Introduction
A third of all Psittaciformes are classified as threatened and over half of all populations are in decline, yet population parameters to support conservation status assessments are missing for many Neotropical parrots (Berkunsky et al. Reference Berkunsky, Quillfeldt, Brightsmith, Abbud, Aguilar, Alemán-Zelaya and Masello2017). Parrots are commonly threatened by habitat loss, persecution, and the pet trade, and many species are in need of conservation support (Berkunsky et al. Reference Berkunsky, Quillfeldt, Brightsmith, Abbud, Aguilar, Alemán-Zelaya and Masello2017). Endemic parrots with small fragmented populations, and those such as Amazon parrots with large body size and long generation times, are disproportionately at greater risk of extinction (Snyder et al. Reference Snyder, Wiley and Kepler1987, Purvis et al. Reference Purvis, Gittleman, Cowlishaw and Mace2000, O’Grady et al. Reference O’Grady, Reed, Brook and Frankham2004, Olah et al. Reference Olah, Butchart, Symes, Guzmán, Cunningham, Brightsmith and Heinsohn2016). Indeed 58% of species in the genus Amazona are currently listed by the IUCN as threatened or ‘Extinct in the Wild’ (BirdLife International 2017).
Amazona lilacina is endemic to Ecuador and was described as a full species in 2014 (Pilgrim Reference Pilgrim2010, del Hoyo and Collar Reference del Hoyo and Collar2014). An initial Red List assessment categorised it as ‘Endangered’ due to its small and fragmented population (BirdLife International 2014) however, detailed status information was lacking, uncertain or outdated. For example, the northernmost limit of the species’ extent of occurrence (EoO), was historically recorded as south-west Colombia (e.g. Juniper and Parr Reference Juniper and Parr1998, Forshaw Reference Forshaw2010), which is now believed to be incorrect. Additionally, its dispersal area and habitat preference were recorded as regions encompassing both mangrove and lowland coastal forest habitats (e.g. Ridgley and Greenfield Reference Ridgley and Greenfield2001a, Athanas and Greenfield Reference Athanas and Greenfield2016), yet a recent study confirmed the presence of a large roost in a non-mangrove habitat (Blanco et al. Reference Blanco, Bravo, Pacifico, Chamorro, Speziale, Lambertucci, Hiraldo and Tella2016).
Evidence suggests population size and trajectory are strongly correlated with extinction risk among vertebrates (O’Grady et al. Reference O’Grady, Reed, Brook and Frankham2004) but, since A. lilacina was described as a full species, the population size has not been estimated and little is known about its trend in recent years. The species was reported to have undergone severe population decline prior to the mid-1980s in response to ongoing habitat loss and trapping pressure (e.g. CITES 1986, Ridgley and Greenfield Reference Ridgley and Greenfield2001b), and by 1998 the population was estimated at just 400–600 individuals (Juniper and Parr Reference Juniper and Parr1998). However, this estimate is now almost 20 years old and its reliability is questioned due both to possible declines and to the recent identification of new roosts (Blanco et al. Reference Blanco, Bravo, Pacifico, Chamorro, Speziale, Lambertucci, Hiraldo and Tella2016, authors’ pers. obs.). Roost surveys have been used to estimate global and local population sizes in many parrots species (e.g. Gnam and Burchsted Reference Gnam and Burchsted1991, Martuscelli Reference Martuscelli1995, Matuzak and Brightsmith Reference Matuzak and Brightsmith2007, Dénes et al. Reference Dénes, J and Beissinger2018) and provide a tool for long-term population monitoring (e.g. Wermundsen Reference Wermundsen1998, Wright et al. Reference Wright, Lewis, Lezama-López, Smith-Vidaurre and Dahlin2019). Amazona lilacina’s communal roosting behaviour thus allows us to update the population estimate and conduct long-term monitoring to assess population trajectory.
In response to the ‘uplisting’ of this species to ‘Endangered’ in 2014, we re-examined its Red List status through personal field observations and collation of information from local experts, NGOs, and communities, over a seven year period to fulfil four objectives:
-
1. update the current known Extent of Occurrence and estimate area of daily dispersal;
-
2. estimate global population size;
-
3. determine change in roost size as an indicator of overall population trend;
-
4. quantify prevalence of pet parrots within the species’ range.
