Protein malnutrition continues to represent a significant world health problem, especially with regard to children, the elderly and those in hospital. Nutritional deficiencies can result in an impairment of cognitive function as well of overall development and motor coordination, and are associated with an increase in morbidity following infectious diseases (Lozoff et al. Reference Lozoff, Jimenez and Wolf1991).
Protein–energy malnutrition is a syndrome in which anaemia may be present. Anaemia has multiple aetiologies: it can be caused by congenital abnormalities or be associated with several diseases, such as chronic renal disease or even other nutritional alterations. Literature describes the frequent occurrence of anaemia in malnutrition, which can be due to Fe and folate deficiency (Macdougall et al. Reference Macdougall, Moodley, Eyberg and Quirck1982; Villalpando et al. Reference Villalpando, Latulippe, Rosas, Irurita, Picciano and O'Connor2003; Gasche et al. Reference Gasche, Lomer, Cavill and Weiss2004; Moretti et al. Reference Moretti, Torre, Antonello, Cattaruzza, Cazzato and Bava2004), vitamin A deficiency, protein deficiency, a decrease in erythropoietin level (Stenvinkel, Reference Stenvinkel2003; Gibson, Reference Gibson2004), haemolysis due to an alteration in the antioxidant mechanisms in erythrocytes, infection and (in tropical regions) infestation with parasites (Buitron et al. Reference Buitron, Hurtig and San Sebastian2004; Stoltzfus et al. Reference Stoltzfus, Chway, Montresor, Tielsch, Jape, Albonico and Savioli2004). Fe deficiency has been considered to be the main cause of this anaemia (Yip, Reference Yip1996; Akman et al. Reference Akman, Cebeci, Okur, Angin, Abali and Akman2004; Khosrof-Ben Jaafar et al. Reference Khosrof-Ben Jaafar, Gharbi, El Fazaa, Beji, Farhat, Cherif, Haddad and Kamoun2004; Mehta, Reference Mehta2004).
It is generally recognised that the aetiology of malnutrition is multifactorial, but the contribution of each nutritional factor to the establishment of several syndromes associated with malnutrition is not yet fully understood. Haemopoietic tissues, as with all those with a high rate of renewal and turnover, present a high demand for nutrients. The need for protein for haemopoiesis, particularly for erythropoiesis, could alone justify the occurrence of anaemia frequently observed in cases of malnutrition.
In our studies, we have observed that malnourished animals present with anaemia, even when the purified diet used contains a balanced content of vitamins and minerals, including Fe (Borelli et al. Reference Borelli, Mariano and Borojevic1995). The aim of the present study was thus to evaluate erythropoiesis in a murine model of protein–energy malnutrition.
Materials and methods
Diets
The murine diets were prepared in our laboratories. Their diet contained fibre, saline mixtures and balanced vitamin mixtures in the same quantity, although the control purified diet contained 400 g/kg protein and the hypoproteic purified diet only 20 g/kg (Fried et al. Reference Fried, Shapiro, Barone and Anagnost1978; Borelli et al. Reference Borelli, Mariano and Borojevic1995). The source of protein used was casein. Except for the protein content, the two diets were identical and isocaloric (Table 1). The final protein content was monitored by the standard micro-Kjeldahl method (Ward, Reference Ward1963).
Table 1 Composition of the experimental diets*
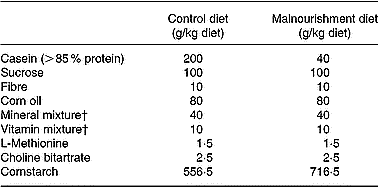
* Isocaloric diets providing 1716·3 kJ/100 g (410·6 kcal/100 g).
† Mineral and vitamin mixtures were prepared according to the 1993 recommendations of the American Institute of Nutrition for adult mice (Reeves et al. Reference Reeves, Nielsen and Fahey1993).
Animals
Male outbred Swiss mice of 2–3 months of age were obtained from the Faculty of Pharmaceutical Sciences at the University of São Paulo. They were placed in individual ‘metabolic cages’ and received the control diet for 21 d. After this period of adaptation, the mice reached a stable body weight. They were subsequently divided into four groups (control, control+erythropoietin (EPO), malnourished and malnourished+EPO) maintained under a regular light/dark cycle of 12 h, a temperature of 22–25°C and a humidity of 55 %, receiving either the control or the low-protein diet and water ad libitum. Their body weight was monitored every 72 h and the consumption of food every 48 h.
