Hyperinsulinaemia is linked to the development of various chronic diseases including obesity, diabetes, CVD and some cancers(Reference Bell and Sears1–Reference Liu, Willett and Stampfer3). It has been reported that hyperinsulinaemia is also involved in the pathophysiology of psychological disorders(Reference Perry, Khandaker and Marwaha4–Reference Lee, Park and Ryoo6). Coexistence of psychological disorders and diabetes might further highlight the role of hyperinsulinaemia in these conditions(Reference Anderson, Freedland and Clouse7). However, the underlying mechanistic link between depressive symptoms and insulin resistance remains unknown.
Given the role of lifestyle-related factors, including diet, in both psychological disorders and diabetes, finding a common dietary factor that might contribute to both conditions is of high interest. Great attention has been given to carbohydrate consumption in this regard. Consumption of a high-glycaemic index (GI) and high-glycaemic load (GL) diet has been associated with increased risk of psychological disorders(Reference Mwamburi, Liebson and Folstein8). This direct association might be explained by the post-ingestive tendency to hypoglycaemia following such a diet, eliciting central dysfunction and depression(Reference Anderson, Freedland and Clouse7). Some studies have also attributed such an association to hyperinsulinaemia, which has been independently related to psychological disorders(Reference Shomaker, Kelly and Pickworth9,Reference Okamura, Tashiro and Utsumi10) . The link between hyperinsulinaemia and psychological disorders might be explained by facilitating the transport of tryptophan, a precursor of serotonin, into the brain(Reference Markus11). Indeed, the synthesis of serotonin is a function of the tryptophan level in the brain, which, in turn, depends upon the uptake of this amino acid from circulating blood. Due to the competition between tryptophan and other large neutral amino acids, it has been suggested that the rate of serotonin synthesis in human brain would also be dependent to the alterations in plasma amino acid ratios, which in turn can be influenced by changes in insulin concentrations(Reference Markus11). Thus, it is evident that hyperinsulinaemia may contribute to the development of serious mental disorders including depression. It must be kept in mind that dietary GI or GL does not consider the role of other insulinotropic factors including certain amino acids and fatty acids(Reference Moghaddam, Vogt and Wolever12,Reference Nuttall and Gannon13) . Therefore, a dietary insulin index (DII), which systematically quantifies postprandial insulin responses to all dietary factors, has been suggested(Reference Holt, Miller and Petocz14). Dietary insulin load (DIL), which is calculated by summing up the product of food insulin index (FII), energy content and consumption frequency of food items, has been indicated to provide a more accurate prediction of insulin demand than carbohydrate content or glycaemic load(Reference Bao, de Jong and Atkinson15). These indices have been linked with several chronic conditions in earlier studies; however, we are aware of no earlier study linking DII and DIL with the risk of psychological disorders. Given the lack of evidence of the association between DII and DIL and psychological disorders along with the high consumption of carbohydrates, in particular refined carbohydrates in the Middle-East, we thought that examining the association between DII and DIL and psychological disorders in this part of the world might provide additional information for nutritionists. We hypothesised that a high DII might be associated with a greater risk of psychological disorders. Therefore, the purpose of the present study was to investigate the association between DII and DIL and psychological disorders among Iranian adults.
Study population and methods
Participants
The present cross-sectional study was conducted within the framework of the Study on the Epidemiology of Psychological, Alimentary Health and Nutrition (SEPAHAN) project, which was performed on a large population of Iranian adults working in fifty different health centres in Isfahan, Iran. Detailed information about SEPAHAN project has been described elsewhere(Reference Adibi, Keshteli and Esmaillzadeh16). Briefly, data collection was done in two separate main phases to achieve greater high accuracy of collected data. At the first phase, data on demographic variables along with dietary intakes were collected for 8691 people. At the second phase, data regarding psychological health were collected. By merging data from both phases, we had complete information for 4763 people(Reference Adibi, Keshteli and Esmaillzadeh16). In the present analysis, we excluded participants who did not have total energetic intakes at the range of 3347–17 573 kJ/d as under-reporters and over-reporters of energy intake (n 1271). Furthermore, women with pregnancy and lactation (n 190) and also participants with missing data on anthropometric information as well as dietary intakes were excluded (n 130). After these exclusions, a dataset of 3172 participants including 1398 men and 1774 women was available for the present analysis. Comparing individuals participated at first and those remained for final analysis, no significant difference was found between the general characteristics(Reference Salari-Moghaddam, Keshteli and Adibi17). All participants provided signed informed written consent forms. The whole project of SEPAHAN was ethically approved by the Bioethics Committee of Isfahan University of Medical Sciences, Isfahan, Iran(Reference Adibi, Keshteli and Esmaillzadeh16).
