Introduction
In recent decades, the consumption of bivalves has increased worldwide (FAO, 2016). Clams are among the most valued and commercially exploited bivalve species in the Mediterranean Sea. Despite historically representing a low percentage of total catches (< 5%), they are the target of different types of commercial fisheries (FAO, 2016). Most of the clam fisheries in this region are small-scale fisheries (i.e. they use small boats on shallow inshore waters performing daily fishing trips). These types of fisheries have historically been poorly managed, monitored and regulated because of their low economic importance, even though they represent socially and ecologically important activities (Guyader et al., Reference Guyader2013).
There is a commercial fishery targeting clams using mechanized dredges along the north-eastern coast of Spain. The wedge clam (Donax trunculus) and the striped venus clam (Chamelea gallina) are the main target species in this area (Baeta et al., Reference Baeta, Breton, Ubach and Ariza2018). Both species inhabit the Mediterranean Sea and Black Sea as well as the eastern Atlantic from the British Isles to Senegal (Saeedi & Costello, Reference Saeedi, Costello and da Costa González2012). They are shallow-burrowing, suspension-feeding organisms that live in high-density shoals (Özden et al., Reference Özden, Erkan and Ulusoy2009). Donax trunculus inhabits highly energetic environments on sandy beaches, where it is exposed to tidal rhythms, intense wave action and sediment instability (McLachlan & Brown, Reference McLachlan, Brown, McLachlan and Brown2006). It is distributed in well-sorted fine sand biocoenoses at depths of 0–2 m in the Mediterranean Sea and between 0–6 m on the Atlantic coast (Gaspar et al., Reference Gaspar2002). Chamelea gallina inhabits a wider variety of sediment types (sand, sandy-mud and mud), and is preferentially distributed on the coastal well-sorted fine sand biocoenosis between 3–12 m depth in the Mediterranean Sea (Papetti et al., Reference Papetti2018) and between 0–6 m on the Atlantic coast (Delgado et al., Reference Delgado, Silva and Juárez2013).
Governance and management of human activities in coastal ecosystems are essential to maximize the benefits that people obtain from these areas (Bundy et al., Reference Bundy, Chuenpagdee, Boldt, de Fatima Borges, Camara, Coll, Diallo, Fox, Fulton, Gazihan, Jarre, Jouffre, Kleisner, Knight, Link, Matiku, Masski, Moutopoulos, Piroddi, Raid, Sobrino, Tam, Thiao, Torres, Tsagarakis, van der Meeren and Shin2017). Knowledge about the impact of fishing activities on the coastal ecosystems is indispensable for sustainable and efficient management of the natural resources (Chuenpagdee et al., Reference Chuenpagdee, Lance, Sara, Norse and Daniel2003). Any fishing activity exerts an impact on marine diversity. However, not all fishing activities have the same impact on coastal ecosystems. Fishing gears are extremely diversified, particularly in the case of small-scale fisheries. Dredging has traditionally been considered among those practices with a greater impact on coastal benthic ecosystems (Collie et al., Reference Collie, Hall, Kaiser and Poiner2000). It includes many different types of fishing gears (e.g. mechanized clam dredging, hydraulic clam dredging, etc.) and each has a specific impact on megabenthic communities. Impacts also strongly depend on many other factors, such as the time scale (i.e. direct, short term and long term) (Piersma et al., Reference Piersma, Koolhaas, Dekinga, Beukema, Dekker and Essink2001); the technical characteristics of dredges (e.g. the length of the teeth, mesh size, etc.); the fishing effort; and local conditions (e.g. depth, granulometry, benthic communities, other stress factors, etc.) (Collie et al., Reference Collie, Hall, Kaiser and Poiner2000). Mechanized clam dredging has been an important activity over the last 70 years on the north-eastern coast of Spain (including Catalonia, Balearic Islands, Valencia and Murcia) (Baeta, Reference Baeta2015). However, its direct impact on megabenthic communities has never been evaluated in this geographic area. Effects of similar fishing gears have been described in the smooth clam (Callista chione) and the surf clam (Spisula solida) associated with megabenthic communities in southern Portugal (Chícharo et al., Reference Chícharo, Chícharo, Gaspar, Regala and Alves2002; Falcão et al., Reference Falcão, Gaspar, Caetano, Santos and Vale2003; Gaspar et al., Reference Gaspar2003). Recently, these impacts were assessed on C. gallina and D. trunculus in southern Spain (Alboran Sea) (Gallardo-Roldán et al., Reference Gallardo-Roldán2015; Urra et al., Reference Urra2017), but effects on the north-eastern coast of Spain have never been studied.
The main objective of the present study is to evaluate the discards and define the direct impact of mechanized clam dredging on the Catalan coast (north-eastern coast of Spain). To this end, we evaluated the direct impact on discarded species of the two main commercial species (D. trunculus and C. gallina) for the three main clam fishing areas in Catalonia.
Materials and methods
Study area
The study was carried out in Catalonia (north-east Spain) located in the north-western Mediterranean Sea (Figure 1). The Catalan coast stretches for over 600 km, from the French border (north-east) to the Ebro Delta (south-west). In Catalonia, the General Fisheries Directorate (GFD) of the ‘Departament d'Agricultura, Ramaderia, Pesca i Alimentació’ (Catalan Government) is in charge of managing clam fisheries. It has a specific management plan for clam dredge fisheries. This plan clearly defines: the users (fishermen), the target species (minimum legal size, maximum catch limits, etc.), fishing gear (mesh size, dredge characteristics and boat specifications) and the clam fishing areas. Each of the fishing areas has its own clam dredge fishing fleet. Within each fishing area, fishermen can target D. trunculus and C. gallina, but rarely target both on the same day, typically choosing the target species depending on many factors (abundance, market price, weather conditions, etc.). Each boat has four clam dredges (frame mouth width 60 cm; height 58 cm; depth 87 cm; mesh size of 11 × 11 mm; the same for both target species), which are simultaneously towed along the bottom of the sea for 20–30 min at a towing speed of 0.2–0.5 knots. Each boat performed between 15–20 tows per day.
