Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease of the joints and bones(Reference Firestein1). Joint inflammation is manifested by swelling, pain, functional impairment, morning stiffness, osteoporosis, and muscle wasting. Bone erosion commonly occurs in the joints of the hands and feet. The joint lesions are characterised by infiltration of immune cells and contain high concentrations of many of the chemical mediators they produce(Reference Feldmann and Maini2). One pharmaceutical treatment for the inflammation involved in RA has involved the use of non-steroidal anti-inflammatory drugs (NSAIDs). NSAIDs target the metabolism of the n-6 fatty acid arachidonic acid (ARA) to prostaglandins (PGs) by cyclooxygenase (COX) enzymes, suggesting a key involvement of these mediators in the pathology of RA. N-3 fatty acids from oily fish and fish oils target ARA availability and metabolism and also influence several other immuno-inflammatory responses involved in RA. Thus, marine n-3 fatty acids could be useful in treating RA. This article will describe the effects of marine n-3 fatty acids on different aspects of immune responses of relevance to RA, and will then describe the effects of marine n-3 fatty acids in animal models of RA. The article will then report on a systematic evaluation of marine n-3 fatty acids in clinical trials with RA patients. Finally, the findings of the systematic evaluation will be compared with the conclusions of meta-analyses of the efficacy of marine n-3 fatty acids in RA. Parts of this article are updated from an earlier one on this topic(Reference Calder3).
Rheumatoid arthritis
RA is a chronic inflammatory autoimmune disease that affects about 1 % of adults. It is more common in women than in men. The joint lesions show infiltration of activated T lymphocytes, macrophages and antibody-secreting B lymphocytes into the synovium (the tissue lining the joints) and there is proliferation of fibroblast-like synovial cells called synoviocytes(Reference Sweeney and Firestein4). These cells and new blood vessels form a tissue termed pannus which leads to progressive destruction of cartilage and bone. This is most likely due to cytokine- and eicosanoid-mediated induction of destructive enzymes such as matrix metalloproteinases. Synovial fluid from patients with RA contains high levels of pro-inflammatory cytokines including tumour necrosis factor (TNF)-α, IL-1β, IL-6, IL-8 and granulocyte/macrophage colony stimulating factor(Reference Feldmann and Maini2). Synovial cells cultured ex vivo spontaneously produce these cytokines for extended periods of time(Reference Feldmann and Maini2). RA is also characterised by signs of systemic inflammation, such as elevated plasma concentrations of some cytokines (e.g. interleukin (IL)-6), acute phase proteins, and rheumatoid factors.
Genetic studies have linked susceptibility to, and severity of, RA to genes in the major histocompatibility class (MHC) II locus(Reference Bowes and Barton5); in humans these genes encode the human leukocyte antigen (HLA) II proteins involved in antigen presentation. RA is associated with specific alleles of the HLA-DRB1 gene, although other HLA-DR alleles may also play a role(Reference Bowes and Barton5). Because the function of HLA-DR is antigen presentation to T lymphocytes, the genetic association indicates a role for T cells in RA(Reference Panayi6). In total, the HLA region contributes 30 to 50 % of the genetic component of RA. The second largest genetic risk for RA lies with a variant in the protein tyrosine phosphatse non-receptor 22 gene, which encodes an intracellular protein tyrosine phosphatase(Reference Bowes and Barton5). The variant may act to reduce the ability to down-regulate activated T cells.
Arachidonic acid, eicosanoids and the links with inflammation and RA
Eicosanoids are amongst the most important mediators and regulators of inflammation(Reference Tilley, Coffman and Koller7). They are formed from 20 carbon polyunsaturated fatty acids (PUFAs). Because immune cells usually contain a high proportion of the n-6 PUFA ARA and low proportions of other 20-carbon PUFAs, ARA is considered to be the major substrate for synthesis of eicosanoids. Eicosanoids include PGs, thromboxanes, leukotrienes (LTs) and other oxidised derivatives. Fig. 1 summarises the pathway of synthesis of these mediators from ARA(Reference Calder8). COX is key to synthesis of PGs and there are two principal COX isoforms. These are COX-1, which is constitutively expressed, and COX-2, which is up-regulated by inflammatory stimuli. Expression of both COX isoforms is increased in the synovium of patients with RA and in joint tissues in rat models of arthritis(Reference Sano, Hla and Maier9). Eicosanoids produced by both the COX and lipoxygenase (LOX) pathways are found in the synovial fluid of patients with active RA(Reference Sperling10). Infiltrating leukocytes such as neutrophils, monocytes and synoviocytes are the key sources of eicosanoids in RA(Reference Sperling10). PGE2 has a number of proinflammatory effects including increasing vascular permeability, vasodilation, blood flow and local pyrexia, and potentiating pain caused by other agents. It also promotes the production of some of the destructive matrix metalloproteinases and stimulates bone resorption. The efficacy of NSAIDs, which are COX inhibitors, in RA indicates the importance of this pathway in the pathophysiology of the disease. These drugs provide rapid relief of pain and stiffness by inhibiting joint inflammation. LTB4 increases vascular permeability, enhances local blood flow, is a potent chemotactic agent for leukocytes, induces release of lysosomal enzymes, and enhances release of reactive oxygen species and inflammatory cytokines like TNF-α, IL-1β and IL-6.

