Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease after Alzheimer’s disease (Hirtz et al., Reference Hirtz, Thurman, Gwinn-Hardy, Mohamed, Chaudhuri and Zalutsky2007), and it is characterized by motor symptoms and nonmotor characteristics. Mild cognitive impairment is common in nondemented PD patients (PD-MCI), affecting 30–50% depending on the progression of the disease (Galtier et al., Reference Galtier, Nieto, Lorenzo and Barroso2016; Monastero et al., Reference Monastero, Cicero, Baschi, Davì, Luca, Restivo, Zangara, Fierro, Zappia and Nicoletti2018). PD-MCI is considered a risk factor in the development of dementia (PDD), with a high conversion rate to PDD in the years following PD-MCI diagnosis (Galtier et al., Reference Galtier, Nieto, Lorenzo and Barroso2016; Hoogland et al., Reference Hoogland, Boel, de Bie, Geskus, Schmand, Dalrymple-Alford, Marras, Adler, Goldman, Tröster, Burn, Litvan and Geurtsen2017). More than 80% of PD patients will develop PDD after 20 years (Hely et al., Reference Hely, Reid, Adena, Halliday and Morris2008).
Subjective cognitive decline (SCD) is very common in the elderly and has gained attention as a predictor of future cognitive decline and AD dementia (Jessen et al., Reference Jessen, Amariglio, Buckley, van der Flier, Han, Molinuevo and Wagner2020). Patients or their caregivers are often the first to notice subtle changes in the patient’s cognitive functioning and the presence of this subjectively experienced cognitive decline may be one of the first signs of cognitive impairment. PD patients frequently report subjective cognitive complaints (Lehrner et al., Reference Lehrner, Moser, Klug, Gleiß, Auff, Pirker and Pusswald2014) but the number of investigations focused on PD-SCD is still limited and their clinical meaning is unclear. The results suggest that PD-SCD is a risk factor for developing PD-MCI (Erro et al., Reference Erro, Santangelo, Barone, Picillo, Amboni, Longo, Giordano, Moccia, Allocca, Pellecchia and Vitale2014; Hong et al., Reference Hong, Sunwoo, Chung, Ham, Lee, Sohn and Lee2014) and PDD (Galtier et al., Reference Galtier, Nieto, Lorenzo and Barroso2019). Thus, the early identification of minor cognitive changes in PD patients that can be useful predictors of PDD should be a high-priority objective for researchers and also for clinicians.
The language domain can be conceptualized as a set of complex behaviors involving several processes. The disorders in motor speech execution caused by an impairment in tone, range of motion and coordination of speech effectors are well described in PD patients (Smith & Caplan, Reference Smith and Caplan2018). Language production and comprehension have also been studied in PD, although they are less-well known compared to other cognitive domains, and many of the results are difficult to interpret. This is partially explained by the diversity of tasks designed to evaluate linguistic functions. Language production, measured by word generation or naming tasks, is usually affected in PD patients, even in the early stages of the disease (Bocanegra et al., Reference Bocanegra, García, Lopera, Pineda, Baena, Ospina, Alzate, Buriticá, Moreno, Ibáñez and Cuetos2017, Reference Bocanegra, García, Pineda, Buriticá, Villegas, Lopera, Gómez-Arias, Cardona, Trujillo and Ibáñez2015). Moreover, a disadvantage in action naming (Bertella et al., Reference Bertella, Albani, Greco, Priano, Mauro, Marchi and Semenza2002; Cotelli et al., Reference Cotelli, Borroni, Manenti, Zanetti, Arévalo, Cappa and Padovani2007; Rodríguez-Ferreiro et al., Reference Rodríguez-Ferreiro, Menéndez, Ribacoba and Cuetos2009) and action generation (Crescentini et al., Reference Crescentini, Mondolo, Biasutti and Shallice2008; Péran et al., Reference Péran, Rascol, Démonet, Celsis, Nespoulous, Dubois and Cardebat2003) compared to nouns has been described. These results are consistent with recent evidence regarding brain functioning and the hypothesis that different categories of content may be represented in different regions of the brain depending on the sensory and motor processes involved in the acquisition of these contents (Auclair-Ouellet et al., Reference Auclair-Ouellet, Lieberman and Monchi2017).
On the other hand, comprehension has been assessed in PD with a variety of sentences of diverse syntactic complexity, with special attention being paid to subordinate clauses. Several studies have reported that deficits in comprehension occur in highly complex sentences that include this type of clause and that performance is influenced by other cognitive processes such as attention, working memory and executive functions (Grossman, Reference Grossman1999; Grossman et al., Reference Grossman, Carvell, Stern, Gollomp and Hurtig1992; Hochstadt, Reference Hochstadt2009; Hochstadt et al., Reference Hochstadt, Nakano, Lieberman and Friedman2006). However, other results have questioned these results, reporting that comprehension deficits in nondemented PD patients also occur in less complex sentences, without a clear association with executive resources (Bocanegra et al., Reference Bocanegra, García, Pineda, Buriticá, Villegas, Lopera, Gómez-Arias, Cardona, Trujillo and Ibáñez2015; Skeel et al., Reference Skeel, Crosson, Nadeau, Algina, Bauer and Fennell2001).
