhai Changqingshu Food Company, Limited, Zhuhai, China), and 0 (control) or 25 g/kg CLA (369·1 mg cis-9, trans-11 CLA, 374·6 mg trans-10, cis-12 and 53·7 mg other isomers/g fatty acids).
Table 1 Composition of experimental diets (g/kg)
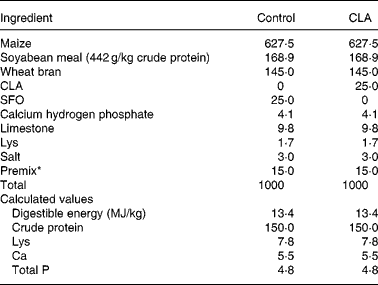
CLA, conjugated linoleic acid; SFO, sunflower oil.
* Providing the following per kg diet: 40 mg Cu as CuSO4, 133 mg Fe as FeSO4, 145 mg Zn as ZnSO4, 58 mg Mn as MnSO4, 3 mg iodine as CaIO3, 0·3 mg Se as NaSeO3, 858 μg vitamin A, 12·5 μg vitamin D3, 22 mg vitamin E, 5 mg riboflavin, 16 mg pantothenic acid, 0·3 mg folic acid, 20 mg niacin and 22 μg vitamin B12.
Collection of pig tissues
Pigs from control and 25 g/kg dietary CLA-treated groups were randomly selected (n 6) to analyse the intramuscular fat content and muscle proteome changes. The animals were killed by electrical stunning and exsanguination. They were then scalded, dehaired, eviscerated and divided into halves. Samples of Longissimus muscle from the ninth to tenth rib were immediately obtained and frozen in liquid N2 for proteomics analysis and fatty acid composition determination. Samples of Longissimus muscle from the eleventh to the last rib were collected and stored at − 20°C to determine the intramuscular fat content.
Measurement of intramuscular fat content
The muscle samples were minced and freeze dried to produce muscle powders. The content of intramuscular fat from these muscle powders was measured using petroleum diethyl ether extract using the Soxtec 2055 fat extraction system (Foss Tecator AB, Höganäs, Sweden) according to the Association of Official Analytical Chemists official method 991.36(26). The boiling point of petroleum diethyl ether used in the present study was 30–60°C.
Fatty acid composition analysis
The fatty acid composition of CLA and sunflower oil was analysed according to our previous method(Reference Jiang, Zhong and Zheng8). Each fatty acid methyl ester was determined as the percentage of a specific peak area to total fatty acid methyl ester peak areas.
Main chemicals for proteome analysis
Phenylmethanesulfonyl fluoride and trypsin were purchased from Roche Applied Sciences (Hoffmann-La Roche AG, Basel, Switzerland). Dithiothreitol, Tris, Coomassie Brilliant Blue G-250, urea, agarose and acrylamide were obtained from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). Thiourea was purchased from Acros Organics Geel (Liège, Belgium). Other chemicals were obtained from Sigma (Sigma-Aldrich, St Louis, MO, USA).
Two-dimensional electrophoresis
Frozen muscle sample (approximately 1 g) was crushed into powders with mortar and pestle in liquid N2. Powders (1 g) were homogenised in 3 ml lysis buffer consisting of 8 m-urea, 2 m-thiourea, 65 mm-3-((3-cholamidopropyl) dimethylammonio)- 1-propane sulfonate, 60 mm-dithiothreitol, 40 mm-Tris, pH 8·6, and 0·5 mm-phenylmethanesulfonyl fluoride by sonication. The solution was centrifuged at 15 000 g for 45 min at 4°C. The non-fat supernatant fluid was centrifuged at 40 000 g for 45 min at 4°C. Extracts were stored at − 80°C until used. Protein concentration was determined as described previously(Reference Bradford27), and bovine serum albumin was used as a standard.
