Depression is one of the most prevalent mental health disorders and a leading cause of disability worldwide. It has been associated with poor quality of life, physical decline, higher risk of premature death and a large economic burden( 1 , Reference Doris, Ebmeier and Shajahan 2 ). In this context, depression is a major global public health problem, and reducing its prevalence by acting on associated modifiable lifestyle factors, including diet, is of major importance( 3 ).
The relationship between the overall quality of the diet and the risk of depression or depressive symptoms has been evaluated in several studies, mostly using a posteriori dietary patterns based on correlations in observed dietary data. Overall, healthier diets (characterised by high consumption of plant foods, whole grain products, olive oil and fish) have been associated with a decreased risk of depression or depressive symptoms, whereas unhealthy Western eating habits (characterised by high consumption of sweet and fatty products, processed meats and refined grains products) have been associated with an increased risk of depression or depressive symptoms( Reference Rahe, Unrath and Berger 4 – Reference Li, Lv and Wei 6 ).
To prevent chronic diseases and to promote overall health, nutritional recommendations have been issued by health authorities, and adherence to these recommendations can be estimated using a priori-defined dietary indexes. In France, based on the recommendations of the Programme National Nutrition Santé (PNNS)( Reference Hercberg, Chat-Yung and Chaulia 7 ) and on the national recommended dietary allowances( Reference Martin 8 ), the Programme National Nutrition Santé-Guideline Score (PNNS-GS) was developed to measure adherence to dietary recommendations for the general population( Reference Estaquio, Kesse-Guyot and Deschamps 9 ), and the Probability of Adequate Nutrient Intake Dietary score (PANDiet) to measure compliance with the recommended nutrient intakes( Reference Verger, Mariotti and Holmes 10 ). Besides those mentioned earlier, various a priori dietary scores have been developed to date, including different versions of the healthy eating index (HEI), the dietary quality index-international (DQI-I), the Dietary Approaches to Stop Hypertension and various scores measuring adherence to the Mediterranean Diet (MD)( Reference Arvaniti and Panagiotakos 11 ). Among them, only scores reflecting adherence to the MD and the HEI have been widely studied in associations with depression. Overall, the studies that have investigated the association between adherence to the MD and depression or depressive symptoms showed a protective effect( Reference Psaltopoulou, Sergentanis and Panagiotakos 12 – Reference Adjibade, Assmann and Andreeva 18 ). Other cross-sectional( Reference Saneei, Hajishafiee and Keshteli 19 – Reference Saneei, Esmaillzadeh and Keshteli 24 ) and some prospective studies( Reference Sanchez-Villegas, Henriquez-Sanchez and Ruiz-Canela 15 , Reference Collin, Assmann and Andreeva 25 – Reference Akbaraly, Sabia and Shipley 27 ) have also evaluated the associations between different a priori dietary scores (measuring adherence to national dietary guidelines) and the risk of depression or depressive symptoms, but only one study has compared such associations across different diet quality scores( Reference Sanchez-Villegas, Henriquez-Sanchez and Ruiz-Canela 15 ). Overall, the different versions of the HEI( Reference Sanchez-Villegas, Henriquez-Sanchez and Ruiz-Canela 15 , Reference Saneei, Hajishafiee and Keshteli 19 – Reference Kuczmarski, Cremer and Hotchkiss 22 , Reference Saneei, Esmaillzadeh and Keshteli 24 , Reference Akbaraly, Sabia and Shipley 27 ) and the Australian Recommended Food Score( Reference Lai, Hure and Oldmeadow 26 ) were significantly inversely associated with the risk of depression or depressive symptoms, whereas the Ireland food pyramid recommendations( Reference Meegan, Perry and Phillips 23 ) were not significantly associated with the risk of depressive symptoms. The PNNS-GS was also significantly inversely associated with recurrent depressive symptoms( Reference Collin, Assmann and Andreeva 25 ). Available studies investigating the association of the HEI, or modified versions, with the risk of depression were mostly cross-sectional, and few studies were based on non-American population. Thus, such relationships should be replicated in other settings.