Mice were submitted to experimental assays after 14 d of eating their respective chows, when the undernourished group had suffered about a 20 % loss of their original body weight (Borelli et al. Reference Borelli, Mariano and Borojevic1995). To collect the different biological samples, the animals were anaesthetised with xylazene chlorohydrate (Rompum, 10 mg/kg; Bayer, Leverkusen, Germany) and with ketamide chlorohydrate (Ketamina, 100 mg/kg; Cristália, Campinas, Brazil). This study was approved by the Commission for Ethics of Animal Studies of the Faculty of Pharmaceutical Sciences at the University of São Paulo.
Erythropoietin
Recombinant human erythropoietin (Cristália) was diluted in a pyrogen-free isotonic solution, distributed into aliquots and stored at − 40°C until used. Animals from the two EPO groups (control+EPO, malnourished+EPO) received 20 IU EPO intravenously every other day for 14 d (Silver & Piver, Reference Silver and Piver1999) during the malnutrition phase.
Blood
Animals were anaesthetised, and whole-blood samples with and without EDTA (1 mg/ml) were obtained via cardiac puncture. Bone marrow cells, sternum, liver and spleen were collected concomitantly with the blood from the animals that had or had not received EPO. Total and differential counts of blood cells were carried out (Dacie & Lewis, Reference Dacie and Lewis1995). The serum was separated by centrifugation, and the total protein content and albumin were determined by standard methods used in medical analysis (Gornall et al. Reference Gornall, Bardawill and David1949; Rodkey, Reference Rodkey1965; Doumas et al. Reference Doumas, Watson and Biggs1971).
Determination of serum erythropoietin
The quantification of erythropoietin present in serum was carried out by ELISA using commercial reagents (Quantikine M murine; R&D Systems, USA) according to the manufacturer's instructions. The serum was separated by centrifugation (2000 g for 10 min at 4°C) and frozen in aliquots at − 40°C until use. All samples were processed in duplicate.
Determination of serum Fe and total Fe-binding capacity
Blood samples obtained without anticoagulant were collected in demineralised containers, and serum Fe concentration as well as total Fe binding capacity (TIBC) and transferrin saturation were determined using the COBAS MIRA plus (Roche, Schweiz, Switzerland) automated system (Goodwin et al. Reference Goodwin, Murphy and Guillemette1966).
Bone marrow, spleen and liver: histology and evaluation of tissue Fe
The sternum, spleen and liver were removed and immediately immersed in Carnoy fixative for 1 h and processed by standard histological techniques (paraffin-embedding). Sections measuring 5 μm from sternum, liver and spleen were stained by haematoxylin and eosin and by the Perls method for the evaluation of tissue Fe (Rosai, 1996), and were evaluated by conventional optical microscopy. All material used was previously demineralised.
Splenic cellularity
The spleen was aseptically removed, placed in a 60 mm Petri dish (Corning Acton, MA, USA) containing Ca-free and Mg- free phosphate buffer solution with EDTA (1 mg/ml; Sigma Chemical Co., St Louis, MO, USA) and gently dissociated. The total number of cells was quantified in a standard haemocytometer (Neubauer® chamber; Herka, Berlin, Germany). Cytocentrifuge smears were stained using standard May-Grunwald and Giemsa solutions (Sigma Chemical Co). Differential cell counts were performed considering 300 cells per slide.
Bone marrow cellularity
For the cell preparations, femurs were removed aseptically. Bone marrow cells were flushed from these using Fisher medium (Sigma Chemical Co.) supplemented with 10 % fetal calf serum (Cultilab, Campinas, Brazil) and heparin 50 U (Liquemine; Roche), and were used in a myelogram as well as in clonogenic assays and flow cytometry. Total cell counts were carried out, followed by differential counts on cytocentrifuge smears prepared as described above.