Despite the different investigations that have focused on the study of linguistic functions in PD patients, and the evidence of language impairment in PDD (Noe et al., Reference Noe, Marder, Bell, Jacobs, Manly and Stern2004), the lack of studies focused on predementia stages of PD, that is, patients with PD-SCD or PD-MCI is surprising. In the studies based on the Movement Disorder Society (MDS) Task Force criteria for PD-MCI (Litvan et al., Reference Litvan, Goldman, Tröster, Schmand, Weintraub, Petersen, Mollenhauer, Adler, Marder, Williams-Gray, Aarsland, Kulisevsky, Rodriguez-Oroz, Burn and Emre2012), language domain has not usually been explored (Pedersen et al., Reference Pedersen, Larsen, Tysnes and Alves2013, Reference Pedersen, Larsen, Tysnes and Alves2017; Weintraub et al., Reference Weintraub, Simuni, Caspell-Garcia, Coffey, Lasch, Siderowf, Aarsland, Barone, Burn, Chahine, Eberling, Espay, Foster, Leverenz, Litvan, Richard, Troyer and Hawkins2015) or assessment has been limited to standardized naming tasks (i.e. Boston Naming test) (Broeders et al., Reference Broeders, de Bie, Velseboer, Speelman, Muslimovic and Schmand2013; Domellöf et al., Reference Domellöf, Ekman, Forsgren and Elgh2015; Marras et al., Reference Marras, Armstrong, Meaney, Fox, Rothberg, Reginold, Tang-Wai, Gill, Eslinger, Zadikoff, Kennedy, Marshall, Chou, Persad, Litvan, Mast, Gerstenecker, Weintraub and Duff-Canning2013; Pan et al., Reference Pan, Ren, Li, Li, Xu, Xue, Hu, Yu, Chen, Zhang, Zhang, Hu, Sun, Liu and Chen2022; Pigott et al., Reference Pigott, Rick, Xie, Hurtig, Chen-Plotkin, Duda, Trojanowski and Weintraub2015; Santangelo et al., Reference Santangelo, Vitale, Picillo, Moccia, Cuoco, Longo, Erro, Amboni, Trojano and Barone2015). Moreover, it is probable that a significant number of studies, previous to the MDS criteria, have included PD patients with MCI in groups of patients without cognitive impairment, complicating the interpretation of these results and clinical value for the characterization of cognitive impairment in PD patients without dementia.
To date, only a few cross-sectional research works have focused on the study of linguistic functions in PD-MCI and none of them include PD patients with SCD. The scarce available results report word-finding difficulties in PD-MCI characterized by less words per minute and more pauses within utterances (Smith et al., Reference Smith, Ash, Xie and Grossman2018). Other authors showed that PD-MCI patients showed an altered performance in action and object naming, whereas PD patients without MCI exhibited a selective difficulty for action naming (Bocanegra et al., Reference Bocanegra, García, Lopera, Pineda, Baena, Ospina, Alzate, Buriticá, Moreno, Ibáñez and Cuetos2017, Reference Bocanegra, García, Pineda, Buriticá, Villegas, Lopera, Gómez-Arias, Cardona, Trujillo and Ibáñez2015). Moreover, patients with and without MCI exhibited comprehension difficulties in sentences with different levels of complexity (with and without subordinate clause). Interestingly, differences between PD patients and controls in action naming and comprehension of sentences without a subordinate clause remained after adjusting for executive functions. On the contrary, differences between groups in subordinate clause sentence comprehension disappeared after executive function adjustment (Bocanegra et al., Reference Bocanegra, García, Pineda, Buriticá, Villegas, Lopera, Gómez-Arias, Cardona, Trujillo and Ibáñez2015).
There are no previous studies, to the best of the authors’ knowledge, focusing on studying the linguistic functions in predementia stages of PD (SCD and MCI) by a long-term follow-up study. Thus, the overall objective here was to conduct a longitudinal study evaluating linguistic functions in a sample of PD patients with SCD and MCI. The aims of the present study were: (1) to investigate language performance in patients with PD-SCD and PD-MCI with a comprehensive battery of linguistic tests; and (2) to explore which of the language subcomponents at the baseline better predict the development of PDD after a mean follow-up of 7.5 years. The hypotheses are that the PD-MCI group, compared to the controls and PD-nSCD, will present more severe production and comprehension language difficulties while the PD-SCD group will present mild language difficulties, primarily at the production level. Selective language disturbances will be useful predictors of dementia development.
Methods
Subjects
The study is part of a larger research project developed by the School of Psychology, University of La Laguna, in collaboration with the Department of Neurology, N.S. La Candelaria University Hospital and the Tenerife Parkinson Disease Association. The sample consisted of 66 participants: 46 patients with idiopathic PD, according to the clinical criteria for the diagnosis of PD (Hughes et al., Reference Hughes, Daniel, Kilford and Lees1992), and 20 healthy normal controls (HC). Patients were recruited consecutively by a neurologist specializing in movement disorders, in the regular neurology consulting department of the above hospital, and were evaluated in the “on” state, using the Hoehn & Yahr Scale (Hoehn & Yahr, Reference Hoehn and Yahr1967) and the Unified Parkinson’s Disease Rating Scale (UPDRS; Fahn & Elton, Reference Fahn, Elton, Fahn, Marsden, Goldstein and Calne1987). The exclusion criteria were as follows: (a) dementia associated with PD (Emre et al., Reference Emre, Aarsland, Brown, Burn, Duyckaerts, Mizuno, Broe, Cummings, Dickson, Gauthier, Goldman, Goetz, Korczyn, Lees, Levy, Litvan, McKeith, Olanow, Poewe, Quinn, Sampaio, Tolosa and Dubois2007) or global cognitive deterioration defined by the Mini-Mental State Examination (MMSE) score <24 (Folstein et al., Reference Folstein, Folstein and McHugh1975); (b) history of major psychiatric disorder; (c) drug or alcohol abuse; (d) visual and/or auditory perception disorders limiting the ability to take the test; (e) history of stroke and/or head injury with loss of consciousness; and (f) deep brain stimulation surgery. Patients and controls were matched in age, education, gender, manual preference and estimated IQ (Information subtest) (Wechsler, Reference Wechsler1997). The Beck Depression Inventory was administered for the assessment of mood state (Beck et al., Reference Beck, Ward, Mendelson, Mock and Erbaugh1961). All participants were informed about the aims of the investigation, participated voluntarily and gave their informed consent. The data were obtained in accordance with the regulations of the local ethics committee and in compliance with the Helsinki Declaration for Human Research. Demographic and clinical characteristics of PD patients and controls are shown in Table 1.