In addition, several vitamins and minerals have been associated with depression in various studies( Reference Lai, Moxey and Nowak 28 , Reference Lim, Kim and Kim 29 ). However, most dietary scores do not include micronutrients, which may hinder the capacity of such scores to adequately reflect achievement of recommended nutrient intakes. To our knowledge, no study has yet examined the association between scores based on recommended nutrient intakes (e.g. PANDiet) or both recommended nutrient and food intakes (e.g. DQI-I) and the risk of depression or depressive symptoms. As nutritional recommendations were not primarily issued to prevent depression, further prospective studies using different nutritional scores are needed to examine whether some specific scores may perform better than others, thus informing preventive strategies.
In the present study, we thus aimed at investigating the prospective association between the overall quality of the diet measured by different scores reflecting adherence to food and nutrient recommendations, including the modified version of the PNNS-GS (mPNNS-GS), the Alternative Healthy Eating Index-2010 (AHEI-2010), PANDiet and DQI-I and the risk of incident depressive symptoms in a large French prospective cohort.
Methods
Study population
We used data from the NutriNet-Santé study, a web-based observational cohort launched in France in 2009, which aims to investigate the relationship between nutrition and health, as well as the determinants of dietary behaviours and nutritional status. The design and methodology of the study have been described in detail elsewhere( Reference Hercberg, Castetbon and Czernichow 30 ). In brief, participants are adult volunteers (aged ≥18 years) with internet access recruited from the general population by a vast multimedia campaign. Upon enrolment and each year thereafter, participants are asked to complete a set of self-administered web-based questionnaires assessing socio-demographic factors, economic conditions, physical activity, dietary intake, anthropometrics and health status. The NutriNet-Santé study is conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of the French Institute for Health and Medical Research (IRB Inserm no. 0000388FWA00005831) and by the National Commission on Informatics and Liberty (CNIL no. 908450 and no. 909216). Electronic informed consent was obtained from all participants. The NutriNet-Santé study is registered in ClinicalTrials.gov (NCT03335644).
Depressive symptoms
Depressive symptoms were assessed using the French version of the validated self-administered Center for Epidemiologic Studies Depression (CES-D) scale( Reference Radloff 31 , Reference Führer and Rouillon 32 ) sent to all participants included in the NutriNet-Santé study two years after inclusion and every two years thereafter (with currently a maximum set of three completed CES-D questionnaires). The twenty items of the scale evaluate the frequency of depressive symptoms during the preceding week, using a four-point scale (0=‘<1 d’; 1=‘1–2 d’; 2=‘3–4 d’; and 3=‘5–7 d’). These are summed to yield a total score between 0 and 60 points, with higher scores denoting more depressive symptoms. The Cronbach’s coefficient α (used to assess the internal consistency or reliability of a test or scale( Reference Cronbach 33 )) was >0·80 for all three measures of the CES-D scale in our study, indicating good internal consistency.
The French validated cut-off value (CES-D ≥17 in men and ≥23 in women) was used to define the presence of depressive symptoms( Reference Führer and Rouillon 32 ). We defined incident cases of depressive symptoms as participants who were free of depressive symptoms at the first CES-D assessment and those who had depressive symptoms at least once during follow-up.
Dietary data and dietary scores computation
At enrolment and every 6 months thereafter, participants were invited to provide three non-consecutive dietary records, randomly assigned over a 2-week period (2 weekdays and 1 weekend day). All foods and beverages consumed at each eating occasion were reported via a validated web-based dietary record tool designed for self-administration( Reference Touvier, Kesse-Guyot and Mejean 34 – Reference Lassale, Castetbon and Laporte 36 ). Portion sizes were indicated using validated photographs( Reference Le Moullec, Deheeger and Preziosi 37 ), household measures or by indicating the exact quantity (g) or volume (ml). Dietary data from the first 2 years of follow-up (corresponding to the time window between baseline and the 1st CES-D assessment) were used in the present study. For each participant, daily mean food consumption was calculated from all available dietary records (mean number of recording days=8·03; sd=2·26), weighted according to the type of day (weekdays or weekend). Energy and nutrient intakes were estimated using the NutriNet-Santé composition table including more than 3000 food items( Reference NutriNet-Santé 38 ). In addition, weekly fish and seafood intake was estimated by a self-administrated frequency questionnaire and alcohol intake (g ethanol/d) was estimated using an alcohol consumption frequency questionnaire when no consumption was reported in dietary records.