Immobilised pH gradient isoelectric focusing was performed in a Protean isoelectric focusing Cell (Bio-Rad Laboratories, Inc.) using Bio-Rad ReadyStrip (17 cm, pH 3–10, non-linear), according to the manufacturer's instructions. The running program was set as described previously(Reference Morzel, Terlouw and Chambon19). Low voltage (100 V) was gradually increased to 8000 V until a total of 60 000 Vh. A total of 800 μg protein was loaded onto the strip. Before isoelectric focusing, the strips were rehydrated overnight with a buffer consisting of 8 m-urea, 2 m-thiourea, 65 mm-3-((3-cholamidopropyl) dimethylammonio)-1-propane sulfonate, 60 mm-dithiothreitol and 40 mm-Tris, pH 8·6. After completion of isoelectric focusing, the strips were equilibrated in 10 ml equilibration solution as described previously(Reference Junghans, Kaehne and Beyer28), and the second dimension SDS-PAGE was carried out in a Protean II xi Cell (Bio-Rad Laboratories, Inc.) with 12 % polyacrylamide (36·5:1 ratio of acrylamide to bisacrylamide). The program was set as 12 mA/gel for 30 min and 25 mA until bromophenol blue reached the end of gel at 12°C. Gels were stained by 0·1 % hot Coomassie Brilliant Blue G-250 in 50 % ethanol for 30 min and were then destained with 25 % ethanol and 5 % acetic acid for 30 min and three times.
Image analysis
Destained gels were used immediately for image analysis or stored at − 80°C for future analysis. Triplicate gels from one pig sample were scanned with Image Master 2D Pt Software v5.0 (GeneBio, Amersham Biosciences, Little Chalfont, Bucks, UK) to analyse qualities and numbers of protein spots. After image scanning, two of three gels were selected according to the better protein spot pattern and higher spot number. Consequently, twelve sample gels from six pigs (two sample gels from each pig) in each treatment were obtained. The processing and quantification of the selected protein spots were performed as described previously(Reference Xu, Tang and Li29).
Protein digestion and HPLC-MS/MS analysis
Protein spots were excised with 1 ml pipette tips and sterile blade from the gels, and were transferred to sterile 1·5 ml Eppendorf tubes for tryptic digestion. Proteins were digested by trypsin(Reference Jensen, Larsen and Roepstorff30), followed by the HPLC-MS/MS analysis as described previously(Reference Puente, Carrière and Kelly31) with some modifications. Briefly, extracts of peptides were dissolved in 20 μl solution consisting of 2 % acetonitrile and 0·1 % ammonia, and they were loaded into a 0·3 × 5 mm C18 micro pre-column (Agilent Technologies, Wilmington, DE, USA). The effluents entered a 75 × 150 mm C18 analysis column (Agilent Technologies), and the peptides were separated by gradient elution (2–40 % acetonitrile and 0·1 % ammonia for 20 min). The MS spectra were automatically acquired by Esquire 3000 plus and processed by Esquire Data Analysis Software (version 3.1). Proteins were identified using the Ms-Fit search engine (http://prospector.ucsf.edu/prospector/mshome.htm) against National Center for Biotechnology Information non-redundant and Swiss-Prot databases. The parameters adopted for searching were as follows: taxonomy: mammals; enzyme: trypsin; peptide tolerance: ± 1·2 Da; MS/MS tolerance: ± 0·6 Da, allowed up to one missed cleavage; peptide charge: 1+; monoisotopic: average. The National Center for Biotechnology accession number, theoretical molecular weight/isoelectric point, determined molecular weight/isoelectric point, molecular weight search score, fold change in the dietary CLA group, molecular function, sequence coverage and P value from statistical analysis between the control and dietary CLA groups were presented to identify each protein. The molecular weight search score ≥ 50 was considered a significant match (P < 0·05). Each identified protein was annotated with its biological process in the gene ontology and AmiGO term tools, according to the source of identified protein. The proteins without gene ontology annotation were sorted according to the literatures available.