Dietary intake assessment
The usual dietary intakes of participants were assessed via a validated Willett-format(Reference Willett18) dish-based 106-item semi-quantitative FFQ (DS-FFQ) which was designed particularly for Iranian adults(Reference Keshteli, Esmaillzadeh and Rajaie19). Details on design, food items and validity of this FFQ have been reported previously(Reference Keshteli, Esmaillzadeh and Rajaie19). In brief, first, we prepared a comprehensive list of foods and dishes commonly consumed by Iranian adults. Then, we chose those foods which were nutrient-rich, consumed reasonably often or contributed to between-person variations from this list. Selecting a food as a usual food item was done according to dietary records and recalls that had been collected in our prior investigations. Finally, 106 food items in five different categories were included in this questionnaire: (1) mixed dishes (cooked or canned, twenty-nine items); (2) carbohydrate-based foods (different types of bread, cakes, biscuits and potatoes, ten items); (3) dairy products (dairies, butter and cream, nine items); (4) fruits and vegetables (twenty-two items) and (5) miscellaneous food items and beverages (including sweets, fast foods, nuts, desserts and beverages, thirty-six items).
We asked individuals to report their dietary intakes of foods and mixed dishes based on nine multiple-choice frequency response categories varying from ‘never or less than once a month’ to ‘twelve or more times per day.’ The frequency response categories for all food items were not constant and varied from six to nine choices. We omitted the high-frequency categories for foods consumed infrequently and increased the number of multiple-choice categories for common foods with a high frequency. Furthermore, in order to increase the accuracy of responses, we used the most popular serving sizes familiar to Iranian adults. Finally, we calculated daily intakes of all foods and dishes and converted to g/d using household measures(Reference Ghaffarpour, Houshiar-Rad and Kianfar20). Then, in order to compute the daily energy and nutrient intakes of each participant, we summed up the energy and nutrient contents of all foods and dishes. Energy and nutrient contents of each food were obtained using the US Department of Agriculture’s national nutrient databank(Reference Kimura, Wada and Okumiya21).
The validity of the DS-FFQ was evaluated in a subgroup of 200 randomly selected participants of SEPAHAN project(Reference Keshteli, Esmaillzadeh and Rajaie19,Reference Salehi-Abargouei, Esmaillzadeh and Azadbakht22) . All participants in the validation study completed the DS-FFQ at study baseline and 6 months later. During this validation study, 3-d detailed dietary records, which were used as a ‘gold standard’, were provided by individuals. According to findings from the present study, the DS-FFQ could provide reasonably valid and reliable measures of long-term dietary intakes in Iranian population; for instance, dietary carbohydrate intake estimated from the DS-FFQ was significantly correlated with the values obtained from the average of 3-d dietary records (r 0·81). Such correlation coefficients were also seen for other food groups and nutrients including Mg (r 0·61), proteins (r 0·72) and legumes and nuts consumption (r 0·69).
Calculation of dietary insulin index and load
After considering the components of mixed dishes, we converted all items in the DS-FFQ into a separate food item. FII refers to the incremental insulin AUC over 2 h in response to the consumption of a 1000-kJ portion of the test food divided by the AUC after ingestion of a 1000-kJ portion of the reference food. FII for each food item was obtained from previous studies published by Brand-Miller et al. (Reference Holt, Miller and Petocz14). For food items in the present study that was not available in the food list published by Brand-Miller et al., we used the FII for similar food items. To determine DIL, we first calculated the insulin load of each food using the following formula:
By summing up the insulin load of each food, DIL was obtained for each person. Then, we calculated the DII for each participant by dividing DIL by total energy intake.
Psychological profile assessment
Anxiety and depression were assessed by the Iranian validated version of Hospital Anxiety and Depression Scale which provided valid measures of mental health on the basis of the previous study(Reference Montazeri, Vahdaninia and Ebrahimi23). This scale is a brief and useful questionnaire to examine psychological disorders in addition to symptom and severity of anxiety disorders and depression(Reference Montazeri, Vahdaninia and Ebrahimi23). It contains fourteen items with a four-point scale for each item and consists of two subscales: anxiety and depression; higher scores indicate the greater degree of anxiety and depression. The possible score range is from 0 to 21 for each subscale. Scores of 8 or more on either subscale were considered to indicate the presence of psychological disorders, and scores of 0–7 were defined as ‘normal’ in the present study(Reference Montazeri, Vahdaninia and Ebrahimi23). Overall, our previous investigations revealed that the questionnaire provides relatively valid measures of mental health(Reference Montazeri, Vahdaninia and Ebrahimi23).