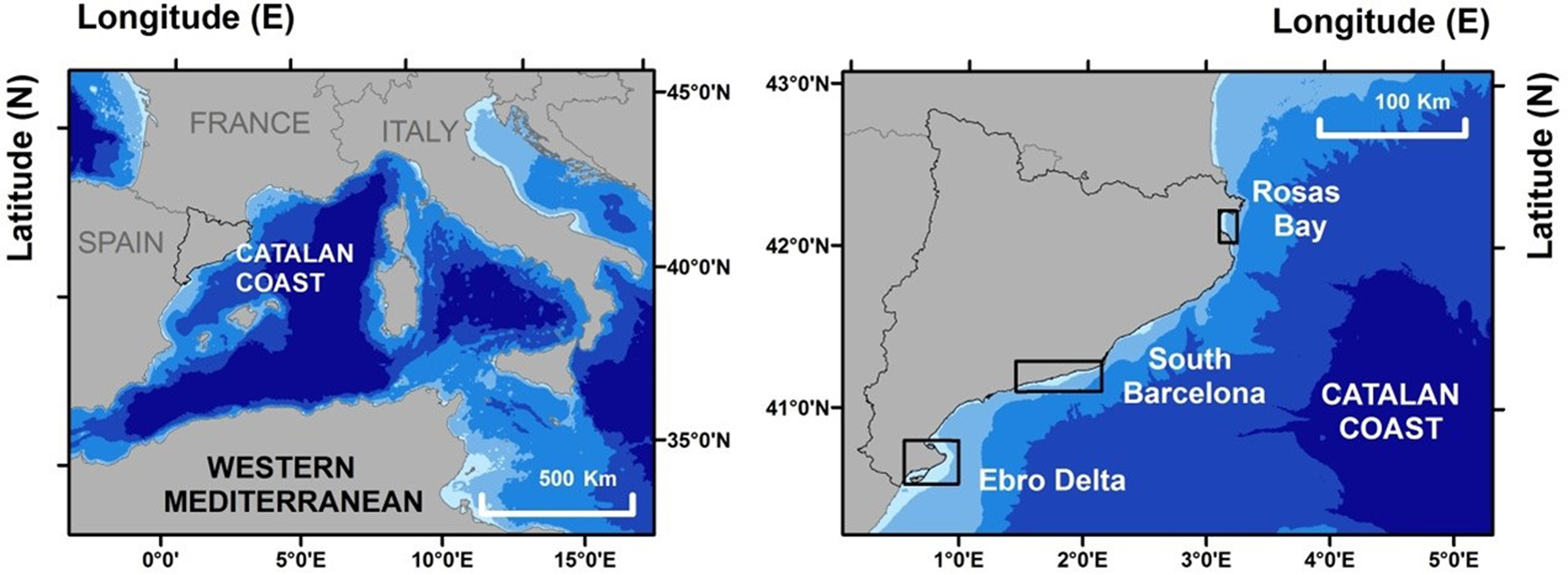
Fig. 1. Map of the study area along the Catalan coast (north-western Mediterranean Sea), showing the three clam fishing areas studied: Rosas Bay (RO), South Barcelona (SB) and Ebro Delta (ED).
There are three fishing areas currently open to commercial exploitation (Figure 1). These areas are located in shallow waters (<10 m) and are geographically isolated. Clam fishing is forbidden outside of these areas, which are: (1) Rosas Bay (RO): the smallest geographic area with a length of 15 km and with only one fishing port with clam fisheries, Rosas; (2) South Barcelona (SB) includes 70 km of coast, mainly fine sand beaches, and three fishing ports in Barcelona, Sitges and Vilanova; and (3) the Ebro Delta (ED): with an extension of 52 km of fine sandy beaches forming a delta with four ports with clam fishing activity (Alcanar, La Ràpita, Deltebre and l'Ampolla).
Sampling process
Three transects were defined in each clam fishing area (perpendicular to the coastline). Transects were selected as representative of the entire clam fishing area and were consecutively numbered from north-east to south-west (T1–T3). Each transect included two stations: the first at 1 m depth to target D. trunculus, and the second at 5 m depth to target C. gallina. Therefore, we set up a total of six stations in each clam fishing area (T1-1, T1-5, T2-1, T2-5, T3-1 and T3-5). The same fishing gear and fishing operation was used in the collection of both species.
Samples were collected at each station on board standard clam boats of the clam fishery (7–12.7 m in length and 20–45 CV). All tows were performed in parallel to the coastline. Four clam dredges (frame mouth width 60 cm; height 58 cm; depth 87 cm) (Figure 2) were used simultaneously in each tow (mesh size of 11 × 11 mm), which lasted 10–15 min at a towing speed of 0.2–0.5 knots. The catch of the four clam dredges were combined into a unique sample. The geographic position was recorded using GPS and the mean transect length was 95.54 m (SE ± 1.47). Two tows were made at each station for all surveys (i.e. 12 samples per fishing area). Surveys were performed for a specific period (September and November 2016 and January 2017) in each of the three delimited clam fishing areas (RO, SB and ED). Therefore, we obtained a total of 36 samples.

Fig. 2. (A) Small-scale boat involved in clam fishery with the four clam dredges on board; (B) traditional clam dredge used in the north-eastern coast of Spain.
All the samples collected were carefully transported to the laboratory. Each sample was sorted on a deck. The organism content was separated, counted, weighed with a digital balance (precision of 0.01 g), and measured with a digital caliper (precision of 0.1 cm). Abundance (ind m−2), biomass (g m−2) and frequency of occurrence (FO) were calculated to quantify the faunistic composition of the megabenthic community. Damage caused to individuals of commercial and non-commercial species was assessed using a three-level scale, depending on the damage degree to the taxa: undamaged (State 1); with minor or partial damage (State 2); and with lethal damage (State 3) following criteria listed in Table 1. These criteria were defined according to previous studies (Gaspar et al., Reference Gaspar2003; Urra et al., Reference Urra2017) with few modifications.
Table 1. Criteria to evaluate the direct damage of traditional clam dredges by taxonomic group
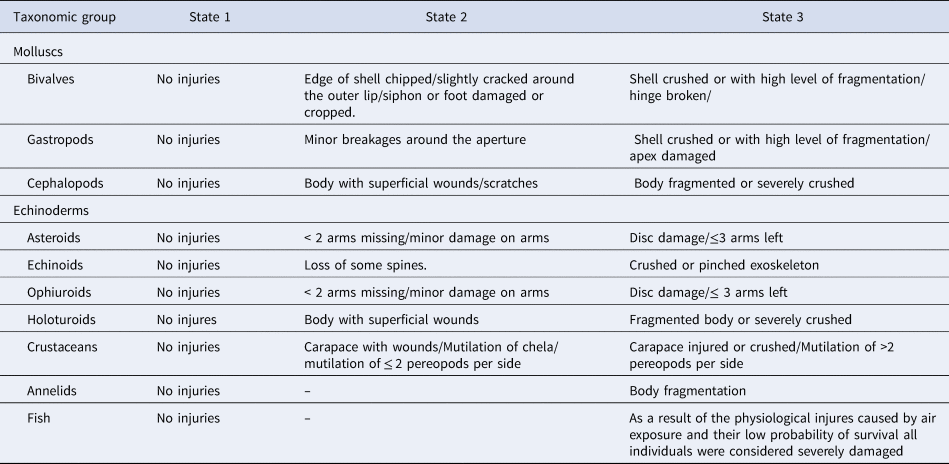
State 1: encompasses all individuals without visible damage (could be stressed by the activity); State 2: organisms with minor or partial damage. In most cases, damage occurred in parts with regenerative capacity; i.e. crustaceans, brittle stars and sea stars, as well as siphon or foot cropping in the case of bivalves; State 3: organisms with lethal damage caused by the clam dredge.
