Adiponectin, a hormone expressed mostly in adipose tissue and encoded by the APM1 gene (chromosome 3q27), plays an important role in regulating insulin sensitivity, glucose and lipid metabolism besides its anti-inflammatory and anti-atherogenic properties( Reference Ahima 1 ). It has been suggested that the synthesis and secretion of adiponectin are influenced by body fat distribution, sex and ethnicity. Low levels of adiponectin are found in patients with obesity, type 2 diabetes mellitus and coronary artery disease( Reference Ahima 1 , Reference Schulze, Shai and Rimm 2 ). More recently, we also found that the presence of the metabolic syndrome and the increasing number of its components are associated with decreased adiponectin concentrations( Reference von Frankenberg, do Nascimento and Gatelli 3 ). Therapeutic strategies that target the metabolic syndrome and its components have been shown to increase adiponectin concentrations, such as lifestyle modification involving moderate- or high-intensity physical activities and weight loss( Reference Rossmeislova, Malisova and Kracmerova 4 , Reference Simpson and Singh 5 ).
Although different nutrients may affect adiponectin concentrations, it is not clear how changes in the amount and quality of macronutrients affect its concentrations( Reference Mantzoros, Williams and Manson 6 ). In one study( Reference Arvidsson, Viguerie and Andersson 7 ) where subjects were randomised to receive hypoenergetic moderate-fat/moderate-carbohydrate v. low-fat/high-carbohydrate diets, no changes in adiponectin concentrations were observed over 10 weeks of dietary intervention. In other intervention studies, the comparison between diets with low and high fat content showed conflicting results. While adiponectin concentrations were not affected in one study( Reference Keogh, Brinkworth and Noakes 8 ), intake of a low-fat diet was associated with a 30 % increase in the concentrations of adiponectin in another study( Reference Wycherley, Brinkworth and Keogh 9 ). These differences probably suggest that the quality rather than the amount of fat may have a significant influence on adiponectin concentrations. This may be exemplified by analysing the effect of a Mediterranean diet on adiponectin concentrations. Close adherence to a Mediterranean diet has been associated with higher adiponectin concentrations( Reference Trichopoulou, Costacou and Bamia 10 ). This may be explained not only by its low glycaemic load and moderate alcohol consumption, but also by its composition that is rich in nuts, olive oil and fish, all of which are dietary sources of unsaturated fatty acids( Reference Mantzoros, Williams and Manson 6 , Reference Qi, Rimm and Liu 11 , Reference Silva, de Almeida and Feoli 12 ). As a result, these data pointed out that lipids are outstanding among potential dietary modulators of circulating adiponectin.
Other dietary lipids such as conjugated linoleic acid (CLA), dietary cholesterol and long-chain n-3 PUFA have been associated with a variable response to adiponectin concentrations( Reference Silva, de Almeida and Feoli 12 ). Regarding n-3 PUFA, a well-conducted systematic review and meta-analysis showed that its intake was associated with a significant increase in adiponectin concentrations( Reference Wu, Cahill and Mozaffarian 13 ). However, the results of that meta-analysis need to be interpreted with caution as a significant and unexplained heterogeneity was present between studies included in its results. Therefore, the present meta-analysis aimed to systematically review and analyse randomised controlled trials (RCT) investigating the effects of dietary lipids on circulating adiponectin concentrations in adults.
Methods
A systematic review was conducted using a predetermined protocol established according to the Cochrane Handbook's recommendations( 14 ). Results are reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement( Reference Liberati, Altman and Tetzlaff 15 ).
Search strategy
A literature review was conducted by searching the electronic databases Medline, Embase and Scopus until July 2013 to identify RCT that reported the effect of dietary lipids on adiponectin concentrations in adults. The initial search included the key search terms ‘dietary lipid’ and ‘adiponectin’. It also included the entry terms associated with a high-sensitivity strategy for the search of RCT (available at http://www.sign.ac.uk/methodology/filters.html#random). The complete ‘Medline’ search strategy is described in the online supplementary material.
Inclusion and exclusion criteria
We included only those RCT that analysed the effect of dietary lipids on fasting concentrations of circulating total adiponectin. The outcome was changes in adiponectin concentrations from baseline to the end of the study. Studies that met the initial criteria were identified, regardless of language or publication date.
We excluded the studies that did not report the outcome, were not randomised, or included children or pregnant women. Controlled trials that analysed the interaction between dietary interventions and changes in adiponectin concentrations corresponding to different polymorphisms were also excluded if they did not report the overall outcome regardless of polymorphisms. Although studies that did not report means and standard deviations for the outcome (separately for each group at baseline and at the end of the intervention, or changes from baseline for each group) were included in the review, these studies were not included in the meta-analysis. If data necessary for the review were missing, we contacted the authors by e-mail and/or telephone. The study was excluded if the reply was not received within 4 weeks. Of the thirteen authors who were contacted, nine( Reference Blüher, Rudich and Kloting 16 – Reference Troseid, Arnesen and Hjerkinn 24 ) replied back. Of these nine authors, three( Reference Blüher, Rudich and Kloting 16 , Reference Shademan, Rastmanesh and Hedayati 17 , Reference Troseid, Arnesen and Hjerkinn 24 ) provided the requested data to be included in the present meta-analysis.
Study selection and data extraction
For the present meta-analysis, two reviewers (A. D. v. F. and F. M. S.) independently analysed the titles and abstracts of the articles retrieved from the literature search, reviewed the full text of the published articles, and extracted the data using a standard data extraction protocol. Any disagreements between the reviewers regarding study inclusion were resolved by a third investigator (J. C. d. A. or F. G.).