Fig. 1 Outline of the pathway of eicosanoid synthesis from arachidonic acid. COX, cyclooxygenase; HETE, hydroxyeicosatetraenoic acid; HPETE, hydroperoxyeicosatetraenoic acid; LOX, lipoxygenase; LT, leukotriene; PG, prostaglandin; TX, thromboxane. Taken from Calder(Reference Calder8) with permission.
Fatty acid modification of immune cell fatty acid composition and of eicosanoid profiles
Fatty acids are constituents of phospholipids and phospholipids are components of cell membranes. The bulk phospholipids of immune cells (e.g. neutrophils, lymphocytes, monocytes) isolated from the blood of healthy people consuming typical Western diets have been reported to contain about 10 to 20 % of fatty acids as ARA, with about 0·5-1 % of the n-3 PUFA eicosapentaenoic acid (EPA) and about 1·5-3 % of another n-3 PUFA docosahexaenoic acid (DHA)(Reference Lee, Hoover and Williams11–Reference Healy, Wallace, Miles, Calder and Newsholme17). There are, however, differences between the different phospholipid classes in terms of the content of these fatty acids(Reference Sperling, Benincaso and Knoell13). EPA and DHA are found in seafood, especially oily fish, and in fish oil-type supplements. Thus EPA and DHA may be referred to as marine n-3 PUFAs. The fatty acid composition of human blood leukocytes can be modified by increasing the oral intake of marine n-3 PUFAs. This results in increased proportions of EPA and DHA in blood monocytes, mononuclear cells and neutrophils(Reference Lee, Hoover and Williams11–Reference Rees, Miles and Banerjee21). Typically the increase in content of marine n-3 PUFAs occurs at the expense of n-6 PUFAs, including ARA. Time-course studies suggest that the incorporation of EPA and DHA into human blood leukocytes begins within days and reaches its peak within one or two weeks of commencing increased intake(Reference Yaqoob, Pala, Cortina-Borja, Newsholme and Calder16–Reference Thies, Nebe-von-Caron and Powell18, Reference Kew, Mesa and Tricon20–Reference Faber, Berkhout and Vos22). Studies using multiple doses of fish oil show that the incorporation of EPA and DHA into human blood leukocytes occurs in a dose-response manner(Reference Healy, Wallace, Miles, Calder and Newsholme17, Reference Rees, Miles and Banerjee21, Reference Calder23).
There are many reports of decreased production of PGE2 and of 4 series-LTs by immune cells following a period of fish oil supplementation of the diet of healthy volunteers(Reference Lee, Hoover and Williams11–Reference Sperling, Benincaso and Knoell13, Reference Caughey, Mantzioris, Gibson, Cleland and James15, Reference Meydani, Endres and Woods24, Reference Von Schacky, Kiefl, Jendraschak and Kaminski25). Similar effects are seen in patients with RA, where fish oil supplements decreased LTB4 production by neutrophils(Reference Kremer, Bigauoette and Michalek26–Reference van der Tempel, Tullekan, Limburg, Muskiet and van Rijswijk31) and monocytes(Reference Cleland, French, Betts, Murphy and Elliot30), 5-hydroxyeicosatetraenoic acid production by neutrophils(Reference Cleland, French, Betts, Murphy and Elliot30), and PGE2 production by mononuclear cells(Reference Cleland, Caughey, James and Proudman32).