Dietary under-reporting was identified using the method developed by Black( Reference Black 39 ), which states that for an individual of stable weight energy intake (EI) and energy expenditure (EE) are equal. Thus, the ratio between EI and BMR (EI:BMR) is equivalent to a physical activity level (PAL) for which a minimum cut-off limit below which it is impossible to remain in stable weight has been defined( Reference Goldberg, Black and Jebb 40 ). The EI:BMR was thus calculated for each participant, and dietary under-reporters were defined as participants who reported average energy consumption so that the PAL was below the minimum cut-off. Average EI was calculated from the EI of all validated dietary records and BMR was estimated using Schofield equations based on sex, age, weight and height( Reference Schofield 41 ). Two PAL cut-off limits were considered: a cut-off of 0·88 to identify the ‘extreme’ under-reporters who were systematically excluded and a cut-off of 1·55 for the remaining under-reporters. Of these, some participants who were identified as under-reporters were not considered as such (declaration of unusual consumption, diet for weight loss or recent weight loss >5 kg) and thus were not excluded
Overall diet quality was measured by using four different dietary scores including the mPNNS-GS, the AHEI-2010, the DQI-I and the PANDiet. The mPNNS-GS and AHEI-2010 mostly reflect food-based dietary guidelines( Reference Estaquio, Kesse-Guyot and Deschamps 9 , Reference Chiuve, Fung and Rimm 42 ), the PANDiet reflects nutrient-based reference recommendations( Reference Verger, Mariotti and Holmes 10 ) and the DQI-I reflects both food-based and nutrient-based recommendations( Reference Kim, Haines and Siega-Riz 43 ). The computation of each dietary score has been extensively described elsewhere( Reference Estaquio, Kesse-Guyot and Deschamps 9 , Reference Verger, Mariotti and Holmes 10 , Reference Chiuve, Fung and Rimm 42 , Reference Kim, Haines and Siega-Riz 43 ). It is noteworthy that, for each score, points were assigned using predefined cut-off points (portion sizes or recommended nutrient intakes)( Reference Estaquio, Kesse-Guyot and Deschamps 9 , Reference Verger, Mariotti and Holmes 10 , Reference Chiuve, Fung and Rimm 42 , Reference Kim, Haines and Siega-Riz 43 ). The description and scoring systems of the different scores are presented in Table 1 and detailed in the online Supplementary Material S1.
Table 1 Description and the scoring system of the investigated dietary scores
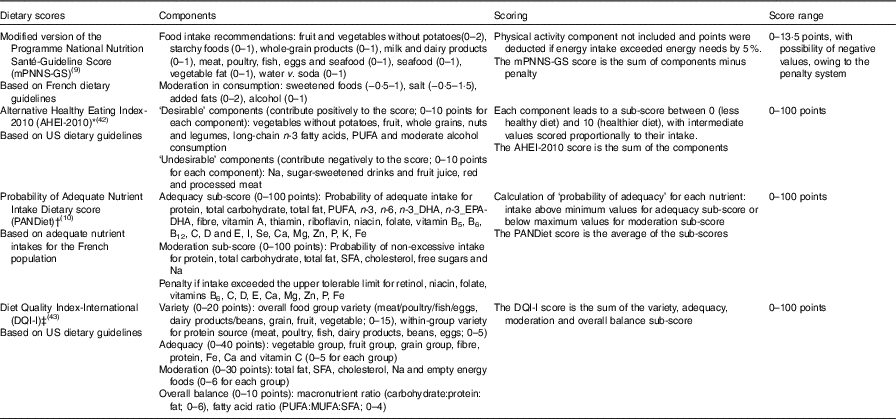
* We did not include trans-fatty acid intakes (not available in our study).
† Probability of adequate nutrient intake
![]() $$F\,\Big( {{{\overline{y} {\minus}r} \over {\sqrt {{\rm SD}r^{2} {\plus}{\rm SD}y^{2} \,/\,n} }}} \Big)$$
F (ranged from 0 to 1, where 1 represents a 100 % probability that the usual intake was adequate): ‘Probnorm’ function in SAS,
$$F\,\Big( {{{\overline{y} {\minus}r} \over {\sqrt {{\rm SD}r^{2} {\plus}{\rm SD}y^{2} \,/\,n} }}} \Big)$$
F (ranged from 0 to 1, where 1 represents a 100 % probability that the usual intake was adequate): ‘Probnorm’ function in SAS,
![]() $\overline{y} $
is the mean intake, sd
2
y the day-to-day variability of intake, n the number of dietary record days, r the nutrient reference value, sd
2
r the interindividual variability.