The extracted data included the number of participants, study design, trial duration, and patients’ demographic and anthropometric characteristics (age, sex, height, weight, BMI, presence of obesity, the metabolic syndrome, hypertension, and dyslipidaemia). Data on total energy, macronutrients (type and amount) and dietary compliance were collected from the description of the intervention and control diets. Data extracted for dietary fat composition included the following: total, saturated, monounsaturated and polyunsaturated fats (g or percentage of total energy intake); n-3 PUFA (g); n-6 PUFA (g); cholesterol (mg). However, data for n-3 and n-6 PUFA were not available in most of the included studies. Data on means and statistical dispersion for adiponectin concentrations at baseline and at the end of the study were extracted. Percentage changes in adiponectin concentrations at the end of each study were calculated for all studies that presented baseline adiponectin values.
The included studies were grouped according to the following interventions: (1) total dietary lipid intake; (2) dietary/supplementary n-3 PUFA; (3) CLA supplementation; (4) other dietary lipid interventions.
Assessment of bias and quality of studies
The quality of the studies was assessed independently by two reviewers (A. D. v. F. and F. M. S.), and any disparity was resolved by a third reviewer (J. C. d. A. or F. G.). Biases were classified into six domains: selection; performance; detection; attrition; reporting; other( Reference Liberati, Altman and Tetzlaff 15 , Reference Higgins and Green 25 ). The ‘other’ domain included the assessment of dietary compliance. The risk of bias in each domain was classified as high, low or unclear. Regarding dietary compliance, the risk of bias was classified as ‘low’ if the study described the method for the assessment of dietary compliance.
Statistical analyses
Changes in adiponectin concentrations were reported as absolute differences between the values of arithmetic means and standard deviations at baseline and at the end of the study( Reference Follmann, Elliott and Suh 26 ). Heterogeneity between studies was assessed by Cochran's Q test, and a P for trend ≤ 0·10 was considered statistically significant. The I 2 test was also performed to evaluate the magnitude of heterogeneity, which was considered high if I 2≥ 50·0 %. Pooled estimates of the weighted mean differences (WMD) between dietary intervention and control groups were calculated using a random-effects model of DerSimonian & Laird( Reference DerSimonian and Laird 27 ) because a significant heterogeneity between the included studies was identified in preliminary models. Furthermore, this approach provides a more conservative assessment of the average effect size.
Potential sources of heterogeneity between trials were assessed by meta-regression analyses. Variables were chosen based on biological relevance before the meta-analysis was conducted. All meta-regression models included the following variables: age (less than the mean value, or equal to or greater than the mean value); sex (male, %); study location (Europe/North America v. others); time of the follow-up (equal to or less than the mean value, or greater than the mean value); BMI ( < 30 and ≥ 30 kg/m2); differences in weight change between groups. Blinding of participants/personnel was included in the n-3 PUFA meta-analysis as a meta-regression variable. This variable was neither included in the total dietary lipid meta-analysis as blinding was not clear in all studies, nor in the CLA supplementation meta-analysis as the risk of bias was low in all the studies. Additionally, specific variables were included in the three different meta-analyses according to relevance and availability. For total dietary lipid intake, a cut-off point for the amount of lipid intake was not defined as exclusion criteria. The difference in total energy intake, total dietary lipid intake between groups (difference in total energy intake < 1256 v. >1256 kJ/d (300 kcal/d)), median percentage point difference in lipid intake between groups ( ≤ 10 v. >10 % of lipid intake), and mean carbohydrate content in control groups ( < 30 v. >30 % of total energy intake) were included in the meta-regression models. Mean carbohydrate content was analysed only in the control group because it is expected to be dependent on the differences in the amount of lipid intake between groups. For n-3 PUFA and CLA supplementation, the amount of supplementation and the type of oil used as a placebo were also considered in the meta-regression models.
Subsequently, sensitivity (subgroup) analyses were conducted by including the variables with a positive adjusted R 2 value in meta-regression analyses, to determine how much of the between-study difference could be explained by these variables.
Publication bias was assessed by funnel plot asymmetry and Begg's or Egger's tests( Reference Peters, Sutton and Jones 28 – Reference Egger, Davey Smith and Schneider 30 ). The bias was considered significant if P< 0·10( Reference Begg and Mazumdar 29 , Reference Egger, Davey Smith and Schneider 30 ). The non-parametric trim-and-fill method was used to assess the potential influence of publication bias on sensitivity analyses, and provided a theoretical pooled estimate accounting for estimated missing studies( Reference Peters, Sutton and Jones 28 ).
All statistical analyses were performed using Stata 11.0 software (Stata). Significance was set at P< 0·05, and 95 % CI are quoted throughout.
Results
A total of 4930 studies were identified from the literature search (Fig. 1). On the basis of the titles and abstracts, ninety-one studies were selected for the full-text review, of which fifty-three fulfilled the final inclusion criteria. The included studies were grouped according to the following interventions: (1) total dietary lipid intake( Reference Arvidsson, Viguerie and Andersson 7 – Reference Wycherley, Brinkworth and Keogh 9 , Reference Blüher, Rudich and Kloting 16 , Reference Brons, Jensen and Storgaard 18 , Reference Varady, Bhutani and Klempel 19 , Reference Cardillo, Seshadri and Iqbal 31 – Reference Rajaie, Azadbakht and Saneei 39 ); (2) n-3 PUFA intake( Reference Sofi, Giangrandi and Cesari 21 , Reference Vargas, Almario and Buchan 22 , Reference Troseid, Arnesen and Hjerkinn 24 , Reference Krebs, Browning and McLean 40 – Reference Spencer, Finlin and Unal 55 ); (3) CLA supplementation( Reference Shademan, Rastmanesh and Hedayati 17 , Reference Risérus, Vessby and Arner 56 – Reference Joseph, Jacques and Plourde 61 ); (4) other dietary lipid interventions( Reference Bendsen, Stender and Szecsi 20 , Reference Taylor, Noto and Stringer 23 , Reference Nelson, Stevens and Hickey 62 – Reference Somerset, Graham and Markwell 71 ). The main results of the studies included in the meta-analysis are presented in Tables 1–3, whereas those included only in qualitative analyses are presented in online supplementary Table S2.