To assess psychological distress, we used the Iranian validated version of the General Health Questionnaire (GHQ) which contained twelve items(Reference Montazeri, Harirchi and Shariati24). Each item constitutes a four-point rating scale (less than usual, no more than usual, rather more than usual or much more than usual). We used the bimodal scoring method (0-0-1-1) in order to calculate the total score of psychological distress for each participant. The scores obtained by this method ranges from 0 to 12; higher scores indicate a greater degree of psychological distress. In our study, we considered the score of 4 or more as having psychological distress(Reference Schmitz, Kruse and Heckrath25). The validity of these scores to identify patients with psychological distress was examined in earlier studies(Reference Goldberg, Gater and Sartorius26). Based on comparison with clinical cases, the investigators reported that the cutoff score of 4 or more was more accurate to effectively identify persons with mental illness(Reference Papassotiropoulos, Heun and Maier27). Therefore, in order to determine the thresholds linked with optimum sensitivity and specificity of the GHQ-12, we considered this cutoff score as well.
The convergent validity of GHQ-12 was examined in 748 Iranian young people. A significant inverse correlation was observed between the GHQ-12 and global quality of life scores (r −0·56, P < 0·0001)(Reference Montazeri, Harirchi and Shariati24).
Assessment of covariates
We used a self-administered questionnaire in order to obtain data on age, sex, marital status (single/married), education (high school diploma or below/above high school diploma), smoking status (non-smoker/former smoker/current smoker), family size (≤4/>4 members), homeownership (owner/non-owner), disease history (diabetes, asthma, colitis, stroke, myocardial infarction, heart failure and cancers), current use of antipsychotic medications (including nortriptyline, amitriptyline or imipramine, fluoxetine, citalopram, fluvoxamine and sertraline) and dietary supplements (including intake of Fe, Ca, vitamins and other dietary supplements). Assessing physical activity of study participants was carried out via a General Practice Physical Activity Questionnaire which is a simple validated screening tool for grading adult people’s physical activity by focusing on current general activities. In the present analysis, participants were classified into two categories: physically active (≥1 h/week) and physically inactive (<1 h/week). The validity of the General Practice Physical Activity Questionnaire for assessment of habitual physical activity levels has been examined elsewhere(Reference Adibi, Keshteli and Esmaillzadeh16). To gather information on anthropometric measures including weight and height, we used a self-reported questionnaire. BMI was calculated as weight in kg divided by the height in m2. The validity of self-reported weight and height was examined in a pilot study on 200 participants from the same population. This validation study revealed that correlation coefficients for self-reported weight and height v. technician-measured values were 0·95 (P < 0·001) and 0·83 (P < 0·001), respectively. Also, the correlation coefficient for computed BMI from self-reported values and the one from measured values was 0·70 (P < 0·001). Therefore, self-reported values of anthropometric indices provide reasonably valid measures in the present study(Reference Aminianfar, Saneei and Nouri28).
Statistical analysis
In the present study, we first obtained energy-adjusted DIL and DII by the use of the residual method(Reference Willett, Howe and Kushi29). In this method, a linear regression model was constructed, in which total energy intake was considered as an independent variable and DII and DIL as a dependent variable. Then, the mean DII and DIL in the whole study population plus residuals from this regression model were considered as energy-adjusted DII and DIL. The bivariate Pearson correlation revealed that these values were no longer correlated with total energy intake. Then, we categorised men and women by quartiles of energy-adjusted DIL and DII, and all statistical analyses were separately done for both sex. We applied the one-way ANOVA to examine significant differences in continuous variables including age, BMI and the prevalence of psychological disorders across quartiles of DIL and DII. The χ 2 test was used to assess the distribution of men and women in terms of categorical variables across quartiles of DIL and DII. To compare dietary intakes of food groups and nutrients across quartiles of DIL and DII, ANCOVA was applied. To determine the association of DIL and DII with psychological disorders, binary logistic regression was used in different models. In the first model, we adjusted for age (continuous) and energy intake. Further adjustment was done for marital status (single/married), education (under university/university graduated), smoking status (non-smoker/former smoker/current smoker), family size (≤4/>4 members), homeownership (owner/non-owner), diabetes mellitus (yes/no), dietary supplement use (yes/no) and antipsychotic medications (yes/no) in the second model. In the final model, BMI (continuous) was additionally controlled to see if the associations are independent of obesity. All confounding variables were established risk factors for psychological disorders based on literature. The first quartile of DIL and DII was considered as the reference category in all analyses. To find the overall trend of OR across increasing quartiles of DIL and DII, we considered these quartiles as an ordinal variable in the logistic regression models. Education-stratified analysis (under university/university graduated) was also done. Moreover, in additional analysis, DII and DIL, as well as scores of psychological disorders, were considered as continuous variables and the associations were examined through linear regression analysis. All statistical analyses were conducted using SPSS software (version 19.0; SPSS Inc.). P values were considered significant at <0·05.