$\overline{y} $
is the mean intake, sd
2
y the day-to-day variability of intake, n the number of dietary record days, r the nutrient reference value, sd
2
r the interindividual variability.
‡ For nutrients included in the DQI-I computation, we used the recommended intakes for the French population (similar to those used in the PANDiet).
Baseline covariates
Socio-demographic data collected using the validated web-based questionnaire( Reference Vergnaud, Touvier and Mejean 44 ) provided data on sex, date of birth, marital status (living alone, cohabiting or separated/divorced/widowed), educational level (less than high school diploma, high school diploma or university level), occupational categories (never-employed/other activity, self-employed, employee, intermediate profession and managerial staff), residential area (rural or urban) and smoking status (never, former or current smoker). Monthly household income was also provided and estimated per consumption unit (CU) according to a weighting system where one CU is attributed for the first adult in the household, 0·5 CU for other persons aged 14 years or older and 0·3 CU for children under 14 years( 45 ). Categories of monthly household income per CU were defined as follows: <1200, 1200–1800, 1800–2700, >2700 euros and a category of participants who refused to disclose their income. Weight and height data were collected by a validated self-administered anthropometric questionnaire( Reference Lassale, Peneau and Touvier 46 ) and BMI was calculated as the ratio of weight to squared height (kg/m2). Participants were classified as underweight or normal weight (BMI<25 kg/m2), overweight (25≤BMI<30 kg/m2) or obese (BMI≥30 kg/m2)( 47 ). Physical activity was assessed using a short form of the French version of the International Physical Activity Questionnaire( Reference Craig, Marshall and Sjostrom 48 ), a validated tool based on three specific types of physical activity: walking, activities of moderate intensity and activities of vigorous intensity. EE expressed in metabolic equivalent task minutes per week was estimated and classified as low physical activity (<30 min of physical activity; equivalent to brisk walking/d), moderate physical activity (≥30 and <60 min) or high physical activity (≥60 min), according to the French guidelines for physical activity( Reference Estaquio, Kesse-Guyot and Deschamps 9 ). Prevalent and incident cases of cancer and CVD were self-reported during follow-up and validated by a medical committee. Type 2 diabetes was also self-reported.
In this study, data were missing for some covariates (7 for marital status, 70 for occupational categories, 309 for residential area, 191 for educational level and 411 for physical activity); however, the proportion of missing values was <1 % for all variables. Missing data for covariates were handled using the Hot Deck method, which consists of replacing the missing value with that of respondents with the same characteristics( Reference Andridge and Little 49 ).
Statistical analysis
Selection of the study sample
This study focused on participants who received at least two times the CES-D questionnaire (included between 2009 and 2011, n 124 925). Among the 35 782 eligible participants for inclusion (participants with at least two completed CES-D questionnaires during follow-up and without depressive symptoms at the first CES-D measurement to ensure a prospective design), we included participants who had available data for computation of the dietary scores and who had not reported depression or treatment with anti-depressants during the dietary data collection (before the first CES-D assessment). Thus, a final study sample of 25 837 men and women was obtained (Fig. 1).

Fig. 1 Flow chart of participant selection. Center for Epidemiologic Studies Depression (CES-D) Scale.
Characteristics of the participants
Participants included in this study were compared with excluded eligible participants using χ 2 tests or t tests as appropriate. Participants’ characteristics and nutritional factors were compared across tertiles of the mPNNS-GS using linear contrast or Cochran–Mantel–Haenszel tests. For descriptive purposes, nutrient intakes were energy-adjusted using the residual method( Reference Willett and Stampfer 50 ).