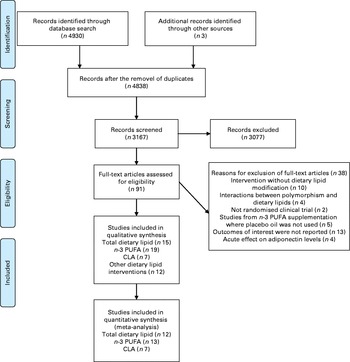
Fig. 1 Flow chart of the literature search and the study selection process( Reference Moher, Liberati and Tetzlaff 77 ). CLA, conjugated linoleic acid. For more information, visit www.prisma-statement.org. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Table 1 Characteristics of the studies investigating changes in adiponectin concentrations by modifying the amount of total dietary lipid intake

I, intervention; C, control; MetS, metabolic syndrome; AHA, American Heart Association; DM, diabetes mellitus; NA, not available.
* Significant change from baseline (P< 0·05).
† Significant difference between the intervention and control groups (P< 0·05).
‡ Adiponectin concentrations expressed as means and standard deviations.
Table 2 Characteristics of the studies investigating changes in adiponectin concentrations by n-3 PUFA intake

I, intervention; C1, control 1; C2, control 2; I1, intervention 1; C, control; I2, intervention 2; DM, diabetes mellitus; PCOS, polycystic ovary syndrome.
* Significant change from baseline (P< 0·05).
† Significant difference between the intervention and control groups (P< 0·05).
‡ Adiponectin concentrations expressed as means and standard deviations.
§ Adiponectin concentrations expressed as ng/ml.
Table 3 Characteristics of the studies investigating changes in adiponectin concentrations by conjugated linoleic acid (CLA) intake

I, intervention; C, control; DM, diabetes mellitus.
* Significant change from baseline (P< 0·05).
† Significant difference between the intervention and control groups (P< 0·05).
‡ Adiponectin concentrations expressed in as means and standard deviations.
Total dietary lipid intake
Of the total selected studies, fifteen( Reference Arvidsson, Viguerie and Andersson 7 – Reference Wycherley, Brinkworth and Keogh 9 , Reference Blüher, Rudich and Kloting 16 , Reference Brons, Jensen and Storgaard 18 , Reference Varady, Bhutani and Klempel 19 , Reference Cardillo, Seshadri and Iqbal 31 – Reference Rajaie, Azadbakht and Saneei 39 ) investigated the effects of a diet with a low-fat content (20–37 % of energy from lipids) on the circulating concentrations of adiponectin compared with a control diet with a high fat content (35–61 % of energy from lipids), as shown in Table 1. To test how differences in lipid quantity (expressed as the percentage of daily energy) may affect adiponectin concentrations, the diet with the lowest fat content was classified as an intervention diet in each study.
The median follow-up time was 14 weeks (5 d–144 weeks). These studies included seventeen to 322 participants (mean age 50 years). Most (71·4 %) of the studies included both sexes( Reference Keogh, Brinkworth and Noakes 8 , Reference Blüher, Rudich and Kloting 16 , Reference Varady, Bhutani and Klempel 19 , Reference Cardillo, Seshadri and Iqbal 31 , Reference Keogh, Brinkworth and Clifton 33 – Reference Yeung, Appel and Miller 36 , Reference Heggen, Klemsdal and Haugen 38 , Reference Rajaie, Azadbakht and Saneei 39 ). The mean difference in total dietary lipid intake between the intervention and control groups was 12·0 % of the total energy intake. Of these studies, seven( Reference Blüher, Rudich and Kloting 16 , Reference Cardillo, Seshadri and Iqbal 31 , Reference Ng, Watts and Barrett 32 , Reference Vetter, Wade and Womble 35 , Reference Summer, Brehm and Benoit 37 – Reference Rajaie, Azadbakht and Saneei 39 ) did not describe the lipid type and four( Reference Arvidsson, Viguerie and Andersson 7 , Reference Brons, Jensen and Storgaard 18 , Reference Yeung, Appel and Miller 36 , Reference Heggen, Klemsdal and Haugen 38 ) had no information about energy consumption. Differences in energy intake were not found to be significant in most of the studies, but were statistically significant only in one study( Reference Ng, Watts and Barrett 32 ). Among all the other studies that did not report a statistical difference in energy intake between the intervention and control groups, two studies were found to have an energy intake difference of 2173 and 1382 kJ (519 and 330 kcal). In the first study, there were no changes and differences in adiponectin concentrations between the groups throughout the study( Reference Al-Sarraj, Saadi and Calle 34 ), while in the other study, there was an increase in adiponectin concentrations within the groups, but not between the groups( Reference Vetter, Wade and Womble 35 ).
The risk of bias in the studies included in the quantitative analysis is summarised in online supplementary Table S1. The risk of selection bias was unclear in the majority of the studies, taking into account the lack of information about random sequence generation and allocation concealment. Performance bias was also unclear in all studies. Information about the blinding of outcome assessors was described in only one study( Reference Rajaie, Azadbakht and Saneei 39 ). Regarding attrition bias, the rates of dropouts and/or withdrawals were less than 20 % in nine studies( Reference Arvidsson, Viguerie and Andersson 7 , Reference Wycherley, Brinkworth and Keogh 9 , Reference Blüher, Rudich and Kloting 16 , Reference Brons, Jensen and Storgaard 18 , Reference Ng, Watts and Barrett 32 – Reference Al-Sarraj, Saadi and Calle 34 , Reference Summer, Brehm and Benoit 37 , Reference Heggen, Klemsdal and Haugen 38 ). Reporting bias was low in all studies. Dietary compliance was assessed in most studies.