Statistical analyses
Abundance data of the faunistic composition was evaluated using a Multi-Dimensional Scaling analysis (MDS, resemblance by Bray–Curtis similarity) by target species (1 and 5 m depth) and by clam fishing area (RO, SB and ED). The minimum stress value of MDS was 0.11. The resulting groups of the MDS were tested with an Analysis of Similarities (ANOSIM) (Clarke & Warwick, Reference Clarke and Warwick2001). Similarity percentages (SIMPER) were used to define the species that typified each group (for each target species and clam fishing area) and the species that contributed to the dissimilarities between the different groups (Clarke & Gorley, Reference Clarke and Gorley2006). MDS, ANOSIM and SIMPER analyses were performed using PRIMER©. Abundances were log(x+1) transformed as a pre-treatment.
The direct impact of mechanized clam dredging on the discarded species was analysed using a Generalized Linear Mixed Model (McCullagh, Reference McCullagh1984). The effects of each variable (target species, clam fishing area and survey) on the proportions of damaged individuals (i.e. State 2 and State 3) per species was tested. GLMM was first applied to all discarded species and was then applied specifically to the three most abundant discarded species (i.e. Mactra stultorum, Liocarcinus vernalis and Echinocardium mediterraneum). The explanatory variables (target species, clam fishing area and survey) and their interactions were used as fixed factors. Transects were considered as a random variable nested with the variable target species in all the analyses. The species E. mediterraneum was not tested for the response variable State 2 due to the lack of organisms in this state. Additionally, E. mediterraneum was not tested in ED for the response variable State 3 due to its low presence. GLMM tests were performed with the software R©. The packages used were: lme4, lmerTest, pbkrtest and lsmeans.
Results
Faunistic composition of the discarded species
A total of 60 species were identified during the surveys of the catch of the mechanized clam dredging fishery associated with C. gallina and D. trunculus. Discards account for most of the catch for both target species. The proportion of the total discarded species was particularly high for C. gallina (75.95%) and biomass (81.51%) and slightly lower for D. trunculus (54.85%) and biomass (66.33%) (Table 2). Discards were mainly dominated by bivalves (22%), gastropods (20.3%), crustaceans (17%), fish (17%), echinoderms (6.8%), cnidarians (1.7%) and polychaetes (1.7%). However, other taxonomic groups such as cephalopods, nemerteans, sipunculids, and cnidarians were also observed in lower proportions (13.5%).
Table 2. %N indicates the proportion of the total discarded species
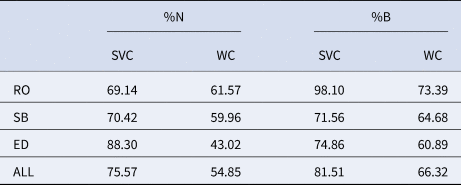
%B indicates the proportion of the total discarded biomass in wedge clam (WC) and striped venus clam (SVC) fisheries of Rosas Bay (RO), South Barcelona (SB), Ebro Delta (ED) and all clam fishing areas together (ALL).
The MDS showed two well-defined groups, separated by target species (i.e. C. gallina and D. trunculus) (Figure 3A) due to depth differences (1 and 5 m). The number of species was higher when fishermen targeted C. gallina (54 species) than in the case of D. trunculus (45 species). However, the frequency of occurrence was higher just in the case of Rosas Bay, but other areas are nearly equal and sometimes less (Table 3). Analysis of Similarities (ANOSIM) demonstrated that faunistic composition associated with the target species differed significantly between the two target species (P < 0.01, global R = 0.58). A SIMPER analysis showed the importance of the bivalves M. stultorum and Acanthocardia tuberculata and the crustacean decapod Diogenes pugilator associated with C. gallina; together they accounted for 69.42%. It also showed the importance of the bivalve M. stultorum, the echinoderm Echinocardium mediterraneum and the crustacean decapod L. vernalis associated with D. trunculus, which together accounted for 79.62%.
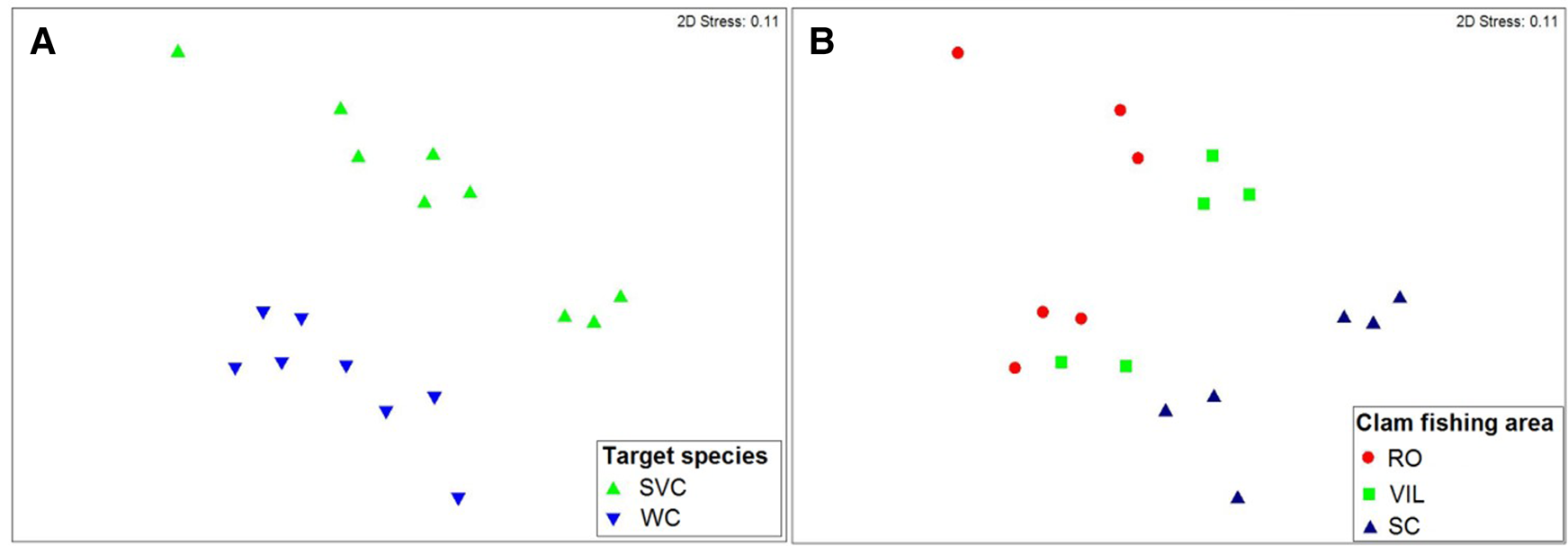
Fig. 3. Multidimensional scaling ordination (MDS) of abundance samples by (A) target species: SVC (C. gallina) and WC (D. trunculus) and (B) clam fishing area: Rosas Bay (RO), South Barcelona (SB) and Ebro Delta (ED).