Methods
Study area and roost sites
Amazona lilacina is reliant on lowland coastal forests (Ridgley and Greenfield Reference Ridgley and Greenfield2001b) where it feeds on a variety of fruits and seeds, and nests in cavities formed in the trunks and branches of tree species such as pigío Cavanillesia platanifolia and ceibo Ceiba trichistandra (Kunz Reference Kunz1996, Berg and Angel Reference Berg and Angel2006). Although we know little about this species’ reproductive behaviour, adults appear to explore cavities in October/November and produce one or two chicks that fledge between mid-February and late-March (Kunz Reference Kunz1996, Berg and Angel Reference Berg and Angel2006). As with several other Amazona species, with the exception of breeding birds, or at least females during the incubation and early chick stages, it returns to communal roost sites every evening e.g. A. brasiliensis (Cougill and Marsden Reference Cougill and Marsden2004), A. auropalliata auropalliata (Matuzak and Brightsmith Reference Matuzak and Brightsmith2007), and A. amazonica (de Moura et al. Reference Moura, Silva and Vielliard2012). For A. lilacina, these roost sites mainly occur on mangrove islands where birds join together every night (Berg and Angel Reference Berg and Angel2006). Birds tend to arrive at sunset, flying in loose-knit flocks made of paired birds, single birds, triplets or small groups, often making loud contact calls as they fly. For this reason roost locations are often well known by local communities, who hear the birds as they arrive and depart the following morning. In contrast, during the day, birds are secretive and extremely difficult to locate as they feed silently and high in the canopy in small groups (Ridgley and Greenfield Reference Ridgley and Greenfield2001b).
For this study, we identified four roost sites that are occupied throughout the year. We believe they contain a large proportion, if not all, of the remaining global population of this species and they are separated from each other by at least 50 km (Figure 1). Roost 1 is located on a mangrove island in Manabí Province and was brought to our attention by Fundación Jocototo in 2012. Roost 2 is located in Santa Elena Province and is known to us through the work of Guillermo Blanco and José Tella (Blanco et al. Reference Blanco, Bravo, Pacifico, Chamorro, Speziale, Lambertucci, Hiraldo and Tella2016). This is the only roost we know of that does not occur in mangroves. Instead, the birds roost in stands of the locally known ‘algarrobo’ tree Prosopis julifora. Roost 3 is perhaps the most well-known roost, located north-west of the Gulf of Guayaquil, in the El Salado Mangrove Reserve where mangrove islands have been frequented by A. lilacina since at least the early 1990s (Berg and Angel Reference Berg and Angel2006). Roost 4 is situated south-east of the Gulf of Guayaquil on an island within the Manglares Churute Ecological Reserve. It was located in 2016 through our community questionnaires.
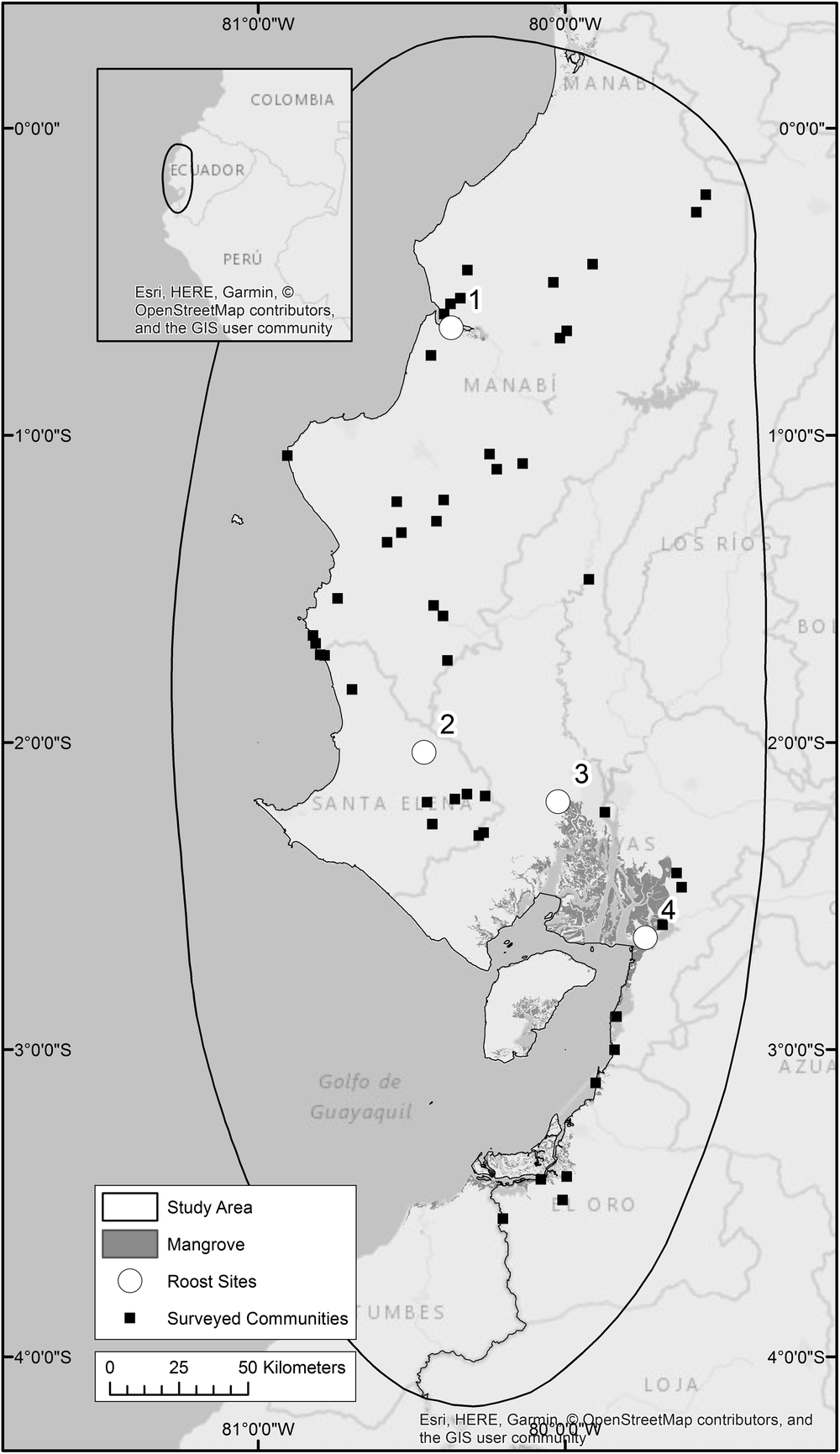
Figure 1. Four A. lilacina roosts are believed to contain the majority of the global population, three of these occur on mangrove islands. Mangroves (Hamilton and Casey Reference Hamilton and Casey2016) and communities taking part in researcher-led questionnaires are indicated.
Field observations