Among the fifteen selected studies, twelve( Reference Arvidsson, Viguerie and Andersson 7 – Reference Wycherley, Brinkworth and Keogh 9 , Reference Blüher, Rudich and Kloting 16 , Reference Brons, Jensen and Storgaard 18 , Reference Cardillo, Seshadri and Iqbal 31 , Reference Ng, Watts and Barrett 32 , Reference Al-Sarraj, Saadi and Calle 34 , Reference Vetter, Wade and Womble 35 , Reference Summer, Brehm and Benoit 37 – Reference Rajaie, Azadbakht and Saneei 39 ) reported sufficient data and were thus included in the meta-analysis. The remaining three studies( Reference Varady, Bhutani and Klempel 19 , Reference Keogh, Brinkworth and Clifton 33 , Reference Yeung, Appel and Miller 36 ) were excluded due to the lack of sufficient data for quantitative analysis.
Among these three studies that were excluded from the quantitative analysis, one( Reference Yeung, Appel and Miller 36 ) showed a greater increase in adiponectin concentrations in the control group than in the intervention group, another( Reference Varady, Bhutani and Klempel 19 ) showed an increase in the concentrations of adiponectin in the control group than in the intervention group, and in the last one it was not possible to describe the differences between intervention and control groups because the results were not described separately by groups( Reference Keogh, Brinkworth and Clifton 33 ).
Overall, the intervention diet (28–37 % of the total energy intake from fat) did not increase adiponectin concentrations compared with the control diet (39–61 % of the total energy intake from fat) (WMD − 0·04 (95 % CI − 0·82, 0·74) μg/ml; I 2 83·7 %, P for heterogeneity < 0·001; Fig. 2(a)) . Given the significant heterogeneity between the included studies, we performed a meta-regression analysis by including one variable per model: age (adjusted R 2 − 9·6 %, P= 0·63); sex (adjusted R 2 − 16·5, P= 0·90); study location (adjusted R 2 − 9·8 %, P= 0·61); follow-up time (adjusted R 2 − 12·7 %, P= 0·87); BMI (adjusted R 2 − 10·3 %, P= 0·91); weight-loss difference between the intervention and control groups (adjusted R 2 − 11·1 %, P= 0·66); energy intake differences between the intervention and control groups (adjusted R 2 − 5·7 %, P= 0·41); percentage point difference in total dietary lipid intake between the intervention and control groups (adjusted R 2 − 15·1 %, P= 0·65); carbohydrate content in the control group (adjusted R 2 − 16·4 %, P= 0·88). In three studies( Reference Ng, Watts and Barrett 32 , Reference Al-Sarraj, Saadi and Calle 34 , Reference Summer, Brehm and Benoit 37 ), a significant change in body weight between the intervention and control groups was observed at the end of each trial. We also performed a sensitivity analysis with body weight used as a variable, which showed no significant change in the results. Publication bias was not observed in the present meta-analysis (Begg's test, P= 0·89; Egger's test, P= 0·21), and asymmetry was also not detected, as shown in the funnel plot (Fig. 3(a)).
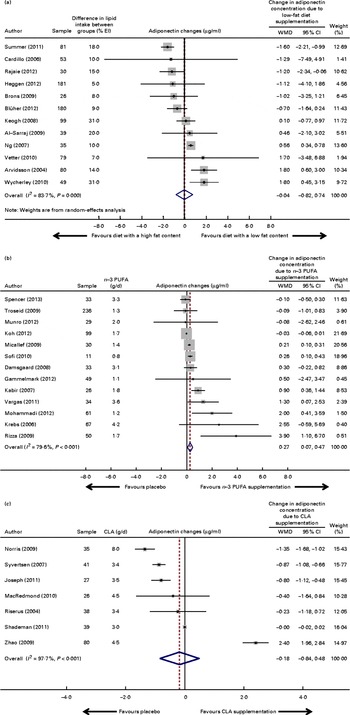
Fig. 2 Forest plots (meta-analyses, random-effects models) of the effect of fatty acid interventions on circulating adiponectin concentrations (μg/ml): (a) diet with a low fat content; (b) n-3 PUFA supplementation; (c) conjugated linoleic acid (CLA) supplementation. % EI, percentage of energy intake. For the Troseid et al. ( Reference Troseid, Arnesen and Hjerkinn 24 ) study, data for the main effect of fish oil intake on adiponectin concentrations were obtained directly from the authors and used in the pooled meta-analysis. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)

Fig. 3 Funnel plots of changes in circulating adiponectin concentrations in randomised trials with (a) a diet with a low fat content, (b) n-3 PUFA supplementation and (c) conjugated linoleic acid (CLA) supplementation. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
n-3 PUFA intake
Of the total selected studies, nineteen analysed the effect of n-3 PUFA intake on adiponectin concentrations: sixteen( Reference Sofi, Giangrandi and Cesari 21 , Reference Vargas, Almario and Buchan 22 , Reference Troseid, Arnesen and Hjerkinn 24 , Reference Krebs, Browning and McLean 40 – Reference Damsgaard, Frokiaer and Andersen 42 , Reference Sneddon, Tsofliou and Fyfe 45 – Reference Simão, Lozovoy and Bahls 52 , Reference Guebre-Egziabher, Debard and Drai 54 , Reference Spencer, Finlin and Unal 55 ) with n-3 PUFA supplementation and three( Reference Kratz, Swarbrick and Callahan 43 , Reference Ramel, Martinez and Kiely 44 , Reference Zhang, Wang and Li 53 ) with diets composed of n-3 PUFA-rich foods. The details of these studies are summarised in Table 2. The median follow-up time was 10 weeks (3–24 weeks). These studies included twenty-six to 324 participants, and most studies (54 %) included both sexes.