Statistical models
The associations between the mPNNS-GS, PANDiet, DQI-I and the AHEI-2010 (all modelled as tertiles to simplify the interpretation of results as low, medium and high adherence) and incident depressive symptoms were assessed using Cox proportional hazards regression models for interval censored data. Hazard ratios (HR) and 95 % CI were estimated. Linear trend tests across the tertiles of dietary scores were performed by modelling the tertiles of dietary scores as ordinal variables. We also modelled the dietary scores as standardised continuous variables (individual score value minus the mean score value and divided by sd of the population) for comparison purposes. Age was used as the primary time-scale variable, with entry time defined as the age at the first CES-D measurement. For non-cases, exit time was defined as the age at last completed CES-D questionnaire, whereas for cases it was defined as the average of the age between the first occurrence of depressive symptoms and the age at the previous measurement( Reference Finkelstein 51 ). The first model was adjusted for age, sex, marital status, educational level, occupational categories, monthly household income per CU, residential area, EI without alcohol, number of recording days and inclusion month. The second model was additionally adjusted for smoking status, physical activity and BMI (continuous variable). A final model (model 3) was performed to additionally account for cancer, type 2 diabetes and cardiovascular events during follow-up.
Sensitivity analyses
Supplementary analyses were performed to test the robustness of our findings. First, we evaluated the association between the dietary scores and incident depressive symptoms among the following: (A) participants who did not report treatment with anti-depressants during follow-up, as the use of anti-depressants could conceal the presence of depressive symptoms; and (B) participants who had completed ≥6 d of dietary records. Second, we defined the presence of depressive symptoms in both men and women by using a cut-off value of 16( Reference Radloff 31 ), and an alternative cut-off value of nineteen validated for the French population( Reference Morin, Moullec and Maiano 52 ). All statistical analyses were conducted using SAS (version 9.4; SAS institute Inc.) with a significance level of 0·05 for two-sided tests.
Results
Sample characteristics
The study sample consisted of 19 985 women and 6240 men with a mean age of 45·5 (sd 13·9) and 53·0 (sd 13·5) years, respectively, at inclusion. Compared with excluded participants (n 9557) from the eligible population (participants with at least two completed CES-D questionnaires during the follow-up and without depressive symptoms at the first CES-D measurement), included participants had higher levels of education, were more physically active, more likely to be men, co-habiting, managerial staff and never smokers. They were also more likely to have a BMI value in the normal-weight range, a household income per unit consumption ≥1800 euros and less likely to have a chronic disease (online Supplementary Table S1).
During follow-up (mean follow-up=5·9 years, sd 1·2), we identified 2166 first cases of depressive symptoms. Baseline characteristics of our study population across tertiles of the mPNNS-GS are presented in Table 2. Participants with high adherence (tertile 3) compared with those with low adherence (tertile 1) were older, more physically active, less likely to live alone and more likely to be women, non-smokers, managerial staff or having an intermediate profession, to have a household income per unit consumption ≥1800 euros and a chronic disease. In addition, a higher mPNNS-GS was associated with a higher EI from carbohydrates and proteins, higher intakes of total PUFA, fibre, most vitamins and minerals, but a lower EI from lipids and a lower intake of saturated and MUFA (Table 3). Similar associations were observed for the other scores examined in this study, with the exception of the AHEI-2010, which was positively associated with intakes of MUFA (online Supplementary Table S2).
Table 2 Baseline characteristics of 26 225 participants according to the tertiles of the modified Programme National Nutrition Santé Guideline Score (mPNNS-GS), NutriNet-Santé study(Numbers and percentages; mean values and standard deviations)
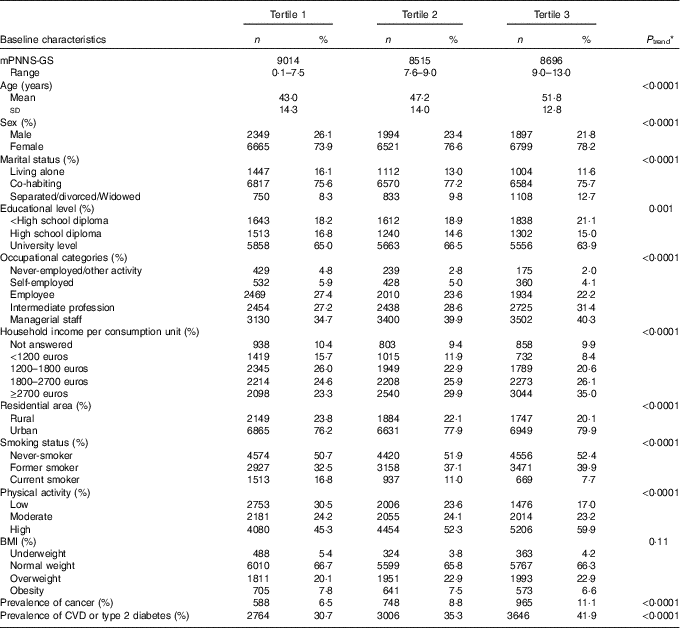
* P trend values are based on linear contrast or Cochran–Mantel–Haenszel tests.