Analysis of mixed colony-forming units
The mixed colony-forming unit (CFU-MIX) assays were carried out according to the procedures of Heyworth & Spooncer (Reference Heyworth, Spooncer, Molineux and Testa1993) to evaluate the number of haemopoietic progenitors in the sample. Bone marrow cells were harvested and plated (5 × 105 cells/ml) in triplicate into 35 mm Petri dishes (Nunc, Roskilde, Denmark) with methylcellulose medium (4000 Kg F n− 1 s− 1; Fischer, NJ, USA), 10 % fetal calf serum (Gibco, Grand Island, NY, USA), 1 % bovine serum albumin (Boehringer Mannheim, IN, USA), 10− 4m-methylprednisolone (Abbott, Brazil), 10− 2m-2-mercaptoethanol (Gibco), 0·2 ng/ml recombinant murine granulocyte–macrophage colony-stimulating factor (Sigma Chemical Co.), 0·2 ng/ml recombinant murine granulocyte colony-stimulating factor (Sigma Chemical Co.), 1 IU recombinant human EPO (Cristália) and 0·1 ng/ml interleukin-3 (Sigma Chemical Co.).
Cells were incubated in a humidified atmosphere of 95 % air and 5 % CO2 at 37°C for 21 d. Internal negative controls were made containing only the culture medium and cells. The number of clusters and colonies (Chimelli et al. Reference Chimelli, Bello and Scaravilli1994) was evaluated on the seventh, fourteenth and twenty-first days after culturing using an inverted microscope. Total cell counts were monitored, and differential counts were carried out on cytocentrifuge smears prepared as described above. Only viable cells with normal morphology were counted, whereas those with pyknotic nuclei and signs of degeneration were discarded.
Flow cytometric profiling of cells in the bone marrow and mixed colony-forming unit growth
Flow cytometry was used to determine the fraction of bone marrow and CFU-MIX growth cells positively labelled with antibodies against CD45 (LY-5, clone 30-F11), CD34 (clone RAM 34), Ter-119 (clone C55BL), CD71 (clone C2), CD117 (clone 2 B8) and CD2 (clone RM2-5) acquired from Becton Dickinson Pharmigen (San Diego, CA). Antibodies to Ter-119 and CD71 were conjugated with phycoerythrin; anti-CD45, anti-CD34, anti-CD117 and anti-CD2 were conjugated with fluorescein isothiocyanate. The isotype controls were rat immunoglobulins 2a kappa, fluorescein isothiocyanate (clone R35-95), for CD34; immunoglobulin G2b kappa, fluorescein isothiocyanate (clone A95-1), for CD45, CD117 and CD2;:immunoglobulins G1 kappa, phycoerythrin, for CD71 and Ter-119.
For the immunophenotyping experiments, total cell suspensions obtained from the bone marrow and from samples obtained on the seventh and fourteenth days after culturing in the CFU-MIX assays. The samples and negative chain controls were incubated with 5 μg monoclonal antibody/106 cells per ml, and kept from light for 20 min at 25°C. After this period, the erythrocytes were lysed by adding 2 ml 10 % Lysing Solution (Becton Dickinson) and incubated again, sheltered from light, for 15 min. Next, the tubes were centrifuged at 400 g for 10 min, the supernatant was discarded, and the cell sediment was washed twice with PBS containing 0·1 % azide. The sediment was resuspended in 500 μl 1 % paraformaldehyde (Sigma Chemical Co.), and the cells were acquired in a flow cytometer. Dual-parameter flow cytometry utilised a fluorescence activated cell sorter (Calibur, Becton Dickinson), equipped with an argon laser. Excitation occurred at 488 nm for both fluorescein isothiocyanate and phycoerythrin. Fluorescence measurements were obtained from 1 × 104 cells. Data were analysed using the software package Cell Quest. The results were expressed as a percentage of gated populations.
Statistics
The dependent variables are normally distributed. The results were submitted to statistical analysis (ANOVA, P ≤ 0·05) using GraphPad Prism software. The data obtained in the CFU-MIX assays were analysed using three-way ANOVA and compared with each other using the Duncan test (P ≤ 0·05).
Results
Diet consumption, body weight and serum protein
Mice maintained on the hypoproteic diet reduced their food consumption by up to 19 %, resulting in a significant reduction in protein consumption, body weight loss and a decrease in serum protein and albumin concentrations (Table 2).
Table 2 Protein consumption, change in body weight, serum protein, albumin concentration, total number of erythrocytes, Hb concentration, haematocrit, percentage of reticulocytes, total number of leucocytes in the blood, total number of cells and total number of erythroblasts in the bone marrow and spleen of control, control with erythropoietin (EPO), malnourished and malnourished with EPO animals (Means values and standard deviations)

For details of diets and procedures, see pp. 308–309.
a Mean values were significantly different between the control group without EPO and other groups (P ≤ 0·05).
b Mean values were significantly different between the control group with EPO and other groups (P ≤ 0·05).
c Mean values were significantly different between the malnourished group without EPO and the malnourished group with EPO (P ≤ 0·05).