Statistical analysis
Data on intramuscular fat content were analysed by one-way ANOVA (Statistical Analysis Software version 8.1; SAS Institute, Inc., Cary, NC, USA) using treatment as the main effect (0 v. 25 g CLA/kg diet). Body weight was initially added as a covariate in the model; however, it was NS (P = 0·95) and was then removed from the final analysis. The abundance of protein spots in two-dimensional gels was analysed by t test between the two treatments. Pearson correlation coefficients were calculated between the intramuscular fat content and the abundance of some protein spots (5′-AMP-activated protein kinase subunit gamma-3 (AMPK-γ3), medium-chain specific acyl-CoA dehydrogenase (MCAD), carbonic anhydrase 3 (CA3), stearoyl-CoA desaturase (SCD) and aspartate aminotransferase (AST)). Probability values ≤ 0·05 were considered significant.
Results
Carcass traits
Feeding pigs with supplemental 25 g CLA/kg diet resulted in a 54 % greater intramuscular fat content when compared with controls at final slaughter (Table 2).
Table 2 Growth performance, intramuscular fat content and fatty acid composition in Longissimus muscle in pigs fed dietary conjugated linoleic acid (CLA)*
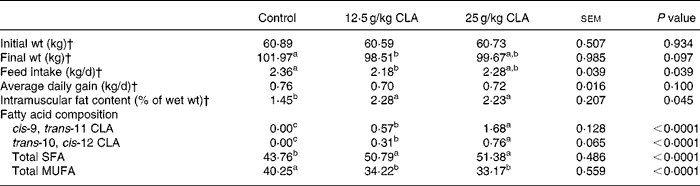
a,b,c Mean values with unlike superscript letters in the same line differ significantly (P < 0·05).
* Reference to Jiang et al. (Reference Jiang, Zhong and Zheng8).
† The data of initial weight and final weight were presented as means of twenty-four pigs. The data of feed intake and average daily gain were presented as six pens. The data of intramuscular fat content were presented as six pigs.
The proteomes in Longissimus muscle
After image analysis, a total of 589 (sd 41) protein spots were detected in the control gels, and 598 (sd 36) protein spots were detected in the CLA-supplemented gels. A representative pattern of protein separation is shown in Fig. 1. Compared with the control group, dietary CLA significantly affected (P < 0·05) the abundance of twenty-six proteins, which are marked in Fig. 1. Of these proteins, fourteen and twelve of them exhibited increased (Table 3) and decreased (Table 4) abundance, respectively. Proteins with a greater abundance were found to participate in six different biological processes, whereas those with a lower abundance were matched to four biological processes. The striking difference was observed for fatty acid oxidation that was up-regulated and for fatty acid synthesis that was down-regulated, respectively, in CLA-treated group v. controls. Transport and miscellaneous processes were shared by the two protein lists, whereas energy expenditure was found only in proteins with a greater abundance in the CLA-treated pigs than that in the controls.

Fig. 1 Proteomic image of a representative Longissimus muscle sample from pigs fed a diet supplemented with conjugated linoleic acid (25 g/kg diet). Proteins were separated by pH 3–10 in the first dimension and 12 % SDS-PAGE in the second dimension. Proteins with significant changes in abundance compared with the unsupplemented control are indicated by numbers in the image.
Table 3 Proteins with a greater abundance in pig skeletal muscle after dietary conjugated linoleic acid (CLA) supplementation

No., number; MW, molecular weight (Da); pI, isoelectric point; MOWSE, molecular weight search; SC, sequence coverage (%); GO, gene ontology.
* Fold change, relative volume of spot in the dietary CLA group/relative volume of spot in the control group.
† P value, statistical analysis between the control and dietary CLA groups.
Table 4 Proteins with a lower abundance in pig skeletal muscle after dietary conjugated linoleic acid (CLA) supplementation

No., number; MW, molecular weight (Da); pI, isoelectric point; MOWSE, molecular weight search; SC, sequence coverage (%); GO, gene ontology.
* Fold change, relative volume of spot in the dietary CLA group/relative volume of spot in the control group.
† P value, statistical analysis between the control and dietary CLA groups.
By Pearson correlation analysis, it was found that the enhanced intramuscular fat content was positively correlated with the increased CA3 and AST protein abundance in Longissimus muscle (CA3, r 0·774, P = 0·003; AST, r 0·702, P = 0·011). No significant correlation was observed between intramuscular fat content and each of the following proteins: SCD, MCAD and AMPK-γ3 (P>0·05).