Table 1. Demographic data and clinical characteristics of PD patients and healthy controls
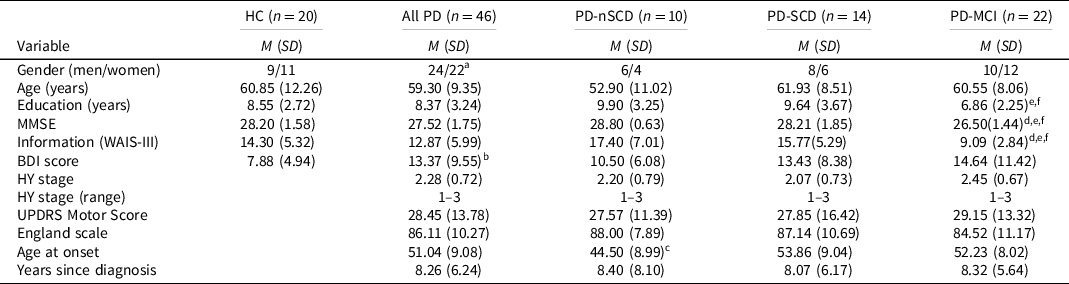
Note. n = number of the sample in each group; HC = healthy controls; PD = Parkinson’s disease; PD-nSCD = PD patients without subjective cognitive decline; PD-SCD = PD patients with subjective cognitive decline; PD-MCI = PD patients with mild cognitive impairment; M = mean; SD = standard deviation; MMSE = Mini-Mental State Examination; WAIS-III = Wechsler Adult Intelligence Scale third edition; BDI = Beck Depression Inventory; HY = Hoehn & Yahr scale; UPDRS = Unified Parkinson’s Disease Rating Scale.
a Pearson’s chi-squared test was not significant.
b Comparisons between healthy controls and PD group was significant.
c Comparisons between PD-nSCD and PD-SCD was significant.
d Comparisons between HC and PD-MCI was significant.
e Comparisons between PD-nSCD and PD-MCI was significant.
f Comparisons between PD-SCD and PD-MCI was significant.
Diagnosis of PD-SCD, PD-MCI and dementia
The participants were evaluated with a neuropsychological protocol including two tests for the attention, executive, memory and visuospatial domains (see supplementary material). PD-SCD was established on the basis of a semi-structured interview, previously published by the authors (Galtier et al., Reference Galtier, Nieto, Lorenzo and Barroso2019). The patients and care partners provided their subjective opinions regarding whether the patient had experienced changes in each of the following cognitive functions: attention, memory, language, visuoperceptual skills and executive functions. Regarding PD-MCI diagnosis, the criteria proposed by the MDS were applied (Litvan et al., Reference Litvan, Goldman, Tröster, Schmand, Weintraub, Petersen, Mollenhauer, Adler, Marder, Williams-Gray, Aarsland, Kulisevsky, Rodriguez-Oroz, Burn and Emre2012). Impairment in neuropsychological tests is demonstrated by the performance of 1.5 standard deviations or more below the mean of the control group. The absence of significant functional decline was confirmed based on a semi-structured interview and clinical impression of the subject’s general cognitive function. The patients’ follow-up assessments were to a mean of 7.5 (median 7.4; interquartile range 6.83–8.00; absolute minimum-maximum 6.30–8.40) years after the baseline. A diagnosis of PDD was made on the basis of the MDS criteria (Emre et al., Reference Emre, Aarsland, Brown, Burn, Duyckaerts, Mizuno, Broe, Cummings, Dickson, Gauthier, Goldman, Goetz, Korczyn, Lees, Levy, Litvan, McKeith, Olanow, Poewe, Quinn, Sampaio, Tolosa and Dubois2007). Decreased global cognitive functioning and deficits severe enough to impair daily life should be present, according to level 1 of the MDS criteria (Dubois et al., Reference Dubois, Burn, Goetz, Aarsland, Brown, Broe, Cummings, Gauthier, Korczyn, Lees, Levy, Litvan, Mizuno, McKeith, Olanow, Poewe, Sampaio, Tolosa and Emre2007).
Linguistic functions assessment
Instruments to assess the linguistic domain were designed by the authors and presented by computer software. Language production was assessed by two tests. The naming task consisted of 60 visual stimuli: 40 items representing elements (noun naming test, NNT) and 20 items depicting action scenes (verb naming test, VNT). Nouns and actions were paired in variables known to affect naming: every action item was paired with two noun items in word frequency and nominal agreement (Alameda & Cuetos, Reference Alameda and Cuetos1995; Cuetos & Alija, Reference Cuetos and Alija2003). The stimuli were line drawings in black and white (Cuetos et al., Reference Cuetos, Ellis and Alvarez1999; Druks & Masterson, Reference Druks and Masterson2000). Participants were instructed to name the concept represented, either the noun corresponding to the drawn element or the verb corresponding to the depicted action. Language production was also assessed by the action generation test (AGT), designed to evaluate lexical access by semantic associations. The AGT consisted of 20 auditory nouns divided into two categories: ten nouns without a phonologic derived action (AGTnf) (e.g. pencil-to write) and ten nouns with a phonologic derived action (AGTf) (e.g. conversation-to converse). Participants were instructed to generate a semantic associated action to each stimuli considering that phonologic derived actions were not allowed. Thus, AGTf entails cognitive inhibitory processes and was considered more difficult compared to AGTnf.