Results
The mean age of men and women was 38·4 (sd 8·2) and 35·1 (sd 7·4) years, respectively. Prevalence of depression, anxiety and psychological distress was 6·5, 3·7 and 16·6 % among men and 12·9, 6·8 and 27·1 % among women, respectively.
General characteristics of men and women across quartiles of DIL and DII are provided in Table 1. Compared with women in the bottom quartile, those in the top quartile of DIL were less likely to be a current smoker. However, men in the highest quartile of DIL were more likely to be university graduated compared with those in the lowest quartile. In terms of DII, women in the fourth quartile of DII were less likely to be a current smoker than those in the first quartile. In addition, men in the highest quartile of DII were more likely to be university graduate than those in the lowest quartile.
Table 1. General characteristics of men and women across quartiles (Q) of dietary insulin load (DIL) and dietary insulin index (DII)
(Mean values and standard deviations; percentages)
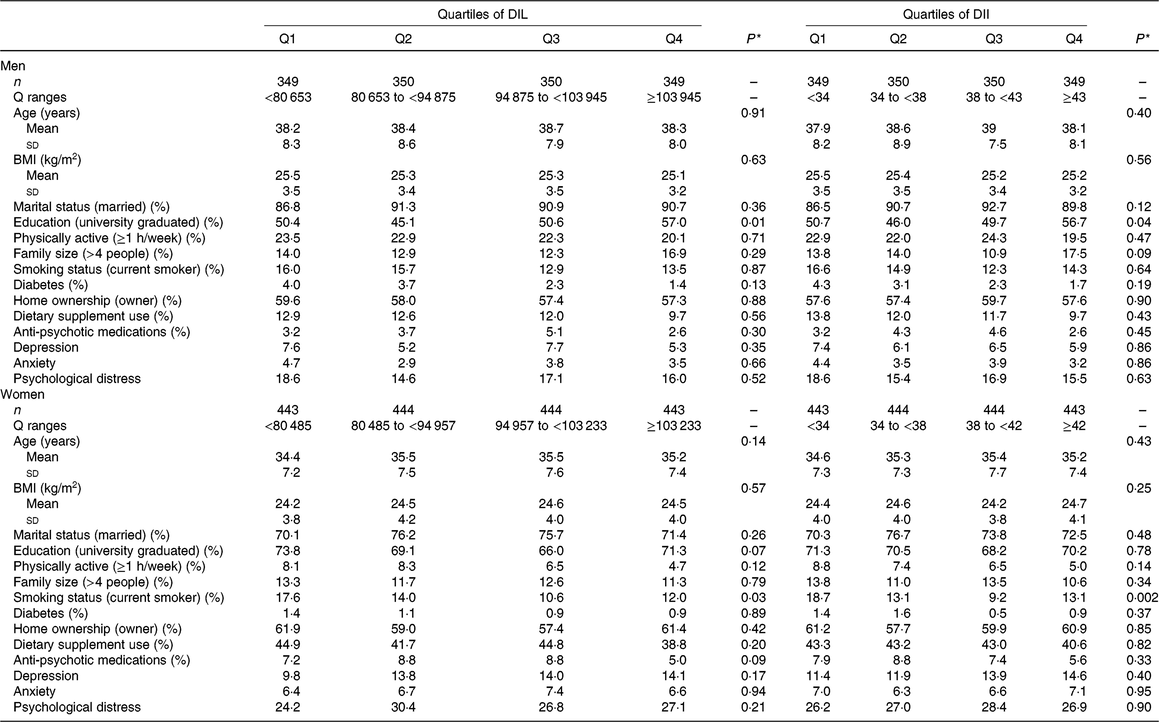
* Obtained from ANOVA or χ 2 test, where appropriate.
Selected food and nutrient intakes of men and women across quartiles of DIL and DII are shown in Table 2. Men and women in the top quartile of DIL had greater intakes of whole grains, refined grains, dairy products and carbohydrate and had lower intakes of fruits, red meat, fish, legume and nuts, energy, protein and fat compared with those in the bottom quartile. Within DII, men and women in the top quartile had greater intakes of whole grains, refined grains, dairy products, carbohydrate and fibre and had lower intakes of fruits, vegetables, red meat, fish, legume and nuts, protein and fat than those in the bottom quartile.