Observational data collected during 10 field trips were used to address Objective 1 (November 2012, January and August 2014, November 2015, August 2016, January and March 2017, February 2018, January and August 2019). Field trips lasted 2–3 weeks during which we investigated potential areas of suitable habitat, verified any recorded sightings of individuals, and monitored known and newly reported roosts. Data collection was informed by: 1) existing information on known distribution and habitat use (Juniper and Parr Reference Juniper and Parr1998, Ridgley and Greenfield Reference Ridgley and Greenfield2001b, Berg and Angel Reference Berg and Angel2006, Forshaw Reference Forshaw2010, Athanas and Greenfield Reference Athanas and Greenfield2016); 2) information on habitat distribution from Google Earth and available ecosystem maps (Ministerio del Ambiente 2012); 3) direct communication with local NGOs, ornithologists, local guides and bird tour companies and 4) communication through researcher-led questionnaires with local communities.
All sightings of perched A. lilacina made by us, Fundación Jambeli staff, and Juan Freile within the last 10 years were georeferenced (sightings of birds in flight were omitted). eBird presence data were lacking, however complete checklists that failed to report A. lilacina were used to gain an idea of absence areas: a total of 34,974 complete checklists for mainland Ecuador were downloaded in February 2019.
Roost surveys
To meet Objective 2, we conducted repeat surveys at all roosts. Although these were not located through systematic survey, they represent the combined current knowledge regarding this species according to the authors, local experts and communities. Initially, we conducted practice censuses at each roost to identify the best vantage points. Surveys were then conducted twice per day and where possible, for a minimum of four days to control for intrinsic variability (minimum of 2, maximum of 20 consecutive surveys). To maximise our chances of counting all individuals leaving or arriving at each roost, morning surveys began before sunrise and lasted for two hours, whilst evening surveys began an hour before sunset and finished when it was too dark for birds to be identified. To reduce observer bias, all surveys were carried out by a combination of the same three researchers (RB, ISP, PC), with one person counting and identifying birds using binoculars, the other keeping record. Roost sites are separated by at least 50 km and it has been suggested for other amazon species that if roosts sites are isolated by > 8 km, daily movement between roosts is unlikely (Cougill and Marsden Reference Cougill and Marsden2004). Still, to account for possible movement of birds between roosts, which could result in counting the same birds twice, only roost surveys conducted during the same weeks of each year were used to estimate population size. Unfortunately, Roost 1 was disrupted and not occupied by amazons during one year of the study, thus an average of counts before and after this disruption, but prior to the next global count, was used. The sum of these counts is presented as an estimated range in minimum global population size during the given time frame. Counts conducted in March are likely to include both adult and juvenile birds returning to the roost after the breeding season, so are suggested to be the most inclusive estimate.
Surveys from 2014 onwards at Roost 3 were conducted from an observation tower within the town of Puerto Hondo, approximately 300 m in front of the roost, allowing a full view of each parrots’ flight path to and from the roost. This tower gives a good view of the roost area and approximately 1.2 km on either side. Morning surveys were conducted, by the same researcher, who attended the vantage point from 05h30 to 07h30. At this roost, birds are only seen flying in one direction (into or out of the roost) and therefore it is unlikely that birds were double counted. A consecutive day counting regime was used - the last four days of each month, which has been previously found to be more precise than counting on random days throughout the month; the regime used in 1999/2000 (Berg and Angel Reference Berg and Angel2006, Cougill and Marsden Reference Cougill and Marsden2004).