Dietary composition was described in ten studies( Reference Sofi, Giangrandi and Cesari 21 , Reference Vargas, Almario and Buchan 22 , Reference Krebs, Browning and McLean 40 , Reference Kabir, Skurnik and Naour 41 , Reference Kratz, Swarbrick and Callahan 43 , Reference Ramel, Martinez and Kiely 44 , Reference Mohammadi, Rafraf and Farzadi 50 , Reference Munro and Garg 51 , Reference Zhang, Wang and Li 53 , Reference Guebre-Egziabher, Debard and Drai 54 ). Comparisons between intervention (diet or supplementation) and fatty acid intake from different sources (placebo) were made in twelve studies( Reference Sofi, Giangrandi and Cesari 21 , Reference Vargas, Almario and Buchan 22 , Reference Troseid, Arnesen and Hjerkinn 24 , Reference Krebs, Browning and McLean 40 , Reference Damsgaard, Frokiaer and Andersen 42 , Reference Sneddon, Tsofliou and Fyfe 45 – Reference Gammelmark, Madsen and Varming 48 , Reference Munro and Garg 51 , Reference Simão, Lozovoy and Bahls 52 , Reference Spencer, Finlin and Unal 55 ). Among the dietary intervention studies, one( Reference Kratz, Swarbrick and Callahan 43 ) used different amounts of n-3 PUFA from plant and marine sources, while two( Reference Ramel, Martinez and Kiely 44 , Reference Zhang, Wang and Li 53 ) used different types of fish.
The risk of selection bias was unclear in the majority of the studies, taking into account the lack of information about random sequence generation and allocation concealment. In general, performance bias was low in most studies. Information about the blinding of outcome assessors was described in only three studies( Reference Troseid, Arnesen and Hjerkinn 24 , Reference Damsgaard, Frokiaer and Andersen 42 , Reference Munro and Garg 51 ). Attrition bias was low in ten studies( Reference Sofi, Giangrandi and Cesari 21 , Reference Troseid, Arnesen and Hjerkinn 24 , Reference Kabir, Skurnik and Naour 41 , Reference Damsgaard, Frokiaer and Andersen 42 , Reference Micallef and Garg 46 – Reference Mohammadi, Rafraf and Farzadi 50 , Reference Spencer, Finlin and Unal 55 ). Reporting bias was low in all studies. Dietary compliance was analysed in the majority of the studies (online supplementary Table S1).
Of these nineteen studies, thirteen( Reference Sofi, Giangrandi and Cesari 21 , Reference Vargas, Almario and Buchan 22 , Reference Troseid, Arnesen and Hjerkinn 24 , Reference Krebs, Browning and McLean 40 – Reference Damsgaard, Frokiaer and Andersen 42 , Reference Micallef and Garg 46 – Reference Munro and Garg 51 , Reference Spencer, Finlin and Unal 55 ) presented the data that could be pooled and used in a meta-analysis. Different oils were used as a placebo: four studies( Reference Sofi, Giangrandi and Cesari 21 , Reference Damsgaard, Frokiaer and Andersen 42 , Reference Rizza, Tesauro and Cardillo 47 , Reference Gammelmark, Madsen and Varming 48 ) used olive oil; three( Reference Troseid, Arnesen and Hjerkinn 24 , Reference Micallef and Garg 46 , Reference Munro and Garg 51 ) used sunola oil; one( Reference Vargas, Almario and Buchan 22 ) used soyabean oil; one( Reference Spencer, Finlin and Unal 55 ) used maize oil; two( Reference Kabir, Skurnik and Naour 41 , Reference Mohammadi, Rafraf and Farzadi 50 ) used paraffin oil; one study( Reference Krebs, Browning and McLean 40 ) used a mixture of linoleic and oleic oil; one study( Reference Koh, Quon and Shin 49 ) did not describe the oil type. Of these thirteen studies, only five( Reference Sofi, Giangrandi and Cesari 21 , Reference Krebs, Browning and McLean 40 , Reference Kabir, Skurnik and Naour 41 , Reference Mohammadi, Rafraf and Farzadi 50 , Reference Munro and Garg 51 ) reported the dietary composition in both intervention and control groups. None of these studies showed any differences in total energy or in the proportion of macronutrient intake between the two groups. Furthermore, no studies described the consumption of n-3 and n-6 PUFA.
Studies that had analysed the effects of n-3 PUFA-rich foods on adiponectin concentrations( Reference Kratz, Swarbrick and Callahan 43 , Reference Ramel, Martinez and Kiely 44 , Reference Zhang, Wang and Li 53 ) were not included in the quantitative analysis. In addition, one study( Reference Sneddon, Tsofliou and Fyfe 45 ) that combined n-3 PUFA intake with CLA supplementation in the intervention group, as well as two studies( Reference Simão, Lozovoy and Bahls 52 , Reference Guebre-Egziabher, Debard and Drai 54 ) in which data extraction was not available were excluded from the analysis. Among these six excluded studies( Reference Kratz, Swarbrick and Callahan 43 – Reference Sneddon, Tsofliou and Fyfe 45 , Reference Simão, Lozovoy and Bahls 52 – Reference Guebre-Egziabher, Debard and Drai 54 ), four did not show any significant change in adiponectin concentrations at the end of the intervention( Reference Kratz, Swarbrick and Callahan 43 – Reference Sneddon, Tsofliou and Fyfe 45 , Reference Guebre-Egziabher, Debard and Drai 54 ). However, in two studies( Reference Simão, Lozovoy and Bahls 52 , Reference Zhang, Wang and Li 53 ), an increase in adiponectin concentrations was observed at the end of the trial.
The pooled data from thirteen studies did show a modest and significant effect of n-3 PUFA supplementation on adiponectin concentrations (WMD 0·27 (95 % CI 0·07, 0·47) μg/ml; I 2 79·6 %, P for heterogeneity < 0·001; Fig. 2(b)). Given the high heterogeneity between the included studies, we performed a meta-regression analysis by including one variable per model. The independent variables were as follows: age (adjusted R 2 − 24·2, P= 0·16); sex (adjusted R 2 − 11·2, P= 0·11); study location (adjusted R 2 − 28·8 %, P= 0·59); follow-up time (adjusted R 2 − 47·0 %, P= 0·58); BMI (adjusted R 2 − 39·5 %, P= 0·42); blinding of participants/personnel (adjusted R 2 − 103·0 %, P= 0·71); amount of n-3 PUFA (g/d; adjusted R 2 − 64·8 %, P= 0·85); EPA (adjusted R 2 − 65·9 %, P= 0·83); docosapentaenoic acid (adjusted R 2 − 87·0 %, P= 0·60); fat type used as a placebo (vegetable oil v. paraffin oil; adjusted R 2 100 %, P= 0·04); change in body weight over the study period between the intervention and control groups (adjusted R 2 − 21·9 %, P= 0·60).