Discussion
Results of the present study indicate that dietary supplementation with CLA increases the abundance of proteins related to energy expenditure and fatty acid oxidation. A major function of these proteins is to degrade macronutrients to provide energy. Our finding is consistent with the reports that whole-body energy expenditure is enhanced by dietary CLA in mice(Reference West, Delany and Camet32, Reference West, Blohm and Truett33) and rats(Reference Choi, Jung and Park34). Recently, Zhai et al. (Reference Zhai, Liu and Li35) have demonstrated that although both cis-9, trans-11 CLA and trans-10, cis-12 CLA increased energy expenditure in cultured 3T3-L1 preadipocytes, only trans-10, cis-12 CLA has a stimulatory effect on fatty acid oxidation. In the present study performed in vivo with pigs fed a mixture of CLA isomers, we found a greater abundance in both proteins involved in energy expenditure such as creatine kinase and creatine kinase M-type playing a key role in the maintenance of energy homeostasis(Reference Hollung, Grove and Færgestad36, Reference Abnous and Storey37) and in the fatty acid oxidation pathway. Especially, a greater abundance in AMPK-γ3, i.e. the predominant AMPK-γ isoforms in white muscle, was also observed here in the muscle of pigs fed CLA, an observation that closely matches with the increase in the same protein reported in 3T3-L1 adipocytes treated with trans-10, cis-12 CLA(Reference Zhai, Liu and Li35). Activation of AMPK-γ3 alone or in heterodimerisation with α2/β2 forms results in oxidation of fatty acids(Reference Barnes, Marklund and Steiler38, Reference Birk and Wojtaszewski39). MCAD is responsible for the first step of β-oxidation of fatty acids in mitochondria(Reference Schuck, Ferreira and Moura40). Notably, as demonstrated for skeletal muscle in the present study, MCAD mRNA levels were elevated in the livers of mice supplemented with CLA(Reference Peters, Park and Gonzalez41) and in rats fed α-linoleic acid-rich diacylglycerol(Reference Murase, Aoki and Tokimitsu42). Likewise, Nall et al. (Reference Nall, Wu and Kim43) recently found that CLA supplementation increased oxidation of fatty acids and glucose in rat liver and skeletal muscle. Thus, MCAD may play a role in mediating the effect of CLA on pigs. The CLA effects observed in the present study are, however, of a shorter magnitude than those observed in vitro for adipocyte cell line(Reference Zhai, Liu and Li35), considering the lower number of spots as involved in these two pathways having a differential abundance in the present study.
Dietary CLA also enhanced protein abundance related to amino acid metabolism. Though mitochondrial AST plays an important role in amino acid metabolic process, it is also a transport protein that is identical to a plasma membrane-bound fatty acid-binding protein(Reference Berk, Wada and Horio44, Reference Stump, Zhou and Berk45). The greater AST abundance observed in the muscle of pigs fed the CLA mixture was also observed in 3T3-L1 cells cultured in the presence of trans-10, cis-12 CLA but not in the presence of cis-9, trans-11 CLA(Reference Zhai, Liu and Li35). Some evidence suggests that plasma membrane-bound fatty acid binding protein/AST plays a role in the transmembrane movement and oxidation of long-chain fatty acids (LCFA) for energy supply(Reference Clarke, Miskovic and Han46). It is possible that an increased abundance of AST in muscle may facilitate the entry of LCFA into muscle cells where LCFA could either undergo mitochondrial β-oxidation or be incorporated into TAG(Reference Hauton, Bennett and Evans47). However, muscle relies predominantly on an exogenous source of LCFA(Reference Koonen, Glatz and Bonen48), due to limited intramuscular storage sites for LCFA utilisation. The exogenous LCFA may in part be derived from backfat. Unfortunately, recent studies have paid more attention to relative than absolute changes of fatty acids in muscle and backfat of pigs fed CLA(Reference Sun, Zhu and Qiao7–Reference Ramsay, Evock-Clover and Steele9, Reference Joo, Lee and Ha11), which makes it difficult to postulate that exogenous LCFA were used to enhance lipid storage in muscle. In support of this hypothesis, we found a positive correlation between the intramuscular fat content and AST. Quantitative determination of the change in fatty acid content of muscle and backfat is still needed to confirm the function of AST in lipid storage.