To facilitate Objective 3, we compiled all available surveys conducted at Roost 3 to assess long-term change in the size of this roost over time. Survey data were available from June 1999 to May 2000 (conducted by Berg and Angel, Reference Berg and Angel2006) and for various months between November 2015 and May 2018.
Community questionnaires
To address Objective 4, information on the presence of pet parrots was gathered through researcher-led questionnaires in 52 communities within the study area (Figure 1). A total of 427 people took part, representing between 4 and 23 households per community. ‘Open Street Map’ (OSM) was used to categorise communities as hamlets, villages, or towns. Communities were selected due to their close proximity to lowland dry tropical forests (Ministerio del Ambiente 2012). Following trial surveys, questionnaires were carried out from January to July 2017. A combination of photographs, questions and sound recordings were used to ascertain if the participant could correctly identify A. lilacina. Participants were then asked: “Are there any pet parrots in your village?” and “Which parrot species are kept as pets?”
Questionnaires were conducted in Spanish and only the researcher (ISP) and participant were present. Due to potential bias in self-reporting behaviour using direct questioning, especially in cases where that behaviour is illegal (Fisher Reference Fisher1993, Nuno and St John Reference Nuno and St John2014), we only asked participants to report the presence or absence of pets in their community as a whole. Participants could decline to contribute and were asked for verbal consent prior to participation once the purpose of the research was explained. Interviews were anonymous and data were coded to ensure that no individuals could be identified.
Data analysis
For Objective 1, observation locations were used to estimate the Extent of Occurrence (EoO) using the IUCN Red List guidelines (IUCN Standards and Petitions Subcommittee 2016). ArcGIS was used to calculate the EoO, defined as “the area contained within the shortest continuous imaginary boundary which can be drawn to encompass all the known, inferred or projected sites of current occurrence of a taxon (IUCN Standards and Petitions Subcommittee 2016). The ‘Minimum Bounding Convex Polygon’ tool was used within ArcToolbox to estimate area of EoO, with no exclusion areas.
To estimate the area of land that birds are likely to disperse over daily, buffers of 10 km were created around observation points; this is suggested to be the approximate diurnal ranging area of A. auropalliata in Costa Rica (Salinas-Melagoza et al. Reference Salinas-Melgoza, Salinas-Melgoza and Wright2012). Buffers were dissolved in ArcToolbox. To analyse possible movement between daily dispersal areas, absence points were created using eBird complete checklists that did not record the species. Data were filtered and extracted using the auk package in R and following suggestions on best practice from Johnston et al. (Reference Johnston, Hochachka, Strimas-Mackey, Ruiz Gutierrez, Robinson, Miller, Auer, Kelling and Fink2019), by restricting checklists to < 5 h duration, > 5 km in length, and with < 11 observers.
For Objective 3, count data from roost surveys conducted using comparable methodology were analyzed to assess any change in the size of Roost 3 from 1999/2000 to 2017/2018. For this analysis, only morning counts were used owing to the conclusions of Berg and Angel (Reference Berg and Angel2006) who found that their morning counts were more consistent, larger, and thus more accurate. Additionally, Cougill and Marsden (Reference Cougill and Marsden2004) showed morning counts to be more precise for estimating size of other amazon roosts. A generalised linear mixed model (GLMM) with a Poisson distribution and ‘month’ as a random effect was fitted to compare counts from the two data sets. All statistical analyses were conducted in R (version 3.6.0; R Core Team, 2019).
For Objective 4, ‘Open Street Map’ (OSM) was used to identify all communities in the study area, in the categories of hamlet/village/town. Predictor variables were calculated for each community (surveyed and not surveyed) using the ‘Euclidean Distance’ and ‘Values to Points’ tools in ArcToolbox. These related to species availability, accessibility and land protection status: distance to nearest sighting/roost, elevation (Jarvis et al. Reference Jarvis, Reuter, Nelson and Guevara2008), distance to nearest road (defined by OSM), and inclusion status within the National System of Forest and Protected Vegetation 2015 (defined by Ministerio del Ambiente). Additionally, mean Normalised Difference Vegetation Index (NDVI) from the monthly MODIS product, MOD13A3, averaged across the period 2010–2015, was included as a proxy of vegetation cover. Random Forests (Breiman Reference Breiman2001) was used to classify surveyed villages with and without pet parrots, and with/without pet A. lilacina. The ‘predict’ function in this package was then used to predict the likelihood of pet parrots and pet amazons being present in the remaining non-surveyed communities within the study area. Communities with a predicted vote score of 0.6 or over, thus a greater than 60% probability, were classed as likely to have pets.