Table 3 Baseline nutritional factors of 26 225 participants according to the tertiles of the modified Programme National Nutrition Santé Guideline Score (mPNNS-GS), NutriNet-Santé study(Mean values and standard deviations)
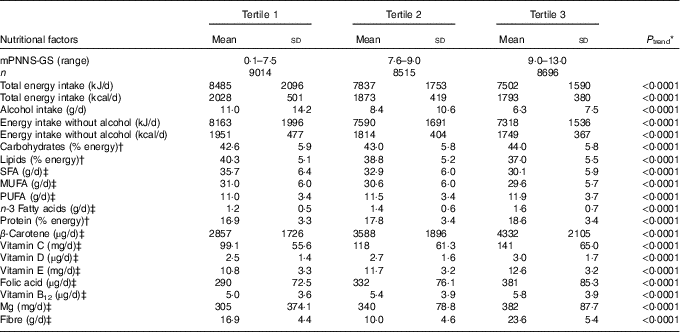
* P trend values are based on linear contrast.
† Values are percentages of total daily energy intake (without alcohol).
‡ Values were adjusted for energy intake without alcohol using the residual method.
Association between the dietary scores and incident depressive symptoms
All dietary scores were significantly but rather modestly correlated with each other, with the highest correlation (0·71) found between the PANDiet and the DQI-I and the lowest (0·52) between the AHEI-2010 and the PANDiet (online Supplementary Table S3). The associations between the dietary scores and depressive symptoms are presented in Table 4. All dietary scores were inversely associated with incident depressive symptoms, but the association was non-significant for AHEI-2010. In the fully adjusted model, an increase of 1 sd in the mPNNS-GS, PANDiet and DQI-I was, respectively, associated with an 8 % (95 % CI 4, 13, P=0·0002), 5 % (95 % CI 1, 9, P=0·02) and 9 % (95 % CI 5, 13, P<0·0001) reduction in the risk of depressive symptoms. When the scores were modelled as tertiles, the highest adherence to the mPNNS-GS, PANDiet and DQI-I was, respectively, associated with a 20 % (95 % CI 10, 28, P=0·0001), 12 % (95 % CI 2, 21, P=0·02) and 21 % (95 % CI 12, 30, P<0·0001) reduction in the risk of depressive symptoms compared with the lowest tertile.
Table 4 Association between the dietary scores and incident depressive symptoms in 26 225 participants, NutriNet-Santé study(Hazard ratios (HR) and 95 % CI)

mPNNS-GS modified Programme National Nutrition Santé Guideline Score; PANDiet Probability of Adequate Nutrient intake Dietary score; DQI-I Diet Quality Index-International; AHEI-2010, Alternative Healthy Eating Index-2010.
* HR for the increase of 1 sd.
† P for linear relation (dietary score as a continuous variable).
‡ Adjusted for age, sex, marital status, educational level, occupational categories, household income per consumption unit, residential area, energy intake without alcohol, number of recording days and inclusion month.
§ Adjusted for all variables in model 1 and smoking status, physical activity and BMI.
|| Adjusted for all variables in model 2 and health events during follow-up (cancer, type 2 diabetes and cardiovascular events).
Sensitivity analyses
In sensitivity analyses including only participants who were not treated with anti-depressants during follow-up, findings were not modified (online Supplementary Table S4). Applying a cut-off value of 16 or 19 to define depressive symptoms in both men and women did not substantially modify the observed associations with continuous standardised scores, but when the scores were evaluated in tertiles only the association between the mPNNS-GS and the DQI-I remained statistically significant in all models (online Supplementary Table S5 and S6). In addition, among the participants who completed ≥6 d of dietary records during the first 2 years of follow-up, only the mPNNS-GS and the DQI-I were significantly associated with a reduced risk of depressive symptoms (online Supplementary Table S7).