Sentence comprehension was examined by the anaphora test (APHT) and the center-embedded subordinate clauses test (CESCT), both instruments designed by the research group. The APHT assesses the ability to make the necessary inferences to comprehend sentences involving pronominal anaphora. The test consisted of twenty sentences, ten of which were nonambiguous (APHTna), in which the anaphora is resolved by the gender key (e.g. Marta gave a painkiller to Enrique as he had a headache) and the other ten were ambiguous (APHTa), where gender does not solve the ambiguity, requiring a semantic interpretation of the sentence to solve it (e.g. Elena laughed at Teresa’s jokes, because she was very funny). Participants were instructed to listen to the sentences and look at the computer screen where two words would appear during each sentence auditory presentation. These words correspond to the characters in the opening sentence, that is, the subject (Marta) and the object (Enrique) of the sentence. After each sentence presentation, participants were asked to answer a question regarding either the subject (Who gave a painkiller?) or the object (Who had a headache?) of the sentence. The CESCT design consists of twenty sentences with two levels of syntactic complexity. Ten sentences were simple declarative in form, without a subordinate clause (CESCTsimple) (e.g. The bellboy greeted the slim receptionist). The other ten sentences were made more complex syntactically by the addition of a center-embedded relative clause (CESCTcomplex), and in which the subject of the main clause is in turn the subject of the relative clause (e.g. The girl who pinched her cousin was naughty). All sentences used the active voice and were considered nonconstrained since the nouns could exchange places without violating the semantic coherence of the sentence (e.g. the girl and the cousin are equally capable of pinching each other). As in the APHT, participants were instructed to listen to the sentences and look at the computer screen where two words would appear during each sentence auditory presentation. These words correspond to the subject (bellboy) and the object (receptionist) of the sentence and participants were asked to answer a question regarding either the subject (Who greeted?) or the object (Who was greeted?) of the sentence.
Statistical analysis
A nonparametric statistic was used to evaluate differences between groups because the Shapiro-Wilk W test showed that data deviated from the standard normal distribution. The Mann–Whitney and Kruskal–Wallis tests were used to compare pairs of groups and multiple groups, respectively. Bonferroni correction for multiple comparisons was applied and effect size measures were calculated. Chi-squared tests were used for categorical data. Correlational analyses were performed using Spearman rank to examine the association between the language performance and other cognitive functions (p < .01). Logistic regression analyses were conducted to examine the performance of linguistic functions in PD patient subgroups and to examine the pattern of linguistic dysfunctions as predictors of PDD. The independent predictive values of the variables were expressed in odds ratio (OR) with 95% confidence interval (CI). p < .05 was set as the level of statistical significance. All the analyses were performed with SPSS-PC software version 24.0 for Windows.
Results
Twenty-two PD patients (47.8%) met the criteria for PD-MCI, fourteen patients (30.5%) were classified with a diagnosis of PD-SCD, and the remaining ten patients (21.7%) were classified as PD-nSCD. The neuropsychological performance for HC and PD patients (PD-nSCD, PD-SCD, PD-MCI) is available as supplementary material. Briefly, the PD-MCI group showed a poor performance, compared to HC and PD-nSCD, in the four evaluated domains (attention, executive, memory and visuospatial). Moreover, the PD-MCI group also performed poorly, compared to PD-SCD, in the executive domain and visuospatial domain. No significant differences were found between PD-SCD and HC in any of the neuropsychological tests.
Linguistic function analyses
Four PD patients did not complete the AGT and APHT. The linguistic functions assessment showed that the PD-MCI group performed poorly, compared to HC, in the naming tests (NNT p = .000, r = .66; VNT p = .004, r = .53) and comprehension tests (APHTna p = .048, r = .41; CESCTsimple p = .002, r = .56; CESCTcomplex p = .002, r = .56). Similar patterns were found between the PD-MCI and PD-nSCD groups. Moreover, significant differences were also found between the PD-MCI and PD-nSCD groups in the action generation test (AGTnf p = .003, r = .61; AGTf p = .031, r = .50). The PD-SCD group only performed poorly, compared to HC and PD-nSCD group, in the AGT (Table 2).
Table 2. Linguistic battery scores for PD patients and healthy controls
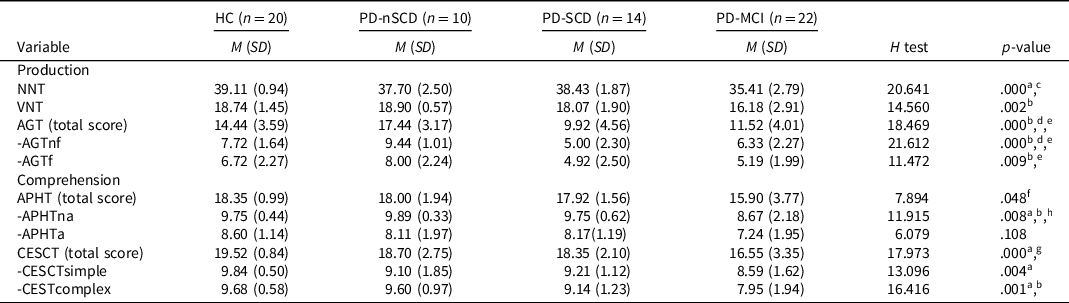
Note. n = number of the sample in each group; HC = healthy controls; PD = Parkinson’s disease; PD-nSCD = PD patients without subjective cognitive decline; PD-SCD = PD patients with subjective cognitive decline; PD-MCI = PD patients with mild cognitive impairment; M = mean; SD = standard deviation; NNT = nouns naming test; VNT = verbs naming test; AGT = action generation test; AGTnf = AGT without a phonologic derived action; AGTf = AGT with a phonologic derived action; APHT = anaphora test; APHTna = APHT nonambiguous; APHTa = APHT ambiguous; CESCT = center-embedded subordinate clauses test; CESCTsimple = CESCT without subordinate clause; CESCTcomplex = CESCT with center-embedded subordinate clause.