Results
In total 132 occurrence points were gathered, and analysis of eBird checklists resulted in confirmation of 4,626 points of species absence (Figure 2a). The estimated Extent of Occurrence is 19,890 km2 within which 5,313 km2 is used by the species during daily dispersal. According to the IUCN’s definition of subpopulations (IUCN Standards and Petitions Subcommittee 2016) we suggest that Amazona lilacina occurs in at least three distinct subpopulations separated by a minimum of 40 km (Figure 2b).
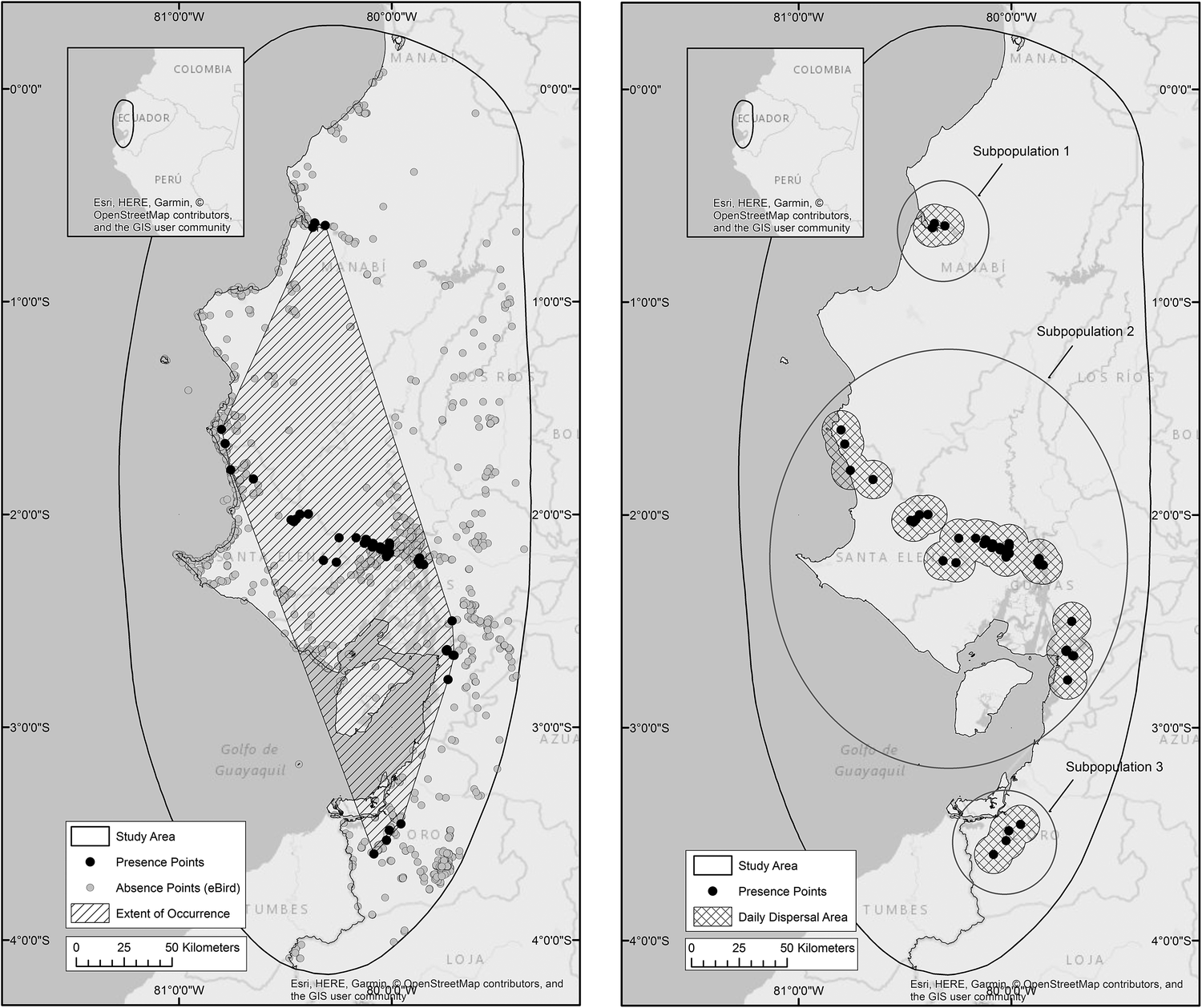
Figure 2a. Presence (n = 132) and absence (n = 4,626) points recorded for A. lilacina. Presence points are joined by a convex hull to estimate the species’ Extent of Occurrence of 19,890 km2. Figure 2b. Occurrence points are surrounded by 10 km buffers to represent a daily dispersal area of 5,313 km2, within three subpopulations.