Table 2. Dietary and nutrient intakes of men and women across quartiles (Q) of dietary insulin load (DIL) and dietary insulin index (DII)
(Mean values with their standard errors)
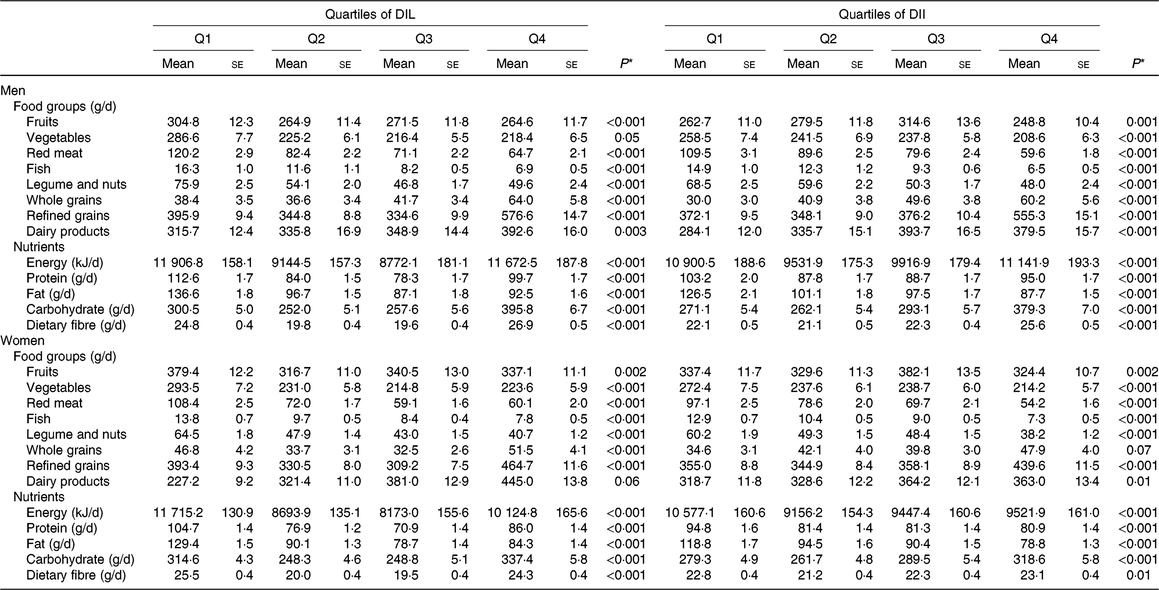
* Obtained from ANOVA.
Multivariable-adjusted OR for depression, anxiety and psychological distress across quartiles of DIL and DII in men and women are indicated in Table 3. After controlling for confounders, women in the top quartile of DIL had higher odds of depression compared with those in the first quartile (OR 1·84; 95 % CI 1·14, 2·96). No other significant association was found between DIL and psychological disorders either in men or in women. In terms of DII, in the fully adjusted model, women in the top quartile of DII were more likely to be depressed compared with those in the bottom quartile (OR 1·65; 95 % CI 1·05, 2·58). Neither in crude nor in adjusted models, we observed other significant relationships between DII and psychological disorders among men and women.
Table 3. Risk for psychological disorders according to quartiles (Q) of dietary insulin load (DIL) and dietary insulin index (DII)
(Odds ratios and 95 % confidence intervals)
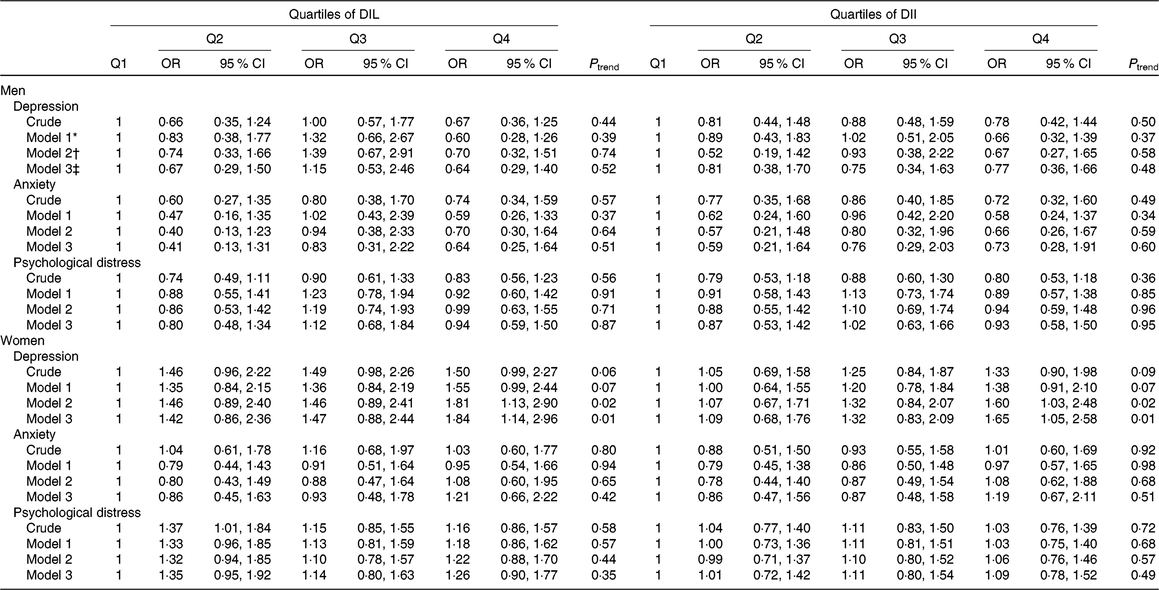
* Model 1: adjusted for age and energy intake.