Table 3. The 10 most abundant discarded species in wedge clam (WC) and striped venus clam (SVC) fisheries of the three clam fishing areas of Catalonia: Rosas Bay (RO), South Barcelona (SB), Ebro Delta (ED) and all clam fishing areas together (ALL)
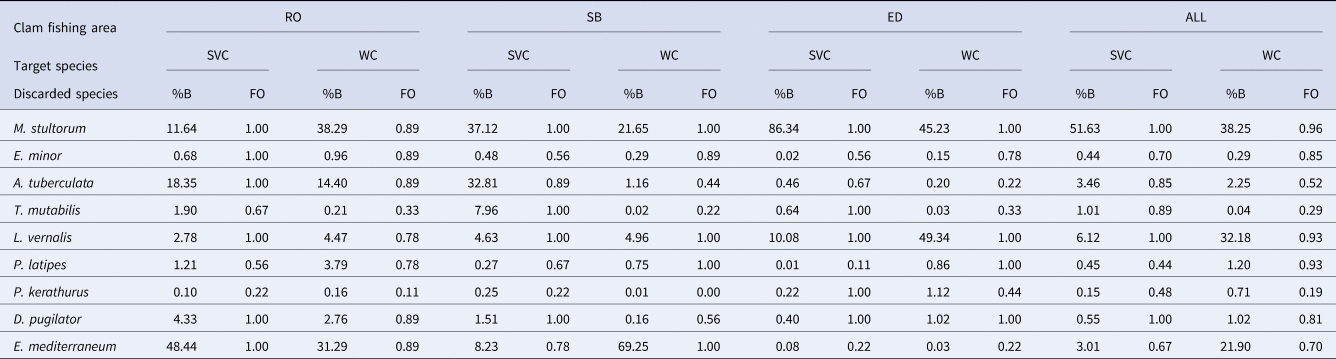
%B indicates the proportion of the total discarded biomass and FO indicates the frequency of occurrence in discards. The species M. stultorum, E. minor and A. tuberculata are bivalves; T. mutabilis is a gastropod; L. vernalis, P. latipes, P. kerathurus, D. pugilator are crustaceans and E. mediterraneum is an echinoderm.
The MDS analysis also showed three groups associated with the three clam fishing areas (RO, SB and ED) (Figure 3B). There is a clear geographic distribution of these three groups, from north-east RO (up to the left) to south-west ED (down to the right). Analysis of Similarities (ANOSIM) indicated that faunistic composition associated with the clam fishing areas differed significantly (P < 0.01, global R = 0.81). A SIMPER analysis showed the importance of the species E. mediterraneum, M. stultorum, A. tuberculata and D. pugilator in RO (84.74%); M. stultorum, E. mediterraneum and L. vernalis in SB (82.71%); and L. vernalis and M. stultorum in ED (90.57%).
Impacts of clam dredging
Taking all the data together (including both target species, clam fishing area and survey) about 66.75% underwent no damage (State 1), 11.11% presented minor or partial damage (State 2) and 25.73% showed lethal damage (State 3). Focusing on the discards associated exclusively with C. gallina, about 67.04% underwent no damage, 10.82% presented minor or partial damage and 22.14% showed lethal damage. Of the discards associated exclusively with D. trunculus, 59.12% underwent no damage, 11.41% showed minor or partial damage and 29.47% showed lethal damage. The impact of the mechanized clam dredging showed significant differences in the proportion of discarded megabenthic fauna with lethal damage between the three clam fishing areas studied (Table 4). It was highest in RO (32%), followed by ED (28%) and SB (20%). No significant differences in the proportion of species showing minor or partial damage were observed. Regarding the impact of the fishery on the discarded megabenthic fauna associated with each target species (D. trunculus and C. gallina) inside each fishing area, significant differences were observed in lethal damage. Considering the differences in the impact of fishing activity by survey (September, November and January), no significant differences were detected in the proportion of species showing lethal damage but significant differences in the proportion of individuals showing minor or partial damage was observed. However, when looking at the interaction of clam fishing area and survey, we observed significant differences in the proportion of both lethal and minor or partially damaged individuals. The proportion of discarded megabenthic fauna showing lethally damaged individuals was highest in January (27%), followed by November (26%) and the lowest in September (24%). Those showing minor or partially damaged individuals tended to increase progressively from September to January (from 10% to 14%).
Table 4. ANOVA tables of the GLMM for all the discarded species
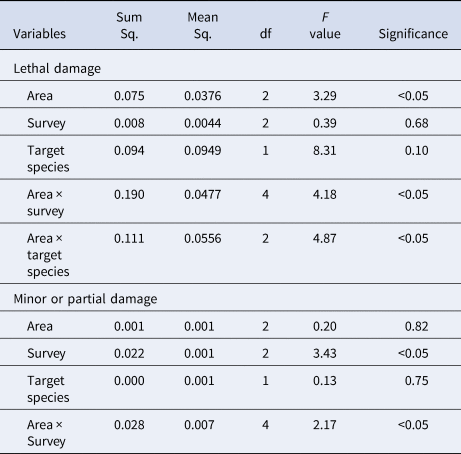
Response variables evaluated the proportion of individuals with lethal damage (State 3) and with minor or partial damage (State 2). Area means fishing clam area (RO, SB and ED); survey means the month of sampling (September, November and January); target species (wedge clam and striped venus clam).