Minimum and maximum counts from each roost survey (Table 1) reflect fluctuations in the number of birds attending each roost during the survey period. Although it is always possible that more roosts exist within the study area, we believe we have identified all remaining large roosts (> 30 individuals) and thus we estimate the remaining global population at 741–1,090, which includes mature and immature birds. We suggest that counts conducted in March (1,090) at the end of the breeding season, represent the population including young birds, and that counts from January (804) represent the population without breeding birds or at least females with eggs or chicks in the early developmental stages. We saw a slight decrease in global population size between March (1,090) and August (1,046) which may represent juvenile mortality.
Table 1. Number of A. lilacina counted during roost surveys at all known roosts. Roost 1 was not present in March 2017. Local reports suggest this was in response to damage caused to the mangrove island roost site by a large earthquake. Thus, the mean of all counts prior to January 2019 is used: 97 and 103 (Nov 2012), 84 and 86 (Aug 2014). The population is estimated at a minimum of 741–1,090 individuals.

When considering all surveys conducted at Roost 3 from 1999 to 2018, there has been an overall decline in the total number of birds (Figure 3). Our GLMM revealed that average counts declined between the two periods of data collection representing a significant drop in roost size in 2017/2018 compared with 1999/2000 (β = -1.02, SE = 0.24, P < 0.001). On average, the difference between monthly counts suggests a 60% reduction in the size of the roost. The maximum roost size in 2000 was 229 birds (Berg and Angel Reference Berg and Angel2006), but just 117 in 2018.
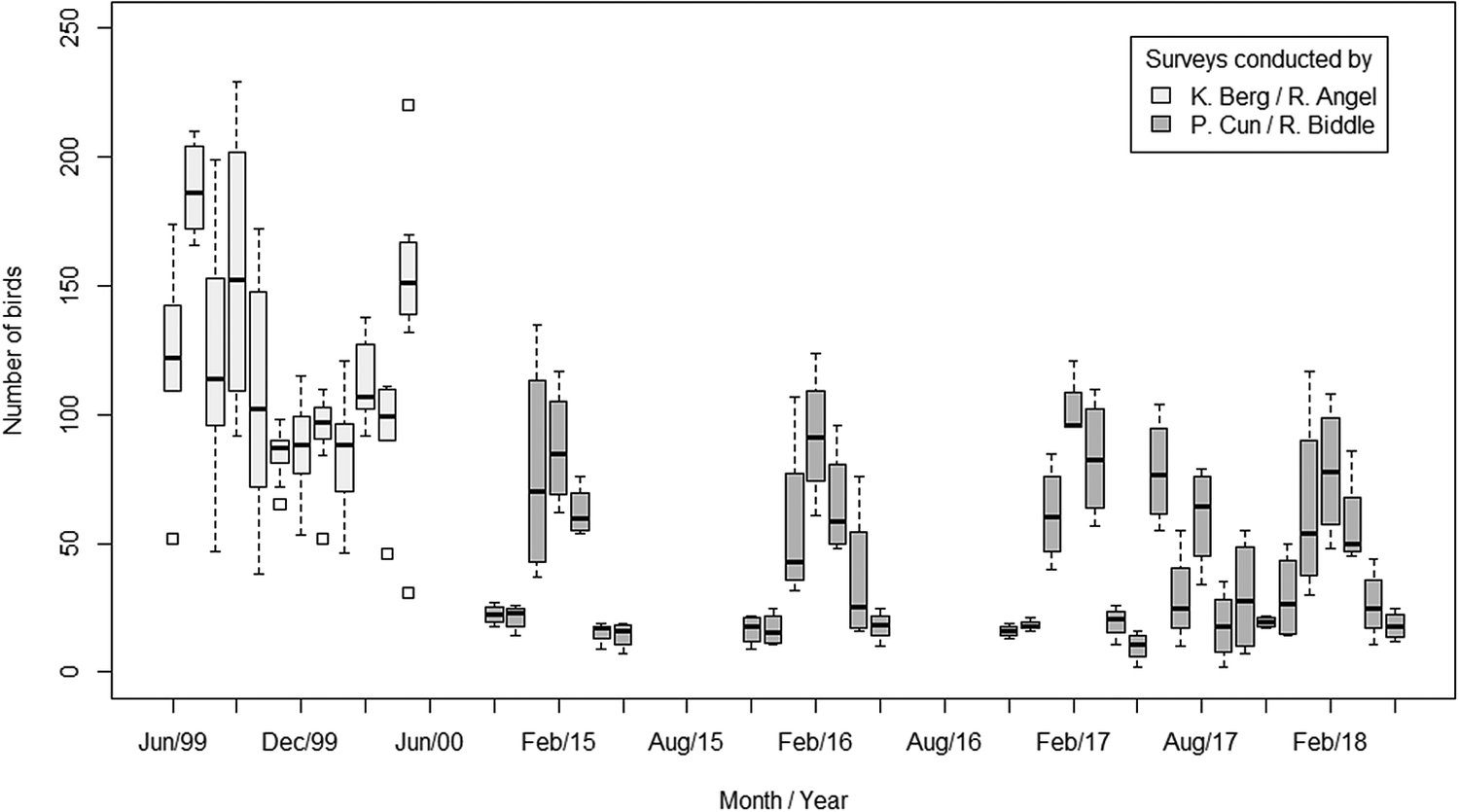
Figure 3. Roost surveys recording the total number of birds departing Roost 3 during morning surveys conducted between June 1999 and May 2018. The average number of birds counted decreased by 60% between 1999/2000 and 2017/2018.