Blood
The animals of the malnourished group showed leucopenia as well as a reduction in erythrocyte count, Hb concentration and haematocrit, and a significant reduction in the number of reticulocytes, suggesting a non-regenerative anaemia (Table 2). Significant morphological differences in the erythrocytes were not found. The administration of EPO did not normalise the haematological parameters of the malnourished animals.
Serum Fe, total Fe-binding capacity, transferrin saturation and Fe tissue deposits
There was no difference between the groups with respect to serum Fe concentration (control, 435·1 (sd 142·3) mg/dl; malnourished, 475·7 (sd 117·9) mg/dl), total Fe-binding capacity (control, 472·7 (sd 141·7) mg/dl; malnourished, 556·3 (sd 136·1) mg/dl) and transferrin saturation (control, 92·5 (sd 8·52 )%; malnourished, 88·2 (sd 14·58) %). The tissue Fe reserve, evaluated by the Perls reaction, was greater in malnourished animals in the spleen (Fig. 1) and bone marrow as well as liver (data not shown).

Fig. 1 Spleen sections stained by Perls method to evaluate tissue Fe in the control (C) and malnourished (M) groups. Arrows indicate Fe as blue points in red pulp ( × 165). For details of diets and procedures, see pp. 308-309.
Bone marrow and spleen cells
Animals in the malnourished group showed marrow and splenic hypoplasia (Table 2) with a pronounced reduction in the myeloid compartment (data not shown) as well as the erythroid compartment. The administration of EPO induced an increase in total cellularity as well as an increase in the erythroid component of the bone marrow and spleen in both the control and malnourished animals. This was similar between the two groups, except for the response of the erythroid compartment of the spleen after administration of EPO, which was smaller in the malnourished animals. The relationship of granulocytic to erythroid precursor cells in the malnourished animals was significantly smaller (P ≤ 0,01), independent of whether the animals had or had not received EPO (data not shown), confirming the depletion of the erythrocytic compartment.
Determination of erythropoietin
No difference was found between the control and malnourished animals with respect to the concentration of circulating erythropoietin (control, 780·9 (sd 295·1) pg/ml; malnourished, 848·4 (sd 205·9 pg/ml).
Mixed colony-forming unit, erythroid burst-forming unit and erythroid colony-forming unit
Results were expressed the number of granulocyte–macrophage colony-forming units, erythroid burst-forming units, erythroid colony-forming units and CFU-MIX in the clonogenic assays using total cells from the bone marrow of the control and malnourished animals. Differential total cell counts were monitored, and counts were carried out on cytocentrifuge smears prepared. In the clonogenic assays, the number of CFU-MIX was significantly lower than in cultures carried out with the cells of malnourished animals, demonstrating the existence of a smaller number of primitive precursors in the bone marrow of malnourished mice. Furthermore, neither erythroid burst-forming units nor erythroid colony-forming units were found (Table 3).
Table 3 Number of granulocyte–macrophage colony-forming units (CFU-GM), erythroid burst-forming units (BFU-E), erythroid colony-forming units (CFU-E), and mixed colony-forming units (CFU-MIX) in the clonogenic assays using total cells in the bone marrow of control and malnourished animals (Representative experiment of three similar experiments)
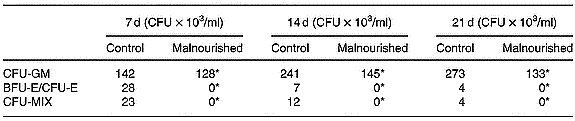
For details of diets and procedures, see p.00. Cultures in semisolid medium were incubated for 21 d at 36·8°C in a humid atmosphere containing 5 % CO2 and were evaluated on the seventh, fourteenth and twenty-first days after culturing using an inverted microscope.
* Mean values were significantly different between the experimental and control groups (P ≤ 0·05).