In support of a role for CLA in stimulating intramuscular lipid accretion, the results of the present study indicate that the abundance of CA3 is increased in muscle in response to dietary CLA supplementation. CA3 catalyses the hydration of CO2 to generate bicarbonate and hydrogen ions for enhancement of fatty acid synthesis and for maintenance of pH homeostasis. Thus, some studies have demonstrated that the CA3 protein is associated with intramuscular fat content(Reference Wang, Zhu and Wang49) and lipid accretion(Reference Wu, Zhou and Deng50), which agreed with our present results from correlation analysis. Consequently, an increase in CA3 abundance may be the factor for up-regulation of intramuscular fat levels in CLA-supplemented pigs.
In the present study, dietary CLA also decreased the relative abundance of proteins involved in fatty acid synthesis. The SCD plays a key role in regulating the synthesis of MUFA, especially oleate and palmitoleate. These two fatty acids are major MUFA components in membrane phospholipids, TAG, wax esters and cholesterol esters(Reference Ntambi and Miyazaki51). Growing evidence shows that dietary CLA affects the ratio of SFA to MUFA in skeletal muscle and adipose tissues(Reference Sun, Zhu and Qiao7, Reference Jiang, Zhong and Zheng8, Reference Joo, Lee and Ha11, Reference Corino, Lo Fiego and Macchioni12, Reference Corino, Magni and Pastorelli52, Reference Weber, Richert and Belury53) due to the inhibition of desaturase. At present, there is limited information about SCD expression or activity in pig skeletal muscle, despite numerous studies of this gene in 3T3-L1 adipocytes(Reference Choi, Kim and Han54), mouse liver and isolated hepatic cells(Reference Lee, Pariza and Ntambi55), human HepG2 cells(Reference Choi, Park and Pariza56) and human breast cancer cells(Reference Choi, Park and Storkson57). Moreover, a reduced SCD abundance was observed in 3T3-L1 cells by trans-10, cis-12 CLA after proteomics analysis(Reference Zhai, Liu and Li35). In adipose tissue, depressed SCD enzyme activity was in part responsible for the depression of adiposity by CLA(Reference Smith, Hively and Cortese58). However, a role for SCD in muscle may be different from that in liver and adipose tissue. According to the idea of Doran et al. (Reference Doran, Moule and Teye59), future studies are warranted to test the role of SCD in muscle and adipose tissue.
Another new and important observation from the present study is that dietary supplementation with CLA affected the expression of proteins related to the immune defence system (e.g. IL-4) and the nutrient transport system (e.g. albumin). For example, albumin would facilitate transport of LCFA to liver and muscle for oxidation and lipid synthesis, respectively. In addition, a reduction in IL-4 would attenuate inflammatory responses and, therefore, oxidative stress in tissues. This is expected to result in improved pork quality. However, spots (no. 39 and 31) named with albumin from rat and cat, respectively, and spots (no. 4 and 19) named with calsequestrin-2 (fragment) from pig had different abundance expression by dietary CLA. The difference may be derived from different sequence coverage to produce different entire amino acids composition between the protein spots with the same name. Furthermore, the spots with the same name exhibited different molecular weight and isoelectric point value.
In conclusion, dietary CLA enhanced the abundance of proteins related to energy metabolism, fatty acid oxidation and synthesis, amino acid metabolism, defence and transport. Increases in the abundance of CA3 and AST in Longissimus muscle may contribute to increased intramuscular fat content in finishing pigs.
Acknowledgements
There were no conflicts of interest in the present study. This research was supported by the National Natural Science Foundation of China (30400318) and the National ‘973’ project of China (2004CB117500). We sincerely thank Guoyao Wu, Professor of Texas A&M University, for help in the revision of our paper. W. Z. and Z. J. were responsible for experiment arrangement and for writing the manuscript. C. Z. was responsible for data analysis. Y. L., L. Y. and S. Z. were responsible for analysing the samples.