Of 52 communities surveyed, presence of pet parrots was confirmed in 37. A total of 11 parrot species, including A. lilacina were recorded: Yellow-crowned Amazon A. ochrocephala, Orange-winged Amazon A. amazonica, Southern Mealy Amazon A. farinosa, Red-lored Amazon A. autumnalis salvini, Bronze-winged Parrot Pionus chalcopterus, Blue-headed Parrot P. menstruus, Red-masked Parakeet Psittacara erythrogenys, Grey-cheeked Parakeet Brotogeris pyrrhoptera, White-winged Parakeet B. versicolurus and Pacific Parrotlet Forpus coelestis. Communities with pet parrots could be classified (out of bag error rate 16%) using variables of elevation and distance to the nearest roost, it is predicted that 1,617 of the 3,231 additional non-surveyed communities within the study area have a greater than 60% probability of containing pet parrots.
Of the 37 communities with confirmed presence of pet parrots, 17 held pet A. lilacina. These 17 could be classified (out of bag error rate 31%) using predictors of distance to roost, distance to sighting and NDVI. It is predicted using this classification, that 79 of the 3,231 additional non-surveyed communities within the study area have a greater than 60% probability of having pet A. lilacina. It was felt the value of 60% across the community as a whole, would equate to a much higher probability of at least one pet being owned. From the above, we suggest that within our study area, approximately 1,645 communities have pet parrots, and at least 96 of these have pet A. lilacina (Table 2).
Table 2. Of the 52 surveyed communities, 37 reported pet parrots and 17 reported pet A. lilacina. Using random forests to predict the occurrence of pet parrots throughout similar communities within the study area, we suggest 1,617 communities have pet parrots and at least 96 have pet A. lilacina.

Discussion
We estimate the Extent of Occurrence (EoO) for the recently recognised Amazona lilacina to be half of that currently listed on the IUCN Red List (BirdLife International 2018), which from available data represents the smallest remaining EoO of any ‘Endangered’ mainland amazon parrot (Birdlife International 2018). We suggest A. lilacina has a population size of between 741 and 1,090 birds and that this population is declining, with Roost 3 showing a reduction in size of 60% over the past 19 years – a similar decline to that seen in other parrot species globally (Berkunsky et al. Reference Berkunsky, Quillfeldt, Brightsmith, Abbud, Aguilar, Alemán-Zelaya and Masello2017). This rate of decline supports the IUCN listing of ‘Endangered’ under criterion A, and if reflected over the whole population may qualify the species for listing as ‘Critically Endangered’. Further research is needed to assess this, however, when comparing our 2019 counts, to unpublished counts from researchers in 2014, we see a decline of 59% also at Roost 2; an area where strong poaching pressure has been observed (G. Blanco, F. Hiraldo and J. L. Tella pers. comm. 2020). We report that local capture for pets is an ongoing threat and support the notion that Ecuador should be prioritised for parrot conservation (Olah et al. Reference Olah, Butchart, Symes, Guzmán, Cunningham, Brightsmith and Heinsohn2016).
As commonly seen in parrot roost counts, our results showed variability in roost size within and across months. This may be explained in part by imperfect detection whereby birds arrive at or depart roosts undetected due to low light levels or weather conditions (Dénes et al. Reference Dénes, J and Beissinger2018). Although every attempt was made to account for this, due to the opportunistic nature of some of our roost surveys, a structured counting regime as suggested by Cougill and Marsden (Reference Cougill and Marsden2004) was not always followed. Additionally, it is possible that some birds may have gathered temporarily in smaller, undetected roosts and thus be missed from main roost surveys. Despite this, we believe the results presented here offer a valuable first estimate of population size and trajectory for this ‘Endangered’ species.