Flow cytometric profiling of cells in the bone marrow, spleen and CFU-MIX culture
Immunophenotypic analysis of the marrow and spleen populations applying AcMO, CD45, CD117, Ter-119, CD71 and CD2 as markers confirmed the depletion of erythroid precursors in malnourished animals. We found an reduced expression of the CD45+ cell population of malnourished animals, especially the population of immature cells that were CD45low. This depletion in primitive cells was confirmed by the lower expression of CD117 (c-kit receptor; Fig. 2). The population that expressed Ter-119 and CD71 (transferrin receptor) was also found to be considerably reduced (Fig. 3). Bone marrow cells of control animals were found to express two populations–CD71high (more mature population) and CD71low (less mature population) – whereas malnourished animals presented a small percentage of CD71low cells and did not show a CD71high population. As the CD71 antigen is also expressed in lymphoid cells, we used CD71/CD2 double-marking in order to separate and quantify the erythroid and lymphoid populations.

Fig. 2 Immunophenotyping of cells in the bone marrow of control (C) and malnourished (M) animals. The results were expressed in a dot plot: (gate R1) referent to the expression of Ter-119, CD71, CD2, CD117 and CD45. For details of diets and procedures, see pp. 308–309. FITC, fluorescein isothiocyanate; PE, phycoerythrin; SSC, side scatter; FSC, forward scatter.

Fig. 3 Immunophenotyping of cells in the bone marrow of control (C) and malnourished (M) animals. The results were expressed in a dot plot: (gate R1) referent to the expression of CD71. Immunophenotyping shows positivity for the control group, which permits the distinction between a CD-71low population and a CD-71hi population, one of low intensity (C1) and one of high intensity (C2), respectively, whereas the malnourished group (M) exhibited only a CD-71low population, with low intensity. For details of diets and procedures, see pp. 308–309. PE, phycoerythrin.
The results of the clonogenic assays and the immunophenotypic profile of cells obtained from the spleen (data not shown) were similar to those obtained when using bone marrow cells.
Discussion
The present study indicates that protein malnutrition produces normochromic, normocytic anaemia, with a significant reduction in reticulocyte and alterations in bone marrow and spleen erythropoiesis. The anaemia found does not show characteristics of either Fe-deficiency anaemia or anaemia that is secondary to chronic disease.
The control group received an adequate protein diet, and the test group received a protein-deficient diet. The deficient group had, however, a markedly lower feed intake and experienced about a 20 % loss of body weight. Thus, in the study, we sought to compensate this effect by preparing a diet exceeding the recommendations of the American Institute of Nutrition AIN-93 (Reeves et al. Reference Reeves, Nielsen and Fahey1993). As a result, even though the animals ingested a lower amount of food, the diet we prepared met the minimum nutrient requirements for adult mice (Reeves et al. Reference Reeves, Nielsen and Fahey1993). As the minimum daily amounts of nutrients were ingested by the animals in the malnourished group, we can conclude that the changes observed in our experimental model were mainly the result of the reduction in protein and energy intake compared with the control group. Animals belonging to the malnourished group lost 25 % of their initial weight in a period of 14–16 d after the introduction of the hypoproteic diet.
Haemopoietic tissue, like all tissues that have a high rate of renewal and cellular proliferation, presents a high demand for nutrients. The sheer need for protein for the process of haemopoiesis could in itself justify the occurrence of anaemia and leucopenia, which are frequently encountered in malnourished human subjects. According to Vilter (Reference Vilter and Olson1975), children with typical protein–energy malnutrition have a normochromic, normocytic anaemia, with Hb levels that lie between 8 and 10 g/dl and normal medullary erythropoiesis, or a discretely hypoplastic marrow with fatty infiltration.
The lack of Fe has been considered to be the main cause of anaemia in malnourishment (Finch, Reference Finch and Olson1975). Other authors have, however, found normal Fe serum levels with an increase in transferrin saturation (Ramdath & Golden, Reference Ramdath and Golden1989) and normal serum ferritin levels, the bone marrow presenting normal or elevated Fe deposits. Liver biopsies obtained post mortem from individuals suffering from marasmus have demonstrated high levels of Fe (Mclarem et al. Reference Mclarem, Fariz and Zeckian1968; Waterlow, Reference Waterlow1996). Fondu et al. (Reference Fondu, Hariga-Muller, Mozes, Neve, Van Steirteghem and Mandelbaum1978) concluded that the anaemia was caused by a reduction in the mean life of the erythrocytes, suggesting that an increase in erythrocyte fragility was the result of a decrease in Se and vitamin E ingestion. Furthermore, these same authors suggested that among the causes of anaemia in protein–energy malnutrition was an adaptation of the organism to the reduction in the demand for O and also to chronic infections, which are frequently present.