The studies in humans demonstrating a reduction in ARA-derived eicosanoid production by oral marine n-3 fatty acids have typically used fairly high intakes (several g/day). A dose-response study in healthy volunteers reported that an EPA intake of 1·35 g/d for 3 months was not sufficient to influence ex vivo PGE2 production by endotoxin stimulated mononuclear cells, whereas an EPA intake of 2·7 g/day significantly decreased PGE2 production(Reference Rees, Miles and Banerjee21). These data suggest a threshold of intake of EPA to elicit an anti-inflammatory effect; this threshold would be between 1·35 and 2·7 g/day.
EPA is also a substrate for the COX and LOX enzymes that produce eicosanoids (Fig. 2), but the mediators formed have a different structure from the ARA-derived mediators. Neutrophils from healthy volunteers supplemented orally with fish oil for several weeks produced much increased amounts of 5-series LTs(Reference Lee, Hoover and Williams11–Reference Sperling, Benincaso and Knoell13). In patients with RA given marine n-3 PUFAs, there was generation of the usually undetectable LTB5 and 5-hydroxyeicosapentaenoic acid by stimulated neutrophils(Reference Sperling, Weinblatt and Robin29–Reference van der Tempel, Tullekan, Limburg, Muskiet and van Rijswijk31) and monocytes(Reference Sperling, Weinblatt and Robin29). The functional significance of generation of eicosanoids from EPA is that EPA-derived mediators are often much less biologically active than those produced from ARA(Reference Goldman, Pickett and Goetzl33–Reference Bagga, Wang, Farias-Eisner, Glaspy and Reddy35) and they may even act as antagonists of the ARA-derived mediators(Reference Tull, Yates and Maskrey36).
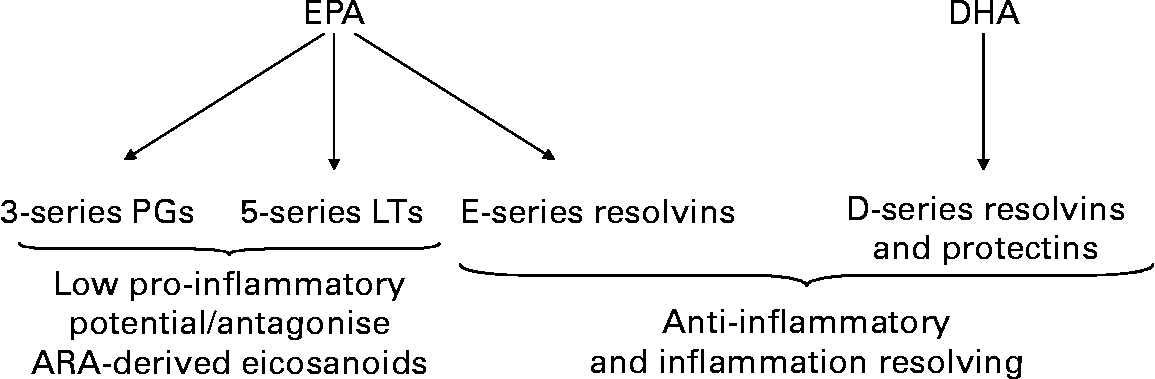
Fig. 2 Overview of eicosanoid and resolvin synthesis from eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Abbreviations used: LT, leukotriene; PG, prostaglandin.
Resolvins: Novel anti-inflammatory and inflammation resolving mediators produced from EPA and DHA
EPA and DHA both give rise to a family of lipid mediators termed resolvins (Fig. 2). The pathways of synthesis of resolvins are complex and not fully elucidated yet, but they involve the action of both COX and LOX enzymes and may be modified in the presence of aspirin. E-series resolvins are produced from EPA and D-series resolvins from DHA. DHA is also a substrate for similar molecules called protectins, also known as neuroprotectins. A large number of studies using cell culture and animal models have convincingly shown that E- and D-series resolvins and protectins are anti-inflammatory, inflammation resolving and immunomodulatory(Reference Serhan, Clish and Brannon37–Reference Marcheselli, Hong, Lukiw and Hua Tian41). There is very limited human data on resolvins and they have not yet been studied in the context of RA.