Subsequently, we performed a sensitivity analysis with fat type as placebo (unsaturated oil or paraffin oil), which revealed that n-3 PUFA supplementation was still associated with an increase in adiponectin concentrations. Studies that had used unsaturated oil as placebo showed an effect of n-3 PUFA supplementation on adiponectin concentrations (WMD 0·23 (95 % CI 0·04, 0·42) μg/ml; I 2 40·2 %, P for heterogeneity = 0·09) as well as those that had used paraffin oil as placebo (WMD 1·19 (95 % CI 0·24, 2·13) μg/ml; I 2 39·5 %, P for heterogeneity = 0·20). Studies that had used paraffin oil as placebo showed a greater increase (0·96 μg/ml) in adiponectin concentrations than those that had used vegetable oils as placebo.
Significant evidence of publication bias was found by Egger's test (P= 0·01) but not by Begg's test (P= 0·95). Visual inspection of the funnel plot confirmed the existence of asymmetry (Fig. 3(b)). In fact, a theoretical pooled estimate of 0·08 (95 % CI − 0·13, 0·30) μg/ml (P= 0·46) was obtained by using the trim-and-fill correction method after the addition of six theoretically unreported studies.
Conjugated linoleic acid supplementation
Of the total selected studies, seven( Reference Shademan, Rastmanesh and Hedayati 17 , Reference Risérus, Vessby and Arner 56 – Reference Joseph, Jacques and Plourde 61 ) assessed the effect of CLA (mixture containing cis-9, trans-11 and trans-10, cis-12) supplementation on adiponectin concentrations. The median follow-up time was 13·0 weeks (8–24 weeks). These studies included twenty-eight to eighty participants, aged 18 to 80 years. The details of these studies are summarised in Table 3.
In most studies, CLA supplementation (intervention) was compared with unsaturated fatty acid supplementation (placebo) such as olive oil( Reference Risérus, Vessby and Arner 56 , Reference Syvertsen, Halse and Hoivik 57 , Reference MacRedmond, Singhera and Attridge 60 ), safflower oil( Reference Norris, Collene and Asp 58 , Reference Joseph, Jacques and Plourde 61 )or soyabean oil( Reference Shademan, Rastmanesh and Hedayati 17 ). Only one study( Reference Zhao, Zhai and Wang 59 ) compared CLA supplementation with saturated fatty acid intake (placebo, mixture of fatty acids in capsules). The median CLA supplementation was 4·1 (range 3·0–8·0) g/d, with an equal mix of the two predominant isomers. Only two studies( Reference Norris, Collene and Asp 58 , Reference Zhao, Zhai and Wang 59 ) described the dietary composition.
The risk of selection bias was unclear in the majority of the studies, taking into account the lack of information about random sequence generation and allocation concealment. Performance bias was low in most studies. Information about the blinding of outcome assessors was described in only two studies( Reference Norris, Collene and Asp 58 , Reference Joseph, Jacques and Plourde 61 ). Attrition bias was low in five( Reference Shademan, Rastmanesh and Hedayati 17 , Reference Risérus, Vessby and Arner 56 , Reference Syvertsen, Halse and Hoivik 57 , Reference Zhao, Zhai and Wang 59 , Reference MacRedmond, Singhera and Attridge 60 ) out of seven studies( Reference Shademan, Rastmanesh and Hedayati 17 , Reference Risérus, Vessby and Arner 56 – Reference Joseph, Jacques and Plourde 61 ). Reporting bias was low in all studies. Dietary compliance was analysed in the majority of the studies (online supplementary Table S1).
All these seven studies( Reference Shademan, Rastmanesh and Hedayati 17 , Reference Risérus, Vessby and Arner 56 – Reference Joseph, Jacques and Plourde 61 ) were pooled in the meta-analysis. The pooled data did not show any significant effect of CLA supplementation on circulating adiponectin concentrations (WMD − 0·18 (95 % CI − 0·84, 0·48) μg/ml; I 2 97·7 %, P for heterogeneity < 0·001; Fig. 2(c)). A high level of heterogeneity was detected. The visual inspection of the funnel plot revealed the existence of asymmetry (Fig. 3(c)), suggesting a publication bias, although neither Begg's test (P= 0·76) nor Egger's test (P= 0·48) showed any evidence of the same. In fact, a theoretical pooled estimate of − 0·64 (95 % CI − 1·83 to 0·55) μg/ml (P= 0·29) was obtained by using the trim-and-fill correction method after the addition of one theoretically unreported study.
Given the significant heterogeneity between the included studies, we performed a meta-regression analysis by including one variable per model: age (adjusted R 2 − 21·1, P= 0·75); sex (adjusted R 2 − 5·6, P= 0·44); study location (adjusted R 2 41·9 %, P= 0·07); follow-up time (R 2 29·5 %, P= 0·13); BMI (adjusted R 2 11·6 %, P= 0·23); change in body weight over the study period between the intervention and control groups (adjusted R 2 − 9·0 %, P= 0·53); amount of CLA supplementation ( < 4·8 v.>4·8 g/d; adjusted R 2 11·6 %, P= 0·23); fat type used as a placebo (unsaturated v. saturated fat; adjusted R 2 56·0 %, P= 0·02).
Subsequently, a sensitivity analysis was performed with fat type used as a placebo (unsaturated or saturated fat). The analysis revealed a reduction in adiponectin concentrations with CLA supplementation after the removal of one study that had used saturated fat as placebo (WMD − 0·74 (95 % CI − 1·38, − 0·10) μg/ml; I 2 97·3 %, P for heterogeneity < 0·001). However, the analysis showed a high level of heterogeneity among the studies that used unsaturated fat as placebo.