The sea urchin E. mediterraneum (89%) and the bivalve Ensis minor (74%) were the species most affected by the clam dredging activity (Figure 4A). Damaged individuals of both species showed exclusively lethal damage; no minor or partial damage was observed. Individuals of E. mediterraneum usually appeared with crushed or pinched exoskeletons (Figure 5), whilst individuals of E. minor were segmented towards the middle of the body or in small fragments. Other affected species were the crustacean caramote prawn P. kerathurus (45%), the crabs L. vernalis (35%) and P. latipes (34%) and the bivalves M. stultorum (34%) and A. tuberculata (13%). Impacted individuals of caramote prawn showed only lethal or severe damage, such as segmented individuals or individuals with critical physical injuries (contusions) on the cephalothorax or the abdomen. No individuals with minor or partial damage were detected. Crabs (L. vernalis and P. latipes) showed similar proportions of individuals with minor or partial damage (between 15–17%) and lethal or severe damage (between 18–19%). Individuals with minor or partial damage showed wounds on the carapace, the mutilation of chela or the mutilation of ≤ 2 pereopods, while crabs with lethal or severe damage exhibited injured or crushed carapaces or the mutilation of >2 pereopods. The bivalves M. stultorum with minor or partial damage (15%) showed mainly cropped siphons and some displayed shells that were slightly cracked around the outer lip. Those showing lethal or severe damage (19%) had critical breaks in the shell, broken or crushed hinges or the entire individual was smashed. Bivalves A. tuberculata with minor or partial damage (11%) showed damaged or cropped feet, whereas individuals with lethal or severe damage (2%) showed the separation of shell valves or holes in the shell.
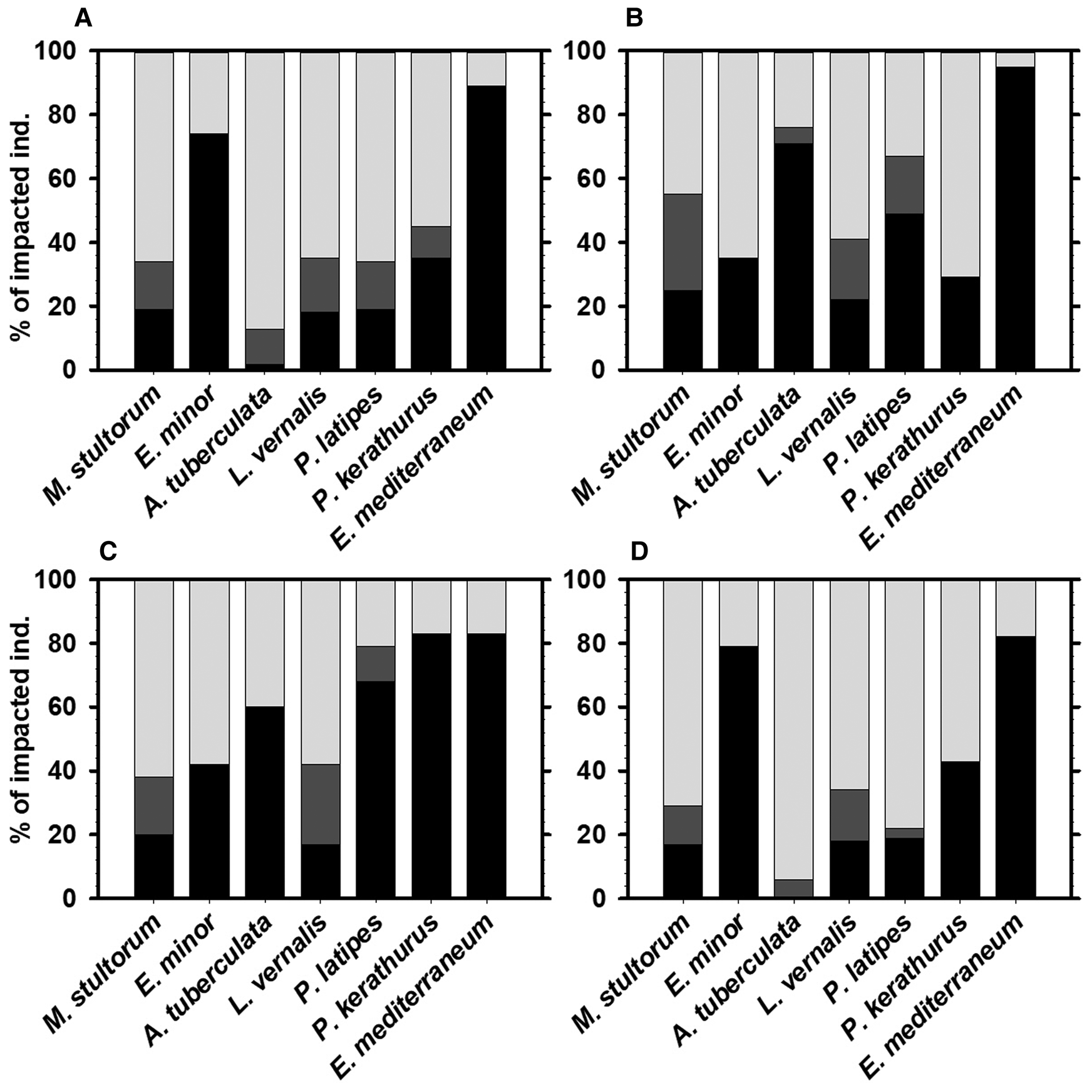
Fig. 4. Proportion of impacted individuals of the seven most common species for (A) all clam fishing areas together; (B) Rosas Bay (RO); (C) South Barcelona (SB) and (D) Ebro Delta (ED). Light grey columns represent individuals in State 1 (undamaged individuals), dark grey columns represent individuals in State 2 (individuals with minor or partial damage), and black columns represent individuals in State 3 (those with lethal damage).