a The comparison between HC and PD-MCI was significant.
b The comparison between PD-nSCD and PD-MCI was significant.
c The comparison between PD-SCD and PD-MCI was significant.
d The comparison between HC and PD-SCD was significant.
e The comparison between PD-nSCD and PD-SCD was significant.
f HC versus PD-MCI not significant after Bonferroni correction.
g PD-nSCD versus PD-MCI not significant after Bonferroni correction.
h PD-SCD versus PD-MCI not significant after Bonferroni correction.
PD patients were classified as “altered” or “nonaltered” to explore the percentage of patients who presented a clinically deficient performance in the linguistics tests. Linguistic impairment was demonstrated by the performance of one standard deviation or more below the mean of the control group (Table 3).
Table 3. Percentage of patients with clinically deficient performance on linguistic assessment
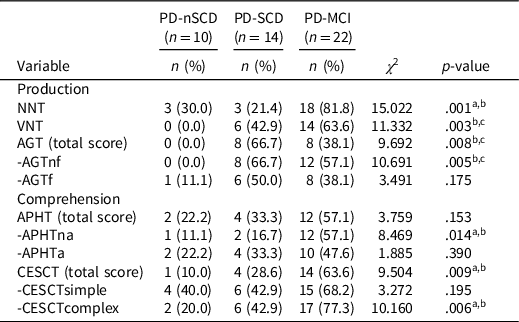
Note. n = number of the sample in each group; PD-nSCD = PD patients without subjective cognitive decline; PD-SCD = PD patients with subjective cognitive decline; PD-MCI = PD patients with mild cognitive impairment; NNT = nouns naming test; VNT = verbs naming test; AGT = action generation test; AGTnf = AGT without a phonologic derived action; AGTf = AGT with a phonologic derived action; APHT = anaphora test; APHTna = APHT nonambiguous; APHTa = APHT ambiguous; CESCT = center-embedded subordinate clauses test; CESCTsimple = CESCT without subordinate clause; CESCTcomplex = CESCT with center-embedded subordinate clause.
a The comparison between PD-MCI and PD-SCD was significant.
b The comparison between PD-MCI and PD-nSCD was significant.
c The comparison between PD-SCD and PD-nSCD was significant.
The paired difference between groups showed a significantly greater percentage of PD-MCI patients who presented a clinically deficient performance, compared to PD-SCD and/or PD-nSCD subjects, in the production tests (NNT, VNT, AGTnf) and comprehension tests (APHTna and CESCTcomplex). No significant differences were found in the percentage of patients with a clinically deficient performance in CESCTsimple, that was high in the three groups. In addition, a significantly greater percentage of PD-SCD patients (similar to PD-MCI group) presented a clinically deficient performance in the VNT and AGTnf, compared to PD-nSCD subjects, who did not perform in a clinically altered manner.
Linguistic functions as a predictor of PD dementia
Conversion to dementia during the follow-up study was more frequent in patients with PD-MCI (50%) compared to patients with PD-SCD (33.3%) and more frequent in the PD-SCD group compared to patients with PD-nSCD (14.3%). The percentage of patients who converted to dementia and those who did not, together with their baseline clinical characteristics are available as supplementary material. Seven PD patients did not participate in the follow-up study (two PD-MCI, two PD-SCD and three PD-SCD).
Logistic regressions were used to explore the association between linguistic performance and dementia development. According to the results shown in Table 4, an altered VNT (OR = 12.00) and AGTnf (OR = 5.71) were significant predictors of dementia. Regarding comprehension tasks, an altered CECSTsimple was the test that was most associated with risk of dementia development (OR = 3.25), although it did not reach statistical significance. The remaining comprehension tasks were not statistically significant either. Considering the results shown in Table 4, logistic regression was conducted to explore whether a specific pattern of linguistic alterations added an increased risk to the development of dementia. The results show that the combination of altered VNT-AGTnf-CESCTsimple (Wald = 8.54; p = .003; OR = 29.33; 95% CI 3.041, 282.904) was associated to an increased risk to dementia development, compared to performance in isolated tasks. PD patients with and without altered VNT-AGTnf-CESCTsimple were compared by digit span backward and the Wisconsin test (categories), to explore de association of linguistic performance with working memory and executive functions. PD patients with an altered performance in the linguistic tasks showed a poor performance in the Wisconsin test (p = .022), but not in digit span (p = .590). Logistic regression analysis showed that Wisconsin categories were a significant predictor of altered VNT-AGTnf-CESCTsimple (Wald = 4.44; p = .035; OR = 2.04; 95% CI 1.051, 3.964) whereas digit span (backward) did not reach statistical significance and was not included in the model (p = .838). Correlation analyses of linguistic tests with Wisconsin test and digit span are included as supplementary material.
Table 4. Linguistic functions as predictor of PD dementia development

Note. n = number of the sample in each group; PDD = PD patients with dementia in the follow-up study; PDND = PD patients without dementia in the follow-up study; OR = Odds Ratio; CI = Confidence Interval. NNT = nouns naming test; VNT = verbs naming test; AGT = action generation test; AGTnf = AGT without a phonologic derived action; AGTf = AGT with a phonologic derived action; APHT = anaphora test; APHTna = APHT nonambiguous; APHTa = APHT ambiguous; CESCT = center-embedded subordinate clauses test; CESCTsimple = CESCT without subordinate clause; CESCTcomplex = CESCT with center-embedded subordinate clause.