† Model 2: additionally adjusted for marital status, education, family size, smoking status, physical activity, home ownership, diabetes mellitus, dietary supplement use and antipsychotic medications.
‡ Model 3: further controlled for BMI.
Education-stratified multivariable-adjusted OR for depression, anxiety and psychological distress across quartiles of DIL and DII are shown in Table 4. Neither in crude nor in adjusted models, we observed a significant relationship between DII and DIL and psychological disorders among men and women based on their educational levels.
Table 4. Risk for psychological disorders according to quartiles (Q) of dietary insulin load (DIL) and dietary insulin index (DII)
(Odds ratios and 95 % confidence intervals)
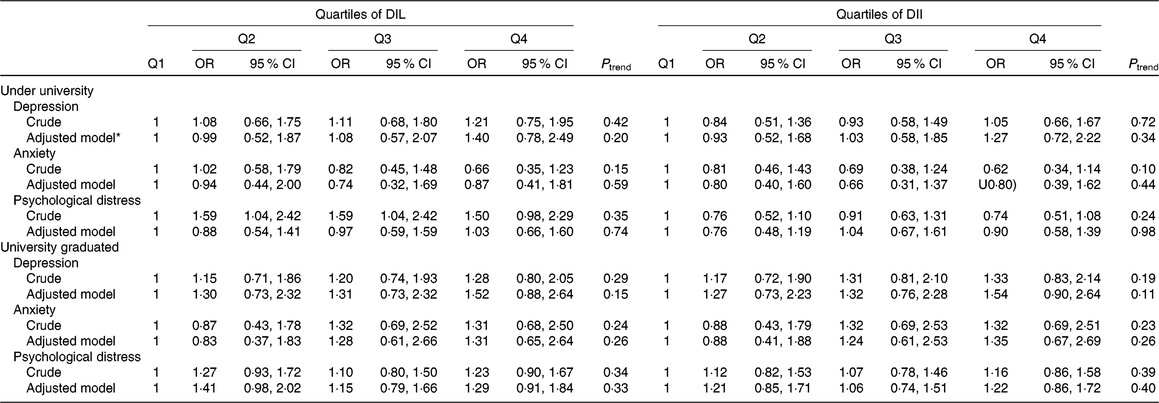
* Adjusted model: adjusted for age, sex, BMI, energy intake, marital status, education, family size, smoking status, physical activity, home ownership, diabetes mellitus, dietary supplement use and antipsychotic medications.
Regression coefficients for the relationship between DIL and DII and psychological disorders, when all were considered as continuous variables, are indicated in Table 5. Although no significant linear association was seen between DIL and DII and depression as well as anxiety scores, we found that DIL and DII were significantly associated with scores of psychological distress.