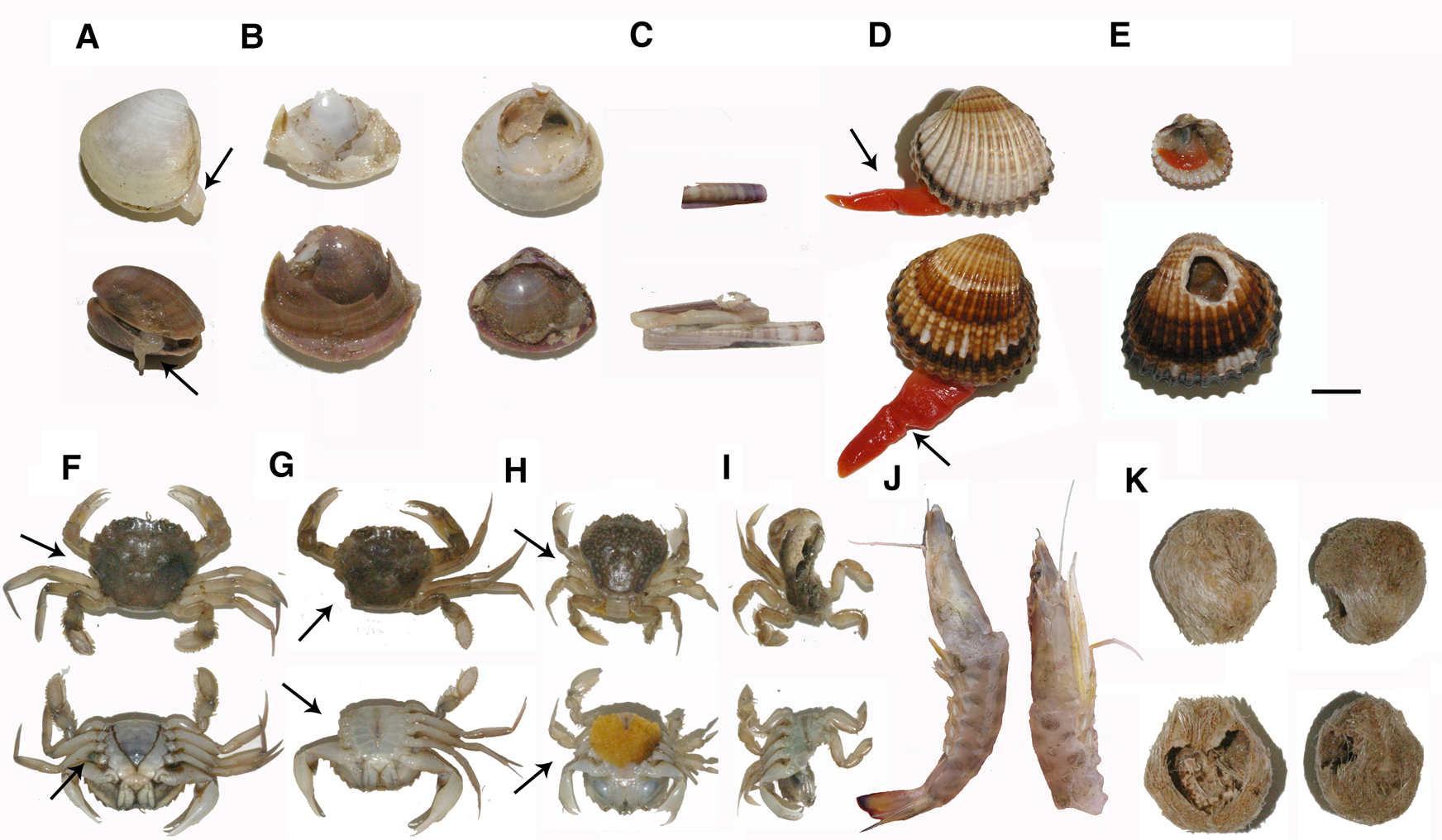
Fig. 5. Examples of common damage found in the most abundant discarded species. (A): M. stultorum, damaged individuals (siphon cropped or with deep scars) (State 2); (B): M. stultorum, broken and crushed individuals (State 3); (C): E. minor, broken individuals (state 3); (D): A. tuberculata, damaged individuals (foot cropped or with deep scars) (State 2); (E): A. tuberculata, broken and crushed individuals (State 3); (F): dorsal and ventral view of L. vernalis, individual with minor damage (1 walking leg missing) (State 2); (G): dorsal and ventral view of L. vernalis, individual with lethal damage (3 walking legs and 1 swimming leg missing) (State 3); (H): dorsal and ventral view of P. latipes, individual with minor damage (1 walking leg missing) (State 2); (I): dorsal and ventral view of P. latipes, individual crushed (State 3); (J): P. kerathurus, individuals with lethal damage (crushed carapace and mutilation of > 2 pereopods per side) (State 3); (K): dorsal and ventral view of E. mediterraneum, individuals with lethal damage on the test (crushed or pinched exoskeleton) (State 3). Black arrows indicate the location of the damage and scale bar width = 1 cm.
Focusing on the less discarded species, the more fragile bivalves such as Pharus legumen, Ensis ensis and Acanthocardia paucicostata suffered similar damage to those described in E. minor, since both species have soft shells. Impacted individuals of these species showed exclusively lethal or severe damage. However, most of the other bivalve species (e.g. Spisula subtruncata, Moerella pulchella, Bosemprella incarnata, Dosinia lupinus, Dosinia exoleta, Glycymeris glycymeris, Pandora inaequivalvis, etc.) only showed minor damage (e.g. siphons or foot damaged or cropped or slightly cracked around the outer lip). Gastropods (e.g. Tritia mutabilis, Tritia reticulate, Bolinus brandaris and Neverita josephinia) usually showed minor damage (e.g. superficial wounds or minor breakages around the aperture), while individuals with lethal or severe damage were very infrequent. Cephalopods (i.e. Sepiola sp., Sepia orbignyana and Octopus vulgaris) were rare in the discards. All cephalopod individuals showed minor damage (e.g. superficial wounds or scratches on the mantle or the arms). Asteroids (i.e. Astropecten irregularis) and Holoturoids (i.e. Holothuria poli) regularly did not show any external damage. Sea stars occasionally showed minor damage in their arms (e.g. < 2 arms missing) and rarely exhibited lethal and severe damage (i.e. disc holes or more than 3 arms missing), whereas sea cucumbers showed uniquely minor damage (i.e. the body slightly scratched or with smaller injuries such as contusions). Ophiuroids (i.e. Ophiura ophiura) showed in many cases minor damage (arms broken or missing), and individuals with lethal and severe damage were very infrequent. In the case of crustaceans, hermit crabs (i.e. Diogenes pugilator) regularly did not show any external damage, although sometimes specimens showed minor damage (i.e. the loss of the left or the right chela). Other crab species (e.g. Derilambrus angulifrons, Portunus hastatus, Liocarcinus depurator) showed similar damage to that described for L. vernalis and P. latipes. Annelids regularly showed lethal and severe damage (i.e. high level of fragmented or crushed bodies). Fish species always displayed lethal and severe damage. For example, Mullus surmuletus, Lithognathus mormyrus, Trachinus sp. and Solea sp. showed large amounts of missing scales. Torpedo torpedo and Raja sp. showed severe holes on the body because of the metallic teeth along the lower leading edge of the dredges and Ophisurus serpens always presented severe wounds with fragmented bodies. The fish species gathered were usually juveniles (i.e. individuals under the minimum landing size).