In addition, logistic regression was conducted to explore whether the pattern of linguistic impairment in combination with executive resources and other cognitive variables, added an increased risk to the development of dementia. The altered VNT-AGTnf-CESCTsimple, digit span (backward), Wisconsin categories, phonemic and semantic fluency, Stroop test (interference index) and MMSE pentagon copying were included in the regression analysis as independent variables. The forward stepwise method was used to exclude nonsignificant variables. The result revealed that the altered VNT-AGTnf-CESCTsimple was significant as an independent predictor of dementia (Wald = 8.54; p = .003; OR = 29.33; 95% CI 3.041, 282.904). Digit span (p = .655), Wisconsin categories (p = .286), phonemic (p = .732) and semantic fluency (p = .301), Stroop interference (p = .539) and pentagon copying (p = .353), did not reach statistical significance, which were not included in the model and, therefore, did not affect the significance of the VNT-AGTnf-CESCTsimple.
A new logistic regression was conducted to study whether linguistic dysfunction (altered VNT-AGTnf-CESCTsimple) in combination with demographic and clinical factors (age ≥ 65, years of education, Information subtest, PD duration, age at onset of the disease and UPDRS motor score), added an increased risk to the development of dementia. The forward stepwise method was used to exclude nonsignificant variables. The results revealed that the altered VNT-AGTnf-CESCTsimple was significant in step one as an independent predictor of dementia (Wald = 8.29; p = .004; OR = 28.00; 95% CI 2.898, 270.541). The altered VNT-AGTnf-CESCTsimple (Wald = 7.84; p = .005; OR = 33.60; 95% CI 2.871, 393.221) and age ≥ 65 (Wald = 3.65; p = .056; OR = 6.10; 95% CI .954, 38.993) were included in step two. Years of education (p = .915), Information subtest (p = .877), PD duration (p = .844), age at onset of the disease (p = .283) and UPDRS motor score (p = .263) did not reach statistical significance, which were not included in the model and, therefore, did not affect the significance of the VNT-AGTnf-CESCTsimple.
Discussion
The aim of the study was to investigate language performance in patients with PD-SCD and PD-MCI and to explore the clinical value of linguistic impairment as predictors of PDD. PD-MCI patients showed an altered execution in language production, characterized by difficulties in noun and verb naming, as well as an impairment of action generation starting from a noun. As a complementary approach to the results of group comparisons, the study of the percentage of patients who presented a clinically deficient performance showed a high percentage of PD-MCI patients with a clinically deficient execution in these processes (naming nouns 82%, naming actions 64%, generating actions 57%). In addition, the PD-MCI group showed an altered performance in language comprehension, which was observed by the altered execution in the anaphora resolution and comprehension of sentences with different levels of complexity. Interestingly, deficit in comprehension did not only occur in sentences with high complexity that included center-embedded subordinate clauses (77% clinically deficient), but was also observed in declarative sentences without relative clauses, in 68% of PD-MCI patients who presented clinically deficient execution. With respect to PD-SCD patients, the group comparisons showed a deficient performance in language production, which was manifested by an altered execution in generating verbs associated with nouns. Moreover, a high percentage of PD-SCD patients, similar to the PD-MCI group, presented a clinically altered execution in naming actions and generating actions. It is worth mentioning that none of PD patients without SCD showed an altered execution in these tasks.
The above results are of interest because the data about language performance is extremely limited in PD-MCI. Moreover, no previous studies have focused on language execution in PD-SCD patients. The production difficulties observed in PD-MCI patients in word generation and naming is consistent with previous investigations focused on PD patients with MCI (Bocanegra et al., Reference Bocanegra, García, Lopera, Pineda, Baena, Ospina, Alzate, Buriticá, Moreno, Ibáñez and Cuetos2017, Reference Bocanegra, García, Pineda, Buriticá, Villegas, Lopera, Gómez-Arias, Cardona, Trujillo and Ibáñez2015; Smith et al., Reference Smith, Ash, Xie and Grossman2018) and also in studies prior to the MDS criteria for PD-MCI (Bertella et al., Reference Bertella, Albani, Greco, Priano, Mauro, Marchi and Semenza2002; Cotelli et al., Reference Cotelli, Borroni, Manenti, Zanetti, Arévalo, Cappa and Padovani2007; Rodríguez-Ferreiro et al., Reference Rodríguez-Ferreiro, Menéndez, Ribacoba and Cuetos2009). Moreover, the specific difficulties in generating verbs associated with nouns observed in PD-SCD is consistent with several previous studies that found a disadvantage for verb production compared to nouns (Crescentini et al., Reference Crescentini, Mondolo, Biasutti and Shallice2008; Péran et al., Reference Péran, Rascol, Démonet, Celsis, Nespoulous, Dubois and Cardebat2003). On the other hand, the pattern of comprehension impairment, not limited to sentences with high levels of complexity is consistent with previous studies that included PD patients without dementia (Johari et al., Reference Johari, Walenski, Reifegerste, Ashrafi, Behroozmand, Daemi and Ullman2019), PD-MCI patients (Bocanegra et al., Reference Bocanegra, García, Pineda, Buriticá, Villegas, Lopera, Gómez-Arias, Cardona, Trujillo and Ibáñez2015) and different investigations prior to the current PD-MCI criteria (Grossman et al., Reference Grossman, Carvell, Gollomp, Stern, Vernon and Hurtig1991; Skeel et al., Reference Skeel, Crosson, Nadeau, Algina, Bauer and Fennell2001). However, other investigations, prior to the PD-MCI criteria, reported that PD patients showed an altered comprehension of sentences with high complexity, especially with center-embedded subordinate clauses, but not in sentences without subordinate clauses (Grossman, Reference Grossman1999; Grossman et al., Reference Grossman, Carvell, Stern, Gollomp and Hurtig1992; Hochstadt, Reference Hochstadt2009; Hochstadt et al., Reference Hochstadt, Nakano, Lieberman and Friedman2006). This discrepancy can be explained by different factors. Firstly, it is likely that a significant number of studies, previous to the current PD-MCI criteria, were conducted with heterogeneous samples of PD patients by the inclusion of subjects with and without MCI. Secondly, numerous investigations have explored comprehension with a wide diversity of experimental tasks in which different sentence parameters have been manipulated, including syntactic complexity, semantic content, reversibility or animacy, among others. In the present study, only syntactic complexity was manipulated by the inclusion or not of a center-embedded subordinate clause. Semantic content was the same for both sentence types of CESCT. The same occurred with reversibility, which are sentences where the action is equally likely to be performed by both characters involved. Regarding animacy, in the simple and complex sentences of CESCT both characters are animate entities (e.g. humans, animals), and as such are more likely to perform actions compared to inanimate entities (e.g. objects) which are more likely to be the object of actions. Thus, the simple sentences of the CESCT can be considered as “more complex” than the simple sentences in some previous studies because these sentence parameters did not facilitate the comprehension.