Reissman (Reference Reissman1964) reported anaemia in malnourished rats and suggested as a mechanism a slow-down in protein synthesis in erythroid cells and/or a reduction in erythropoietin synthesis. Aschkenasy (Reference Aschkenasy1957) observed, in protein malnutrition, the presence of anaemia and leucopenia, and erythroid marrow hyperplasia without a simultaneous elevation in erythropoietin concentration. Anaemia, in experimental malnutrition, presents a decrease in Fe incorporation and in the number of reticulocytes (Fried et al. Reference Fried, Shapiro, Barone and Anagnost1978) and furthermore an interruption in the maturation process of erythroblasts. Studies (Fried & Gurney, Reference Fried and Gurney1965; Seifter et al. Reference Seifter, Rettura, Reissman, Kambosos and Levenson1971; Aschkenasy, Reference Aschkenasy1975) have demonstrated that, in rats, a fall in erythropoietin occurred due to the reduced ingestion of protein, in opposition to the results obtained in our study. Serum concentrations of erythropoietin were found to be both increased (Macdougall et al. Reference Macdougall, Moodley, Eyberg and Quirck1982; el-Nawawy et al. Reference el-Nawawy, Barakat, Elwalily, Abdel-Moneim Deghady and Hussein2002) and decreased (Reissman, Reference Reissman1964; Catchatourian et al. Reference Catchatourian, Eckerling and Fried1980) in protein malnutrition and protein-energy malnutrition, the reduction being attributed to a decrease in the speed of O use by the tissues.
The present data suggest that the anaemia found in adult mice submitted to protein deficiency was not due to Fe deficiency, as the serum Fe concentration of these animals was found to be high, as were the levels of transferrin saturation and ferritin concentration in the bone marrow, liver and spleen. The present results indicate that the response of animals in the control group to the administration of erythropoietin was significantly higher than that of malnourished animals. The morphological analysis of the bone marrow and of the spleen revealed, in malnourished animals, the presence of lympho-haemopoietic hypoplasia, especially of the myeloid compartment, with an emphasis on the erythroid compartment. These findings were corroborated by the results of CFU-MIX, erythroid burst-forming unit growth and immunophenotypic analysis. The immunophenotypic analysis of marrow and spleen population demonstrated a depletion of the erythroid population, indicating a reduction in the number of erythroid progenitors in malnourished animals. Hypoproliferative anaemia is associated with a reduction in the number of erythroid precursors, which can be caused by conditions such as aplastic anaemia, myelofibrosis and infiltrative processes in the bone marrow. As has already been reported in previous studies (Fried et al. Reference Fried, Goldwasser, Jacobson and Plzak1957; Borelli et al. Reference Borelli, Mariano and Borojevic1995), we found severe splenic and marrow hypoplasia with severe structural alterations in the haemopoiesis-inducing microenvironment (Vituri et al. Reference Vituri, Alvarez-Silva, Trentin and Borelli2000).
According to Stohlman (Reference Stohlman1972), bone marrow atrophy can result from abnormalities of stem cells or defects in stromal cells, which would alter the haemopoietic microenvironment. Genetically modified (W/Ww) mice possess an alteration in the proto-oncogene c-kit, a receptor with tyrosine-kinase activity for stem cell factor (a growth factor for stem cells). Mice that possess an alteration known as Steel (SL/SLd) do not express a binding c-kit receptor (CD117), which is the receptor for stem cell growth factor, and furthermore show an alteration in the stroma of the marrow matrix (Shadduck, Reference Shadduck, Beutler, Lichtman, Coller and Kipps1995), as well as anaemia. In the present study, we found a reduction in the CD117+ population among the bone marrow and spleen cells of malnourished mice. The anaemia, leucopenia and bone marrow atrophy found in mice subjected to protein malnutrition suggest a decrease in the proliferative capability of progenitor cells, because the reduction in the marrow compartment was not due to an increase in the efflux of cells into the peripheral blood or to apoptotic processes (data not shown).
All these results suggest that, in essence, this anaemia is not caused by Fe deficiency or erythropoietin deficiency, but is in fact a result of ineffective erythropoiesis.
Acknowledgements
The authors thank Dr Marcelo Macedo Rogero for his suggestions. This investigation was supported by grants from the Fundação de Amparo a Pesquisa do Estado de São Paulo - FAPESP (03/07 322-1), and from the Conselho Nacional de Pesquisa (CNPq), Brazil.