Other dietary lipid interventions
Among the total selected studies, three analysed the effect of fatty acid intake on adiponectin concentrations (saturated fat( Reference Lithander, Keogh and Wang 63 ), α-lipoic acid( Reference Manning, Sutherland and Williams 69 ) and n-6 PUFA( Reference Bjermo, Iggman and Kullberg 68 )) and nine analysed the effect of the food source of lipids on adiponectin concentrations (eggs( Reference Ratliff, Mutungi and Puglisi 64 ), partially-hydrogenated oil( Reference Bendsen, Stender and Szecsi 20 , Reference Vega-Lopez, Matthan and Ausman 65 ), nuts( Reference Kalgaonkar, Almario and Gurusinghe 66 , Reference Aronis, Vamvini and Chamberland 67 , Reference Somerset, Graham and Markwell 71 ) and flaxseed( Reference Taylor, Noto and Stringer 23 , Reference Nelson, Stevens and Hickey 62 , Reference Kontogianni, Vlassopoulos and Gatzieva 70 )). The details of these studies are summarised in online supplementary Table S2. The median follow-up time was 9 weeks (4 d–48 weeks). These studies included fifteen to 160 participants, aged 20 to 80 years. However, these studies were not included in the meta-analysis due to the variability in dietary intervention.
A high consumption of saturated fat( Reference Lithander, Keogh and Wang 63 ), n-6 PUFA( Reference Bjermo, Iggman and Kullberg 68 ) and α-lipoic acid( Reference Manning, Sutherland and Williams 69 )did not show a significant effect on adiponectin concentrations. In contrast, intake of eggs increased the circulating concentrations of adiponectin( Reference Ratliff, Mutungi and Puglisi 64 ). Flaxseed intake reduced adiponectin concentrations in one study( Reference Nelson, Stevens and Hickey 62 ), but did not change its concentrations in other two studies( Reference Taylor, Noto and Stringer 23 , Reference Kontogianni, Vlassopoulos and Gatzieva 70 ). Intake of nuts increased adiponectin concentrations in two studies( Reference Kalgaonkar, Almario and Gurusinghe 66 , Reference Aronis, Vamvini and Chamberland 67 ), with no effect being found in one study( Reference Somerset, Graham and Markwell 71 ).
Discussion
The present systematic review with meta-analysis analysed how different types or amounts of dietary lipids affect circulating adiponectin concentrations. Intervention studies that compared diets with low and high fat content were not associated with any differences in adiponectin concentrations. However, it was observed that n-3 PUFA supplementation modestly increased the circulating concentrations of adiponectin, whereas CLA supplementation reduced the concentrations when compared with unsaturated fatty acid supplementation used as an active placebo.
In the present meta-analysis, a difference of 18·0 % of energy intake from total lipids between the intervention and control groups was not associated with changes in adiponectin concentrations, corroborating the idea that the quality of fat, rather than its amount, might have a more important role in modulating the concentrations of adiponectin. Although we found a high level of heterogeneity between the studies included in the present meta-analysis, this could not be explained by any factor in the exploratory analysis. Differences in carbohydrate content between the low-fat and high-fat dietary arms could also have an impact on adiponectin concentrations. We also performed a meta-regression analysis by including the differences in carbohydrate content between the study arms; however, this could not explain the high level of heterogeneity found between the included studies. In addition, differences in carbohydrate content may affect insulin resistance, which is a potential modifier of adiponectin concentrations( Reference Keogh, Brinkworth and Clifton 33 ). However, it was unlikely to explore the aspects associated with insulin resistance due to the lack of data in most studies.
The protective effect of high intake of oily fish on the risk of type 2 diabetes has been demonstrated in a recent meta-analysis( Reference Zhang, Wang and Li 53 ). Improvement in insulin sensitivity resulting from the intake of n-3 PUFA has been shown to be strongly associated with the increase in adiponectin concentrations. In fact, the utilisation of EPA and DHA in the culture medium of human and rat adipocytes increased the synthesis and secretion of adiponectin by the activation of PPARγ that acts as an insulin sensitiser( Reference Banga, Unal and Tripathi 72 ). In the present meta-analysis, n-3 PUFA supplementation modestly increased the circulating concentrations of adiponectin, suggesting the beneficial effect of this supplementation on adipocyte metabolism. Additionally, the well-known effect of n-3 PUFA intake on reducing TAG and increasing HDL-cholesterol levels( Reference Bernstein, Ding and Willett 73 ) may be partially associated with its effect on adiponectin secretion, which also improves lipid metabolism through the modulation of insulin sensitivity and fatty acid oxidation( Reference Karbowska and Kochan 74 ).
In contrast to the study of Wu et al. ( Reference Wu, Cahill and Mozaffarian 13 ), we found a possible explanation for the heterogeneity identified in the meta-analysis of n-3 PUFA supplementation. While updating the results published by Wu et al. ( Reference Wu, Cahill and Mozaffarian 13 ) by the addition of three studies( Reference Mohammadi, Rafraf and Farzadi 50 , Reference Munro and Garg 51 , Reference Spencer, Finlin and Unal 55 ), we showed using the meta-regression analysis that the type of the placebo oil (vegetable oil v. paraffin oil) could explain part of the heterogeneity found between the studies included in the meta-analysis. Studies that had used paraffin oil as placebo showed a greater increase in adiponectin concentrations than those that had used vegetable oils as placebo. We believe that the biological effect promoted by vegetable oils used as a placebo could reduce the difference in adiponectin concentrations between the intervention and control groups. Interestingly, even after grouping only those studies that used vegetable oils as placebo, the effect of n-3 PUFA intake remains to be significantly associated with an increase in adiponectin concentrations. However, it is likely that studies with negative results were not published. The inclusion of the studies that were not published would probably reduce the effect of n-3 PUFA intake on adiponectin concentrations, as we have already found. Therefore, caution needs to be exercised in the interpretation of the effect of n-3 PUFA intake on adiponectin concentrations.