Amazona lilacina’s northern border was previously recorded as south-west Colombia or the Esmeraldas province of Ecuador (CITES 1986, Juniper and Parr Reference Juniper and Parr1998, Ridgley and Greenfield Reference Ridgley and Greenfield2001, Forshaw Reference Forshaw2010, Athanas and Greenfield Reference Athanas and Greenfield2016), but we suggest, in agreement with local experts, that these more northern birds are in fact A. autumnalis salvini (R. Orrantia, Fundación Jambeli pers. comm. 2013, M. Schaefer, Fundación Jocotoco pers. comm. 2014, R. Ridgley, Rainforest Trust pers. comm. 2015). Within our newly presented EoO we no longer believe the species is restricted to mangrove roosting areas, owing to the discovery of a new roost located > 50 km from any mangroves. However, we do suggest that the species is still highly geographically restricted, with an estimated daily dispersal area of just 5,313 km2 split between three distinct geographically isolated subpopulations. Although movement between these three areas is unlikely due to their separation distance of approximately 40 km, further research into the daily movement and genetic structure of these subpopulations is needed to confirm this.
Historically, threats to this species have been severe: CITES reported thousands of A. lilacina being trapped and exported from the country in the early 1980s (CITES 1986), and Ecuador reported the highest rate of deforestation in South America for the period 2000–2005, with the main cause being clearing of the lowland coastal forests for agricultural crops (Mosandl et al. Reference Mosandl, Günter, Stimm and Weber2008). As early as 1986, the plight of this species was highlighted (CITES 1986) and we believe the population is still at risk and in decline. A likely contributor to this is that the range overlaps with a large proportion (46%) of Ecuador’s human population (INEC 2010). In addition to the direct threat of local capture for pets, anthropogenic effects such as fire, hunting, land trafficking and the development of squatter settlements are reported as the greatest threats to the lowland coastal forests this species relies on (Horstman Reference Horstman2017).
We predict that over half of all communities within the study area have pet parrots, despite it being illegal since the mid-1980s to hunt or trade species included in the CITES Appendices. A few of these could be long-lived individuals, however we expect that to be a minority. Ecuador’s confiscation reports also suggest a large number of parrots in captivity with 91% of all birds confiscated between 2003 and 2014 being Psittacidae and 7% of these A. autumnalis (Ortiz von-Halle Reference Ortiz von-Halle2018). Law enforcement in the form of pet confiscation does not appear to be a strong deterrent, and only once has a case of bird crime resulted in a jail sentence in Ecuador (Ortiz-von Halle Reference Ortiz von-Halle2018). We predict that nearly 100 communities hold A. lilacina but expect this is an underestimation due to difficulties in identifying parrots to species level. We did gather evidence of poaching of A. lilacina chicks and adults during fieldwork, either to generate core income, or incidentally, to fulfil a specific economic need such as buying uniforms at the start of the school term. Additionally, reports of farmers using nets or poison to protect their crops against parrots and historical reports of family relatives shooting macaws and amazons for food were made. Although legal subsistence hunting does not appear a threat to the species, recent concern has been raised regarding its sustainability in the light of changes in human population size, hunting methods, and habitat fragmentation (Suarez and Zapata-Rios Reference Suarez and Zapata–Rios2019).
The lowland coastal provinces where A. lilacina occurs have been identified as having an acute lack of protected areas (Cuesta et al. Reference Cuesta, Peralvo, Merino-Viteri, Bustamante, Baquero, Freile, Muriel and Torres-Carvajal2017). Additionally, the lowland forests, mangroves and algarrobo trees, are all habitats essential for local community income and sustenance, through hunting, fishing for crabs, cutting of firewood or making charcoal. Outside of these habitats, our observations occasionally recorded the species in crop fields, gardens, and even villages where fruit trees have been planted, and in the last couple of years eBird users are more frequently recording the species within the large city of Guayaquil. This species is clearly existing across a highly anthropogenically-influenced landscape, and although there are examples worldwide of parrots adapting to such environments (e.g. Lill Reference Lill2009, Martens and Woog Reference Martens and Woog2017) the effects this may have on their natural behaviours or ecological functions could be significant (Luna et al. Reference Luna, Romero-Vidal, Hiraldo and Tella2018).
Our study highlights an urgent need for a collaborative approach to conservation to reduce A. lilacina’s vulnerability to extinction; with governments, local NGOs and conservation organisations working together to enforce law and to ensure vital remaining fragments of forest are protected, but most importantly, for local communities to be engaged and empowered towards the conservation of this species.
Acknowledgements
Thank you to Dr Robert Ridgley (Rainforest Trust), Dr Nigel Simpson, Francisco Sornoza and Dr Martin Schaefer (Fundación Jocotoco), Rafaela Orrantia and Bob Bivens (Fundación Jambeli), Professor José Tella and Juan Freile, for sharing their knowledge about the species with us. Thank you to the administration staff and park guards of Fundación Pro Bosque and to Belén Chiriboga of Zoo El Pantanal for assisting with logistical support. Additionally, to the Fundación Municipal of Guayaquil para la Regeneración Urbana for allowing access to the Puerto Hondo observation tower outside of public opening hours. Finally, thank you to The North of England Zoological Society for providing all the funds for this research, to Paul Bamford for providing the summary translation, and to the many staff who assisted with data collection.