The results of the group comparisons, combined with clinically deficient execution, are of much relevance to clarify the timing and order of appearance of language impairments. Taken together, these results suggest that PD-SCD language performance is characterized by a specific deficit for action words, accompanied by possible difficulties in sentence comprehension (around 40% of PD-nSCD and PD-SCD showed an altered execution in CESCT). The progression of cognitive impairment, characterized by the affectation of different cognitive domain and PD-MCI diagnosis, is associated with a greater impairment of language domain significantly affecting production (nouns and verbs) and comprehension of sentences with different levels of syntactic complexity. These results are consistent with recent investigations which reported that PD patients without MCI showed a selective difficulty for action verbs compared to nouns (Bocanegra et al., Reference Bocanegra, García, Lopera, Pineda, Baena, Ospina, Alzate, Buriticá, Moreno, Ibáñez and Cuetos2017), accompanied by difficulties in sentence comprehension (Bocanegra et al., Reference Bocanegra, García, Pineda, Buriticá, Villegas, Lopera, Gómez-Arias, Cardona, Trujillo and Ibáñez2015). PD-MCI diagnosis was associated with a more generalized language impairment (Bocanegra et al., Reference Bocanegra, García, Lopera, Pineda, Baena, Ospina, Alzate, Buriticá, Moreno, Ibáñez and Cuetos2017, Reference Bocanegra, García, Pineda, Buriticá, Villegas, Lopera, Gómez-Arias, Cardona, Trujillo and Ibáñez2015). Another recent study focused on asymptomatic PD mutation carriers, that is, individuals unaffected by PD but with mutations in PARK2 or LRRK2. The preclinical PD sample showed deficits in sentence comprehension in the absence of other linguistic or executive difficulties (García et al., Reference García, Sedeño, Trujillo, Bocanegra, Gomez, Pineda, Villegas, Muñoz, Arias and Ibáñez2017). This result reinforces the assumption that deficit in language comprehension can be present even in the early stages of the disease.
A second objective of the present investigation was to study the clinical value of linguistic impairment as predictors of PDD development. The data reported in the present investigation shows that impairment in action naming (OR = 12.00) and action generation (OR = 5.71) was related to a greater risk of PDD development. Alteration in comprehension of simple declarative sentences also was associated with an increased risk of dementia (OR = 3.25), although this did not reach statistical significance. Interestingly, PD patients who were deficient in action words (action naming and action generation) and sentence comprehension exhibited a high risk of PDD development (OR = 29.33), which was greater than the risk associated with only the presence of action naming difficulties.
Different cognitive functions have been associated with an increased risk of dementia. Demographic (older age, education) and clinical factors (age at onset, years since diagnosis, motor symptoms) have also been recognized as variables associated with the evolution of cognitive impairment (Marinus et al., Reference Marinus, Zhu, Marras, Aarsland and van Hilten2018). Moreover, different investigations have associated language difficulties in PD with executive deficit. Thus, an important question is whether language impairment can be considered a more useful predictor of dementia, compared to the above mentioned demographic and clinical factors, as well as other cognitive measures. The result of the regression model showed that the combination of deficits for action words (action naming and action generation) and sentence comprehension was as a significant predictor of dementia, whereas the remaining cognitive tests did not reach statistical significance. Thus, the present results, although preliminary because of the sample size, suggest that the pattern of linguistic dysfunction can be considered as a useful predictor of dementia. As expected, age ≥65 also contributed significantly to the regression model (Marinus et al., Reference Marinus, Zhu, Marras, Aarsland and van Hilten2018). The important role of the executive functions in other cognitive process is well known. However, in the authors’ opinion, the specific implication of executive functions on the interpretation of an evolution pattern of language production/comprehension difficulties in PD is still unclear. The results of the present investigation are consistent with previous studies (Skeel et al., Reference Skeel, Crosson, Nadeau, Algina, Bauer and Fennell2001), and are reinforced by recent investigations with PD-MCI patients (Bocanegra et al., Reference Bocanegra, García, Lopera, Pineda, Baena, Ospina, Alzate, Buriticá, Moreno, Ibáñez and Cuetos2017, Reference Bocanegra, García, Pineda, Buriticá, Villegas, Lopera, Gómez-Arias, Cardona, Trujillo and Ibáñez2015) and a preclinical PD sample which was deficient in linguistic functions in the absence of executive difficulties (García et al., Reference García, Sedeño, Trujillo, Bocanegra, Gomez, Pineda, Villegas, Muñoz, Arias and Ibáñez2017).