CLA fatty acids are lipids derived from fatty tissues of ruminant animals. Some studies suggested that either the trans-10, cis-12 or cis-9, trans-11 isomer increased insulin resistance, but not a mixture of both isomers( Reference Risérus, Vessby and Arner 56 , Reference Riserus, Vessby and Arnlov 75 ). Additionally, it was found that supplementation of the trans-10, cis-12 isomer increases C-reactive protein, a well-defined marker of sub-chronic inflammation associated with insulin resistance, but not the supplementation of isomeric mixture( Reference Riserus, Vessby and Arnlov 75 ). A commercially prepared oil contains a 50:50 mixture of the trans-10, cis-12 and cis-9, trans-11 isomers. All studies included in the present meta-analysis assessed the effect of CLA oil as a mixture containing the cis-9, trans-11 and trans-10, cis-12 isomers compared with placebo. Although we showed no changes in adiponectin concentrations with CLA v. placebo supplementation, data from the sensitivity analysis suggested that CLA supplementation resulted in a reduction of circulating adiponectin concentrations when compared with unsaturated fat supplementation( Reference Shademan, Rastmanesh and Hedayati 17 , Reference Risérus, Vessby and Arner 56 – Reference Norris, Collene and Asp 58 , Reference MacRedmond, Singhera and Attridge 60 , Reference Joseph, Jacques and Plourde 61 ). This result could be attributed to the antioxidant properties of unsaturated fatty acids that might be more effective in modulating the concentrations of adiponectin( Reference Joseph, Jacques and Plourde 61 ). The high level of heterogeneity found between these studies could not be explained by BMI, the amount of CLA supplementation, and the change in body weight over the study period between the intervention and control groups. However, we found the role of blinding of subjects/personnel to be significant in explaining this heterogeneity. As a result, we should be cautious in concluding that there is no effect of CLA supplementation on adiponectin concentrations. Further intervention studies should address the role of CLA as a dietary supplement as well as the mechanisms by which CLA acts to regulate vital steps in the modulation of insulin sensitivity and adiponectin metabolism.
Although other types of fatty acid interventions (diet or supplementation) were identified, they were not included in the meta-analysis due to the lack of sufficient studies. Our data suggest that the consumption of nuts( Reference Kalgaonkar, Almario and Gurusinghe 66 , Reference Aronis, Vamvini and Chamberland 67 ), but not flaxseed( Reference Taylor, Noto and Stringer 23 , Reference Nelson, Stevens and Hickey 62 , Reference Kontogianni, Vlassopoulos and Gatzieva 70 ), is associated with increasing adiponectin concentrations; however, this effect needs to be further explored in RCT.
Although the literature search was conducted using multiple databases and was not restricted to the English language, the present meta-analysis has some limitations. First, despite several attempts to contact the authors of the published articles that had missing data by e-mail or telephone, some studies were excluded from the meta-analysis due to the delay in response. Second, funnel plot asymmetry was apparent with n-3 PUFA and CLA supplementation and may, in part, explain the heterogeneity found between the studies included in the present meta-analysis. To better understand this issue, we performed meta-regression and sensitivity analyses. These analyses revealed that the type of oil used as a placebo (paraffin oil or vegetable oil) in the studies that had used n-3 PUFA supplementation could explain part of the heterogeneity found in the present meta-analysis. Third, the lack of data on the actual consumption of n-3 PUFA has to be taken into account because it may have an influence on adiponectin concentrations. Fourth, differences in dietary composition between the control and intervention groups were not analysed because most of the included studies had limited data, hindering the analysis of the content of other dietary components, such as n-3 PUFA, n-6 PUFA, fibre and whole grains, that have been shown to affect adiponectin concentrations. Fifth, as complete data about the presence of diabetes and the metabolic syndrome, being associated with decreased adiponectin concentrations, were not identified in most of the studies included in the meta-analysis, the results of dietary intervention on subjects with and without them may distinctly affect its concentrations. Lastly, none of the studies included in the meta-analysis presented intention-to-treat analysis, a statistical approach that is usually associated with more conservative results( Reference Hollis and Campbell 76 ).
In conclusion, the present systematic review with meta-analysis of RCT suggests that, among the different interventions on dietary lipid intake, intake of low-fat diets were not associated with differences in adiponectin concentrations. n-3 PUFA supplementation was associated with moderate increases in adiponectin concentrations, whereas CLA supplementation seemed to be associated with a decrease in adiponectin concentrations compared with unsaturated fat intake. Caution needs to be exercised in interpreting these results because important sources of heterogeneity were found in the meta-analyses of n-3 PUFA and CLA supplementation. Therefore, future RCT are necessary to confirm these findings.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514002013
Acknowledgements
We thank Dr Jason H. Y. Wu for his critical suggestions. We thank authors Matthias Blüher, Reza Rastmanesh and Marius Troseid, all of whom provided additional data for the meta-analysis. We also thank authors Charlotte Brons, Krista Varady, Nathalie Bendsen, Francesco Sofi, Sidika Karakas and Carla Taylor, all of whom answered our questions by e-mail.
The present study was supported by the Foundation for Research Support of the State of Rio Grande do Sul (FAPERGS), Hospital de Clínicas de Porto Alegre Research Fund (FIPE 11-226), the National Council of Technological and Scientific Development (CNPq). F. G. was supported by a scholarship from the International Scholarship Program of the Endocrine Society. The funding sources had no role in the design or conduct of this meta-analysis.
The authors’ contributions are as follows: A. D v. F., F. M. S., J. C. d. A. and F. G. designed and performed the study; A. D. v. F., F. M. S., F. G., C. B. L., L. L. R. R. and D. U. analysed the data; A. D. v. F., F. M. S., F. V. d. N., V. P. and F. G. wrote the paper. All the other authors critically reviewed and improved the manuscript.
None of the authors has any conflict of interest to declare.