Discussion
In this large-scale longitudinal study, we investigated the association between adherence to nutritional recommendations (as measured by mPNNS-GS, AHEI-2010, PANDiet and DQI-I) and the risk of incident depressive symptoms over a 6-year follow-up period. We found a significant inverse association between adherence to the mPNNS-GS, the PANDiet and the DQI-I and the risk of depressive symptoms.
Regarding the AHEI-2010, which has been extensively used in the scientific literature, we found a non-significant association, in contrast to other published studies( Reference Sanchez-Villegas, Henriquez-Sanchez and Ruiz-Canela 15 , Reference Saneei, Hajishafiee and Keshteli 19 , Reference Saneei, Esmaillzadeh and Keshteli 24 ). In the Seguimiento Universidad de Navarra Cohort study including 15 093 participants, adherence to the AHEI-2010 at baseline was inversely associated with depression risk (HR quintile 5 compared with quintile 1: 0·72; 95 % CI 0·59, 0·88)( Reference Sanchez-Villegas, Henriquez-Sanchez and Ruiz-Canela 15 ). In the cross-sectional studies published by Saneei et al. including 3363 Iranian adults with a mean age of 36·3 (sd 7·9) years, adherence to the AHEI-2010 was also significantly inversely associated with depression risk( Reference Saneei, Hajishafiee and Keshteli 19 , Reference Saneei, Esmaillzadeh and Keshteli 24 ). In addition, a study based on the Whitehall II cohort including 4215 participants aged 35–55 years at baseline reported a significant inverse association between high adherence to the AHEI and recurrent depressive symptoms only among women (OR for a 1-sd increase: 0·59; 95 % CI 0·47, 0·75)( Reference Akbaraly, Sabia and Shipley 27 ). A possible explanation of our findings concerning the AHEI-2010 could be related to the cut-off values and the portions sizes used in the computation of AHEI-2010, which are different from those of French nutritional recommendations and possibly less discriminant in the French context owing to cultural specificities. For instance, the French nutritional guidelines recommend a daily intake of at least 385 g of n-3 fatty acids EPA+DHA and 5 % of EI from PUFA, whereas the cut-off values used in the AHEI-2010 computation were 250 g and 10 %, respectively.
Besides these studies, only one other investigation has focused on the French food-based guidelines in relation to depressive symptoms. In this prospective study among 3328 participants (baseline mean age of 49·5 years, sd 6·2 and mean follow-up=13years) from the French Supplémentation en Vitamines et Minéraux AntioXydants cohort, a 1-point increase in the mPNNS-GS was associated with a 13 % (95 % CI 6, 20) reduction in the risk of chronic or recurrent depressive symptoms( Reference Collin, Assmann and Andreeva 25 ), in agreement with the reduced risk of incident depressive symptoms found in our study. Our findings are also in agreement with the findings of the Australian Longitudinal Study on Women’s Health, in which maintaining a moderate or high adherence to the Australian Recommended Food Score (including seven food group components; vegetables, fruit, protein foods, grains, dairy products, fats and alcohol) over a 6-year period was, respectively, associated with a 6 % (95 % CI 1, 20) and 14 % (95 % CI 4, 23 %) reduction in the risk of depression( Reference Lai, Hure and Oldmeadow 26 ) among 7877 participants aged 50–55 years at baseline. To our knowledge, no previous study has directly investigated the association between adherence to nutrient-based recommendations such as the PANDiet or the DQI-I and the risk of depression or depressive symptoms.