Discussion
Discarding is an extremely common practice worldwide. The volume of fishery discards is around 230,000 tons per year in the Mediterranean Sea (19% of the total catch) (FAO, 2016). Clam dredging often occurs in areas where the target species is the dominant species in megabenthic communities, and thus often the proportion of discards is relatively low (Butcher et al., Reference Butcher, Matthew, Glaister and Hamer1981). Nevertheless, the discard rate produced by the different types of clam dredging surpasses 40% of the catch in the Mediterranean and the Black Seas (FAO, 2016). Urra et al. (Reference Urra2017) found a proportion of 42% in weight in the D. trunculus fishery in south-west Spain. Our study showed values much higher than these percentages in all three clam fishing areas for both target species, i.e. C. gallina (>80% in weight) and D. trunculus (>66% in weight). Pranovi et al. (Reference Pranovi, Raicevich, Franceschini, Torricelli and Giovanardi2001) noted that the proportion of discards is related to gear and ground characteristics. The clam fisheries along the Catalan coast are currently collapsed or close to collapse, as is the case for most of the clam fisheries in the north-western Mediterranean Sea. The combination of inadequate management along with other factors (e.g. pathologies, pollution and climate change) over the decades, have led the clam stocks to this situation (Baeta et al., Reference Baeta, Breton, Ubach and Ariza2018). We can speculate that our higher values for the discards may be the result of the precarious situation of the target species stocks.
Clam dredging discards include most of the megabenthic communities associated with the target species (Pubill et al., Reference Pubill, Abelló, Ramón and Baeta2011). Our results revealed significant differences in the faunistic composition of megabenthic communities associated with the target species. Both species inhabit different depth ranges, and depth is a major factor influencing shallow soft-bottom benthic assemblages, with an increase in diversity with depth (Urra et al., Reference Urra, Gofas, Rueda and Marina2011). Species are distributed along a depth gradient, conforming to their ability to cope with biological factors (e.g. food availability, competition and predation) and physical factors (e.g. granulometry, hydrodynamics) (Carvalho et al., Reference Carvalho, Cunha, Pereira, Pousão-Ferreira, Santos and Gaspar2012). Results also showed differences by fishing area. These differences could be the result of small differences in local environmental conditions (Blomqvist & Bonsdorff, Reference Blomqvist and Bonsdorff1986) or anthropogenic factors (dredging activities for beach nourishment, other commercial fisheries, recreational activities etc.) (Thrush et al., Reference Thrush1998). Kashenko (Reference Kashenko2006) observed in laboratory experiments that Echinocardium cordatum has low levels of tolerance for changes in salinity, in particular to a decrease in the salinity. Moreover, this author observed that the salinity tolerance range of adults was limited to 33–28‰ (at 19 °C). Our results showed low abundances of E. mediterraneum in ED. This area is located at the mouth of the Ebro River and is subject to changes in salinity for the cyclical fluctuations in river discharge on the coast. Although we do not have information on the salinity tolerance ranges of E. mediterraneum, we suggest that the low abundance of this irregular echinoderm may be linked to these oscillations in the salinity concentration.
Mercaldo-Allen & Goldberg (Reference Mercaldo-Allen and Goldberg2011) highlighted that the intensity of the fishing activities (i.e. dredging and trawling) in benthic ecosystems can also influence differences in the biological communities. Fragile, slow recruiting, near-surface-dwelling, larger and long-lived species are particularly affected by these fishing activities (Macdonald et al., Reference Macdonald, Little, Eno and Hiscockl1996; Thrush et al., Reference Thrush1998; Pranovi et al., Reference Pranovi, Raicevich, Franceschini, Torricelli and Giovanardi2001). There are very few previous documents reporting the species composition of the megabenthic communities associated with the target species in the studied areas. In one example, Vives & Suau (Reference Vives and Suau1962) reported that the most abundant species in the discards of the striped venus clam in the Ebro Delta in the 1950s were the bivalves A. tuberculata, M. stultorum, D. lupinus and G. glycymeris. Although A. tuberculata and M. stultorum remain abundant, D. lupinus was extremely rare in ED (only five individuals were collected during the samplings) and G. glycymeris was not detected in ED. Glycymeris glycymeris is a large bivalve species (50–90 mm in diameter) (Poppe & Goto, Reference Poppe and Goto1993) that can live for over 70 years (Steingrimsson, Reference Steingrimsson1989). Moreover, they do not live deeply buried in the sand (Ansell & Trueman, Reference Ansell and Trueman1967). Dosinia lupinus has a fragile shell (Duineveld et al., Reference Duineveld, Bergman and Lavaleye2007). Species adapted to frequent natural disturbance, e.g. in shallow, sandy areas, are expected to be more resilient to repeated fishing disturbance. However, long-lived species are more impacted by fishing activity and recolonize more slowly after disturbance (Hall et al., Reference Hall, Basford and Robertson1990; Beukema, Reference Beukema1995). Bivalves with fragile shells are among the most impacted by clam dredging activity (Hall-Spencer & Moore, Reference Hall-Spencer and Moore2000; Urra et al., Reference Urra2017). Fish species gathered by the mechanized clam dredges were infrequent but always displayed lethal damage. The main factors affecting discarded fish are related to capture stresses, fishing conditions and biological attributes. Most of the fish specimens were juveniles, which are more sensitive to increased water temperature at the surface, as well as to handling on board (Suuronen, Reference Suuronen2005). Although a discarded fish may survive the discarding process, they are easy preyed upon by scavengers (Ryer et al., Reference Ryer, Ottmar and Sturm2004). Baeta et al. (Reference Baeta, Galimany and Ramón2016) showed that the combination of clam dredging and sand dredging for beach nourishment in the long term affected the entire megabenthic community associated with the clam C. chione and species situated in the upper trophic levels, i.e. the sea stars of the genus Astropecten were among the most affected species. These authors reported changes in sea star composition, distribution and abundance.