The language domain includes a set of complex behaviors that involves several processes related to peri-Sylvian and extra-Sylvian cerebral areas. Current knowledge regarding brain functioning suggests that different categories of content would be represented in different regions of the brain depending on the sensory and motor processes involved in the acquisition of these contents (Goldberg et al., Reference Goldberg, Perfetti and Schneider2006). Semantic representations of action words would be supported by regions that are directly involved in motor planning and execution (i.e. primary motor cortex, premotor cortex), whereas the nouns would be represented in posterior cortical areas (i.e. perceptual/sensory regions) (Auclair-Ouellet et al., Reference Auclair-Ouellet, Lieberman and Monchi2017). PD is characterized by the loss of dopaminergic cells in the substantia nigra, the interruption of the frontal-striatal-thalamic anatomic loop and the consequent deterioration of motor control. This is a possible explanation of the early difficulties in action words reported in previous studies (Bocanegra et al., Reference Bocanegra, García, Lopera, Pineda, Baena, Ospina, Alzate, Buriticá, Moreno, Ibáñez and Cuetos2017, Reference Bocanegra, García, Pineda, Buriticá, Villegas, Lopera, Gómez-Arias, Cardona, Trujillo and Ibáñez2015) and observed in the subsample here of PD-SCD. However, it is now widely recognized that PD evolves into a multi-system disorder that extends beyond the substantia nigra pars compacta, affecting frontal and temporo-parietal cortical areas, as well as subcortical regions (Foffani & Obeso, Reference Foffani and Obeso2018). The dual syndrome hypothesis, differentiates between the following two cognitive syndromes in PD patients: (1) the fronto-striatal, which is associated with an executive dysfunction profile and dopamine depletion; and (2) the posterior cortically based cognitive profile, characterized by dysfunction in language and visuospatial functions, which is linked to nondopaminergic neurotransmitters, and which is associated with an increased risk of dementia (Williams-Gray et al., Reference Williams-Gray, Evans, Goris, Foltynie, Ban, Robbins, Weinberger, Sawcer and Barker2009, Reference Williams-Gray, Mason, Evans, Foltynie, Brayne, Robbins and Barker2013). In line with these results, recent investigations showed that the posterior cortical PD-MCI subtype, characterized by visuospatial, language (assessed by the Boston naming test) or memory deficit, was associated with more extensive structural alterations (i.e. caudate nuclei, thalamus, hippocampus and several white matter tracts) (Devignes, Viard, et al., Reference Devignes, Viard, Betrouni, Carey, Kuchcinski, Defebvre, Leentjens, Lopes and Dujardin2021) and increased basal ganglia intra-network functional connectivity, which could be interpreted as a neurodegeneration compensatory mechanism (Devignes et al., Reference Devignes, Bordier, Viard, Defebvre, Kuchcinski, Leentjens, Lopes and Dujardin2021). The results here are consistent with the dual syndrome hypothesis by showing that PD patients with a pattern of linguistic impairment including deficit in sentence comprehension have a high risk of developing dementia.
Certain limitations of the present study need to be acknowledged. The sample size is relatively small, especially in the PD-nSCD group. The number of participants has limited the methodological approach, especially regarding studying the relationship between different cognitive domains in greater detail. Moreover, although the design of the language instruments was based on the evidence in scientific literature, a previous validation study is not available. Future longitudinal investigations with larger samples and with the inclusion of biomarkers (e.g. neuroimages) could confirm these findings, with special attention being paid to compare the predictive value of linguistic dysfunctions with that of other cognitive domains.
In summary, the present investigation is the first to conduct a comprehensive assessment of linguistic functions in a sample of PD patients with SCD and MCI, and is also the first to study the clinical value of the linguistic impairment as a risk factor of PDD development in a follow-up study. PD-SCD subjects showed a difficulty for action words, which was not observed in PD patients without SCD. PD-MCI diagnosis was associated with a greater impairment of language domain significantly affecting the production of nouns and verbs, as well as the comprehension of sentences with different levels of syntactic complexity. Finally, the coexistence of deficits for action words (action naming and action generation) and sentence comprehension in PD patients can be considered a useful predictor of PDD development. In the authors’ opinion the results of the present investigation are of much value for researchers and also for clinicians. Approximately eight out of ten PD patients will develop dementia after 20 years (Hely et al., Reference Hely, Reid, Adena, Halliday and Morris2008), which has a marked effect on the quality of life of patients and caregivers, with a great societal and financial impact (Leroi et al., Reference Leroi, McDonald, Pantula and Harbishettar2012). The results, although exploratory, suggest that specific patterns of linguistic dysfunctions, that can be present even in the early stages of the disease, can predict future dementia, reinforcing the importance to advance the knowledge of linguistic dysfunctions in predementia stages of PD. These results are high applicable considering that it would not be difficult to incorporate these types of instruments, which are generally brief and easy to apply and interpret, in daily clinical practice.
Acknowledgements
We thank all the patients and healthy volunteers who participated in the study for their cooperation; Professors Manuel de Vega and Alberto Dominguez for their help in the linguistic test design; Nuria Campos, Sandra Larsson, Susana Rodríguez and Ruth Pérez for their help in the sample recruitment.
Funding statement
The study was supported by the University of La Laguna and the Ministry of Science, Innovation and Universities.
Conflicts of interest
None.