Higher adherence to the dietary scores considered in this study reflects a high intake of various vitamins and minerals (generally provided by high consumption of whole-grain products, fruits, vegetables and fish) associated with a reduced risk of several diseases( Reference Mikkelsen, Stojanovska and Prakash 53 – Reference Miki, Kochi and Eguchi 55 ). However, these dietary scores show some slight differences in terms of included components, cut-off values and scoring, leading to different nutrient intakes in tertiles, which could explain the observed differential associations with the risk of depressive symptoms. The main difference is that the mPNNS-GS and AHEI-2010 include mostly food-based components, whereas the PANDiet includes only nutrients. Finally, the DQI-I was specially developed to facilitate cross-country comparisons and includes both food-based components and nutrients. Comparing the mPNNS-GS, the AHEI-2010 and the DQI-I, only the mPNNS-GS includes a system of penalty for energy overconsumption and only the DQI-I takes into account food variety, proportionality in energy sources and fatty acid composition. In addition, the DQI-I and PANDiet do not take into account alcohol consumption. These disparities are illustrated by correlation coefficients, which are not very high (<0·80), although all these dietary scores have the same overall objective, which is to measure the nutritional quality of the diet.
Some limitations of our study should be noted. Given the observational design of our study, we cannot entirely exclude reverse causality although the design is prospective. Second, despite a wide range of confounders included in our statistical models, unmeasured factors related to depression such as personality traits, family history of depressive disorders, stressful life events and sleep disorders( Reference Berk, Williams and Jacka 56 , Reference Lopresti, Hood and Drummond 57 ) might have led to potential residual confounding. Third, participants of the NutriNet-Santé study were volunteers in a nutritional cohort and thus more interested in nutritional issues and healthy lifestyles than the general population. Thus, any generalisation of our findings should be done with caution. Another limitation of our study pertains to the large proportion of participants excluded from the eligible population, which could include a potential bias in the risk estimates. However, we have elected to prioritise the accuracy of the dietary data and to reduce potential reverse causality phenomenon owing to depressive episode during the dietary data assessment. Finally, data on trans-fatty acids were not available in our study, which did not allow to fully compute the original AHEI-2010. Important strengths of this study include the prospective design of our study, its large sample of participants aged 18–86 years and without depression at the beginning, the repeated data assessment of depressive symptoms using a validated tool and the quality of the dietary data based on repeated dietary records, yielding a particularly high accuracy of intake estimations. The wide range of confounding factors also helped to improve the estimations.
To conclude, our study showed that high adherence to food-based and nutrient-based national or international nutritional recommendations was associated with a reduced risk of incident depressive symptoms. Overall, our findings suggest that nutritional recommendations, although designed mostly for the prevention of nutrition-related chronic conditions such as obesity, cardiovascular disorders and cancer, are also associated with a reduction in the risk of depressive symptoms. Thus, promoting better adherence to national dietary guidelines, as well as adequate nutrient intakes, may be useful in a primary prevention strategy of depressive symptoms through modifiable factors in the general population. The results found in this study should be confirmed by further prospective studies.
Acknowledgements
The authors thank all the scientists, dietitians, technicians and assistants for their technical contribution to the NutriNet-Santé study. The authors especially thank Younes Esseddik, Thi Duong Van, Frédéric Coffinieres, Mac Rakotondrazafy, Régis Gatibelza and Paul Flanzy (computer scientists), and Nathalie Arnault, Véronique Gourlet, Dr Fabien Szabo, Julien Allegre, Anouar Nechba and Laurent Bourhis (data-manager/biostatisticians). The authors also thank all the volunteers of the NutriNet-Santé cohort.
The NutriNet-Santé Study is supported by the French Ministry of Health (DGS), the French Public Health Agency, the French National Institute for Health and Medical Research (INSERM), the Medical Research Foundation (FRM), the French National Institute for Agricultural Research (INRA), the National Conservatory for Arts and Crafts (CNAM), the National Institute for Prevention and Health Education (INPES) and the Paris 13 University. M. A. was supported by a doctoral fellowship from the Ecole Doctorale Galilée, Paris 13 University, Sorbonne Paris Cité.
The author contributions were as follows: C. J., S. H., P. G. and E. K.-G. were responsible for the development of the design and protocol of the study; M. A. performed the statistical analysis and wrote the paper; E. K.-G. provided methodological guidance; M. A., C. L., C. J., S. H., P. G., K. E. A. and E. K.-G. were involved in interpreting the results and editing the manuscript for important intellectual content. All authors read and approved the final manuscript.
C. L. has received honoraria for board membership from Lundbeck and for speaking at invited symposia from Daiichi-Sankyo, Janssen, Lundbeck, Otsuka Pharmaceuticals and Servier. None of the other authors has any conflicts of interest to declare.
Supplementary Material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518000910