The percentage of discarded individuals impacted by the clam dredging activity was considerably higher than in previous studies. Gaspar et al. (Reference Gaspar2003) studied the white clam S. solida fishery (8–10 m depth) in Portugal and found lower percentages for both partially damaged (3–7%) and severely damaged individuals (3–6%). Gallardo-Roldán et al. (Reference Gallardo-Roldán2015) described lower percentages of partially damaged individuals (3%) and severely damaged individuals (10%) in the striped venus clam fishery in southern Spain. Urra et al. (Reference Urra2017) observed a proportion of partially damaged individuals (15%) but lower proportions of severely damaged individuals (12%) in southern Spain. These notable differences may be the result of many different factors (e.g. the clam dredge technical design, the fishing operation, the intensity and frequency of the fishing activity, the local environmental conditions, depth, scale of the habitat disturbance, etc.) (Gaspar & Chícharo, Reference Gaspar, Chícharo and Kennelly2007).
The results indicate differences in the proportion of discarded individuals affected by fishing activity (lethal damage) for each target species within each clam fishing area. These differences are the result of variation in the species composition of soft-bottom benthic assemblages due to depth disparity. The results also indicate differences in the proportion of discarded individuals affected by fishing activity in the three clam fishing areas. The three areas have well-sorted fine sand biocoenoses, and fishermen use the same model of clam dredges and gather the target species at the same depth. However, we detected differences in the composition of these benthic communities. The most impacted communities showed a higher number of fragile species, infaunal, sessile and soft-body/shell, than the other fishing areas, in agreement with previous studies (Gaspar et al., Reference Gaspar2003; Gallardo-Roldán et al., Reference Gallardo-Roldán2015; Urra et al., Reference Urra2017). The adoption of adaptive management to each local condition (target species and clam fishing area) may minimize the impact of mechanized clam dredges. An example could be adapting the design of clam dredges to each clam fishing area. Gaspar et al. (Reference Gaspar2003) conducted an experiment with different types of clam dredges and observed significant differences in the percentage of partially damaged and severely damaged individuals. However, small changes in the design of the clam dredges, such as tooth spacing and mesh size, does not seem to affect these percentages (Gaspar et al., Reference Gaspar2002). To minimize the impact on a specific area, Leitão et al. (Reference Leitão, Gaspar, Santos and Monteiro2009) suggested the use of different types of clam dredges, diversifying by-catch and consequently avoiding the cumulative removal and death of specific macrofauna taxa.
There is little information on the survival of the clam dredge discards, once returned to the sea. However, the mortality of the animals released from fishing activity (i.e. individuals showing no damage or minor/partial damage) is probably much higher that obtained in our study. The survival of these organisms depends on the degree of damage suffered during the tow, their size, the time the organism is exposed to air and the damage they suffered on deck. Moreover, they are very vulnerable when returned to the sea, especially if the time needed to reach the sea bottom and rebury (for infauna) or resume normal activity (epifauna) is long (Chícharo et al., Reference Chícharo, Chícharo, Gaspar, Regala and Alves2002; Gaspar et al., Reference Gaspar2003; Broadhurst et al., Reference Broadhurst, Suuronen and Hulme2006; Gaspar & Chícharo, Reference Gaspar, Chícharo and Kennelly2007). Notwithstanding all of these factors, impacts seem to be highly variable among fishing areas, target species, environmental conditions and depth, suggesting an important variability in the discards’ survival and the difficulty of extrapolating data from one area to others. Our study focused on the impact on discarded individuals that entered the dredges and were retained, but the present study did not analyse the mortality of uncaught damaged and dead individuals dislodged and left in the dredge path. Gaspar et al. (Reference Gaspar2003) stated that between 16.88 and 42% of individuals dislodged and left in the dredge path showed partial damage, while between 16.88 and 29.17% showed severe damage. The gastropod Buccinum undatum is frequently discarded by scallop dredging activity in Anglesey (Irish Sea) (Emmerson et al., Reference Emmerson, Hollyman, Bloor and Jenkins2020). This gastropod is returned to the sea without external damage and the first studies estimated 100% survival after being dropped (Kaiser & Spencer, Reference Kaiser and Spencer1995). However, Ramsay et al. (Reference Ramsay, Kaiser and Hughes1998) showed that 18% of these gastropods that were discarded or dislodged along the dredge path were consumed by benthic predators (the sea star Asterias rubens). Leitão et al. (Reference Leitão, Range and Gaspar2014) observed under laboratory conditions that 50% of partially damaged individuals died within 48 h of the fishing activity, in particular two fish species (Trachinus vipera and Dicologlossa acuneata) and one crab species (Polybius henslowii). In our discards, an important proportion of scavengers and predators in the three clam fishing areas was noted, i.e. the crustaceans L. vernalis, Portumnus latipes and D. pugilator and the gastropods of the genus Nassarius. Moreover, other predators that inhabit the same areas and are not gathered with the clam dredges may benefit from the fishing activity, for example: Torpedo torpedo, Trachinus draco, Trachinus radiatus, Echiichtys vipera and Lithognathus mormyrus. In fact, predators and scavengers have been observed to aggregate very quickly along the dredge tracks, preying not only on damaged organisms, but also on undamaged organisms before they have the opportunity to reburrow (Hall-Spencer & Moore, Reference Hall-Spencer and Moore2000).
Regarding the target species, the present study showed low percentages of impact (< 1% both on partially damaged individuals and lethally damaged individuals). Likewise, previous studies also showed low impact on the adult target species; between 0–5% on partially damaged individuals and from 1–6% on severely damaged individuals (Gaspar et al., Reference Gaspar2003; Urra et al., Reference Urra2017). Unfortunately, there is no information on the recruits and juveniles of the target species that are left in the dredge path. The effect of the physical disturbance by the dredges of the soft sediments on the recruits and juveniles should be examined in the future. Piersma et al. (Reference Piersma, Koolhaas, Dekinga, Beukema, Dekker and Essink2001) showed a significant negative effect of clam dredging on the subsequent settlement of the target species Cerastoderma edule; however, these authors did not find significant results for Macoma balthica.
In conclusion, the mechanized clam dredging used in the Catalan Coast had higher impact (both lethal and minor damage) on the discarded megabenthic fauna than found in previous similar studies. The different levels of impact that were observed between the two target species (the wedge clam and the striped venus clam) and among the three clam fishing areas (Rosas Bay, South Barcelona and Ebro Delta) was a result of significant differences in their associated faunistic composition. In all the clam fishing areas, the infaunal and epifaunal species with soft-body or fragile shells were the most impacted by the fishing activity.
Acknowledgements
We would like to thank the clam fishermen from Rosas, Sitges and Sant Carlos de la Rápita and Dr Miquel Riba from the CREAF (Autonomous University of Barcelona) for the statistical advice. We are also grateful to Susana Díez and Joan Solà for assistance and Rachel Ubach for her help with the map.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.