Table 5. Regression coefficients for the relationship between dietary insulin load (DIL) and dietary insulin index (DII) and scores of psychological disorders
(β-Coefficients and 95 % confidence intervals)
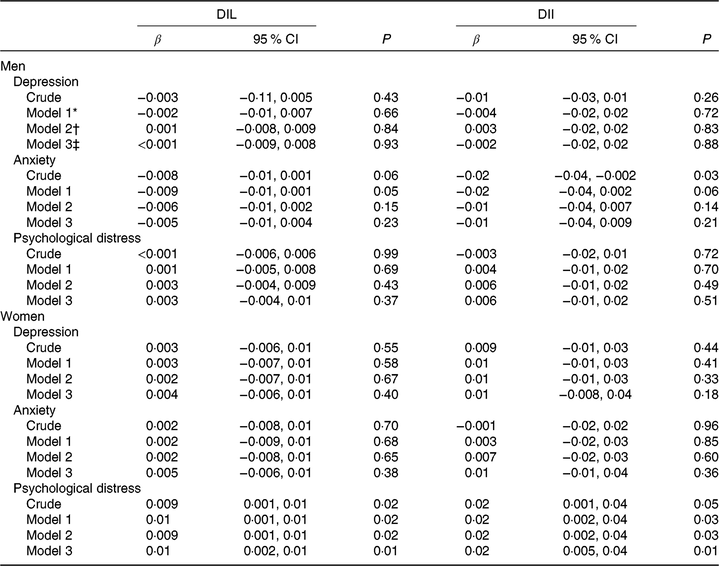
* Model 1: adjusted for age and energy intake.
† Model 2: additionally adjusted for marital status, education, family size, smoking status, physical activity, home ownership, diabetes mellitus, dietary supplement use and antipsychotic medications.
‡ Model 3: further controlled for BMI.
Discussion
In this cross-sectional study, we found that greater DIL, as well as DII, was linked significantly with higher odds of depression among women, even after adjustment for potential confounders. However, no significant association was seen among men. To our knowledge, the present study is the first to examine the relationship between DIL and DII and depressive symptoms worldwide.
It has long been recognised that major depressive disorders are more prevalent among low socio-economic groups(Reference Everson, Maty and Lynch30). However, the apparently adverse associations of DIL and DII with psychological disorders persisted in multivariate models accounting for known risk factors. For instance, we controlled the analyses for several variables of economic situation such as family size and homeownership. In addition, most dietary supplements contain several forms of B vitamins and n-3 fats, which play a neuroprotective role(Reference Firth, Teasdale and Allott31); therefore, we have taken supplement use into account as a confounder to reach an independent association between DII and DIL and psychological disorders. Some intermediary events, including dyslipidaemia or hypertension, might have led to changes in diet and may, therefore, confound the association between DIL and DII and depression. However, in the present study, we excluded all participants with self-reported chronic conditions.
Depression, a common mental illness, is a globally increasing condition associated with poor quality of life and social outcomes(Reference Murphy, Horton and Laird32,Reference Olesen, Gustavsson and Svensson33) . Psychological disorders could be prevented through several strategies emphasising on environmental factors including dietary intakes(Reference Le Port, Gueguen and Kesse-Guyot34). Although the link between dietary GI and GL and mental health status has been evaluated in several studies(Reference Gangwisch, Hale and Garcia35), there is no study examining DIL and DII in relation to these disorders.
In the present study, we found a significant positive association between DIL, DII and depression among women, but not in men. Similar to our observations, a cross-sectional study carried out on 976 homebound elderly US subjects, demonstrated that higher GL and GI were associated with a higher risk of depression(Reference Mwamburi, Liebson and Folstein8). Such a positive relationship between dietary GI and depression was also seen in other publications(Reference Haghighatdoost, Azadbakht and Keshteli36). Dietary GL (but not GI) was inversely linked with depression in 140 elderly Spanish people aged 65–90 years(Reference Aparicio, Robles and Lopez-Sobaler37). Others reported no association between dietary GI and GL during pregnancy and postpartum depression among 865 Japanese women(Reference Murakami, Miyake and Sasaki38). Additionally, an Australian cross-sectional study reported a positive correlation between dietary GI (but not GL) and depressive symptoms assessed by the Mental Health Index in 1981 adults (≥55 years of age)(Reference Gopinath, Flood and Burlutksy39). Likewise, in a meta-analysis, Salari-Moghaddam et al. (Reference Salari-Moghaddam, Saneei and Larijani40) found a significant effect of a high-GL diet on depression based on data from clinical trials, while no relationship was found between dietary GI and GL and odds of depression summarising published cross-sectional studies. As mentioned, all these studies have focused on dietary GI and GL and nothing is available about DIL and DII and risk of depression. To address the insulin hypothesis more directly, it may be more suitable to use food energy, as the constant, allowing all foods, not just those with sufficient carbohydrate content, to be investigated. In this method, all dietary components and their metabolic interactions could be considered(Reference Bell, Petocz and Colagiuri41). Additionally, as the aetiological hypothesis addressing the risk of psychological disorders is primarily related to hyperinsulinaemia, therefore using other dietary surrogate measures for the insulin responses such as GI, GL or total carbohydrate intake is indirect and conceptually suboptimal(Reference Anderson, Freedland and Clouse7). Hence, we used novel dietary insulin scores to quantify directly the postprandial insulin response. In addition, using DIL and DII is of more significance given that several dietary factors such as fructose, certain amino acids and fatty acids as well as gastrointestinal hormones such as gastric inhibitory peptide, glucagon and cholecystokinin are known to mediate postprandial insulin secretion(Reference Holst and Gromada42). Thus, protein- and fat-rich foods might lead to substantial insulin secretion despite producing relatively small blood glucose responses(Reference Collier, McLean and O’Dea43,Reference Nuttall and Gannon13) . However, some other variables, such as cooking methods, which might interact with the stimulatory effect of glucose, should not be ignored.
In the present study, we reached a sex difference in the relationship between DII and DIL and psychological disorders. The reason for this finding is unclear; however, it might be explained, at least in part, by the influence of gonadal steroids on mood(Reference Laurin, Lavoie and Bacon44,Reference Nolen-Hoeksema45) . Another reason for this sex disparity might be the difference in the accuracy of reported dietary intakes between men and women. Previous studies have shown that actual food choices(Reference Beer-Borst, Hercberg and Morabia46), self-reported preferences for foods(Reference O’Doherty Jensen and Holm47) and the accuracy of reported dietary intakes(Reference Marks, Hughes and van der Pols48) are different between men and women.
The underlying aetiology of the association between dietary insulin indices and depressive symptoms remains unclear; however, several probable mechanisms have been given. Insulin regulation through dietary factors might influence mood disorders as reported by experimental and human studies(Reference Shomaker, Kelly and Pickworth9,Reference Okamura, Tashiro and Utsumi10,Reference Szczesny, Slusarczyk and Glombik49) . For instance, in a rat model, inactivation of the insulin receptor in the hypothalamus results in systemic insulin resistance, dyslipidaemia as well as depressive-like behaviour(Reference Grillo, Mulder and Macht50). In addition, it has been suggested that consumption of a high glycaemic diet might also lead to insulin resistance which is in turn linked to a pattern of volumetric and neurocognitive repercussions that are highly similar to those reported in individuals suffering from major depression(Reference McIntyre, Kenna and Nguyen51). Another path by which such diets might contribute to depression is their influence on chronic inflammation(Reference Rumsfeld and Ho52).
The present study has several strengths. As far as we know, this is the first study examining the association between dietary insulin indices and psychological disorders. Additionally, its large sample size of adults, including either sex, should also be considered. Furthermore, in order to reach an independent association between DII and DIL and psychological disorders, we controlled the analyses for several potential confounders such as dietary supplements. Sex-stratified analysis along with the use of a validated FFQ for dietary assessment is among other strengths of the present study. However, the present study had several limitations. The design of our study was cross-sectional, which prohibits us from inferring causality. Therefore, further prospective studies are needed to confirm our findings. In addition, despite the use of a validated FFQ for dietary assessment, some degree of measurement errors and misclassification may have occurred. This is also the case about the outcome of interest and anthropometric indicators in the present study. Even though a significant correlation between self-reported and measured data on different variables was revealed in our validation study, some sort of errors might also occur in this case. In addition, given that no biochemical parameters were measured, some undiagnosed participants with different chronic diseases might be included in the present study(Reference Beagley, Guariguata and Weil53). Furthermore, some sort of co-linearity might have occurred in the last model after adjusting for BMI. However, most previous studies have shown a significant association between obesity and mental disorders(Reference Chauvet-Gelinier, Roussot and Cottenet54). In addition, based on our previous publication, DII was also significantly associated with obesity(Reference Anjom-Shoae, Keshteli and Sadeghi55). This is why we adjusted for BMI in an additional model to reach an independent association. Additionally, due to the limited number of foods with a tested FII value, for foods that were not available in the database, we used the values for similar foods.
In conclusion, adherence to a diet with a high DIL as well as high DII was associated with greater odds of depression in women, but not in men. However, such findings were not seen for anxiety and psychological distress. Further studies, in particular with a prospective design, in other populations are required to confirm these findings.
Acknowledgements
The authors would like to thank authorities in Tehran University of Medical Sciences for financial support of the study.
This research was supported by Tehran University of Medical Sciences, Tehran, Iran.
J. A.-S., A. H. K., H. A., P. A. and A. E. contributed to conception, design, statistical analyses, data interpretation and manuscript drafting. J. A.-S. and A. E. contributed in data analysis, data interpretation and manuscript drafting. A. E. supervised the study. All authors approved the final manuscript for submission.
None of the authors has any conflicts of interest to declare.