Influence of marine n-3 fatty acids on production of inflammatory cytokines
Cell culture studies report that EPA and DHA can inhibit the production of the classic pro-inflammatory cytokines (TNF-α, IL-1, IL-6) by several cell types(Reference De Caterina, Cybulsky, Clinton, Gimbrone and Libby42–Reference Zhao, Joshi-Barve, Barve, Chen, Barve and Chen47), effects supported by animal feeding studies(Reference Calder8). Several studies in healthy human volunteers involving supplementation of the diet with fish oil have demonstrated decreased production of TNF-α, IL-1β and IL-6 by endotoxin-stimulated monocytes or mononuclear cells (a mixture of lymphocytes and monocytes)(Reference Endres, Ghorbani and Kelley12, Reference Caughey, Mantzioris, Gibson, Cleland and James15, Reference Meydani, Endres and Woods24, Reference Trebble, Arden and Stroud48–Reference Gallai, Sarchielli and Trequattrini52), although not all studies confirm this effect(Reference Yaqoob, Pala, Cortina-Borja, Newsholme and Calder16, Reference Kew, Mesa and Tricon20, Reference Rees, Miles and Banerjee21, Reference Thies, Miles and Nebe-von-Caron53–Reference Cannon, Fiatarone and Meydani58). Reasons for the different findings include the low n-3 PUFA dose, the short duration and the small sample size of some of the studies failing to find an effect, but these are unlikely to be the sole explanations. Studies using fish oil in patients with RA report decreased IL-1 production by monocytes(Reference Kremer, Lawrence and Jubiz28), and decreased circulating concentrations of IL-1β(Reference Esperson, Grunnet and Lervang59, Reference Kremer, Lawrence and Petrillo60), TNF-α(Reference Adam, Beringer and Kless61, Reference Kolahi, Ghorbanihaghjo and Alizadeh62) and soluble receptor activator of nuclear factor kappa B ligand(Reference Kolahi, Ghorbanihaghjo and Alizadeh62).
Influence of marine n-3 fatty acids on production of reactive oxygen species
Providing high doses (>3 g/day) of marine n-3 PUFAs to healthy volunteers resulted in decreased production of reactive oxygen species (superoxide or hydrogen peroxide) by blood neutrophils stimulated with different agents(Reference Varming, Schmidt and Svaneborg63–Reference Luostarinen and Saldeen65) and high dose marine n-3 PUFAs decreased hydrogen peroxide production by human monocytes(Reference Fisher, Levine and Weiner66). Studies using lower doses of marine n-3 PUFAs ( < 2·3 g/day) did not see effects on reactive oxygen species production by either neutrophils or monocytes(Reference Healy, Wallace, Miles, Calder and Newsholme17, Reference Thies, Miles and Nebe-von-Caron53–Reference Miles, Banerjee, Dooper, M'Rabet, Graus and Calder55, Reference Schmidt, Varming, Moller, Bulow Pederson, Madsen and Dyerberg67). Rees et al. (Reference Rees, Miles and Banerjee21) identified an EPA dose-dependent decrease in the number of blood neutrophils producing superoxide in elderly subjects, but there was no effect in younger subjects. Fish oil supplements decreased reactive oxygen species production by neutrophils from the blood of RA patients(Reference Magaro, Altomonte and Zoli68).
Influence of marine n-3 fatty acids on T cells
Cell culture studies report that EPA and DHA inhibit proliferation of human T cells and their production of IL-2(Reference Calder and Newsholme69). Animal feeding studies support these observations(Reference Calder8), but human data in this area are inconsistent. Some studies in healthy humans report that increased intake of marine n-3 PUFAs decreases human T cell proliferation(Reference Thies, Nebe-von-Caron and Powell18, Reference Meydani, Endres and Woods24, Reference Molvig, Pociot and Worsaae57) and IL-2 production(Reference Meydani, Endres and Woods24, Reference Gallai, Sarchielli and Trequattrini52), but several other studies show no effect on these outcomes(Reference Yaqoob, Pala, Cortina-Borja, Newsholme and Calder16, Reference Trebble, Arden and Stroud48, Reference Wallace, Miles and Calder49, Reference Kew, Banerjee and Minihane54, Reference Miles, Banerjee, Dooper, M'Rabet, Graus and Calder55, Reference Miles, Banerjee, Wells and Calder70). Again the reasons for these different findings may include the low n-3 PUFA dose, the short duration and the small sample size of some of the studies; differences in the age of the subjects studied might also contribute to the variation in findings(Reference Meydani, Endres and Woods24).
Influence of marine n-3 fatty acids on antigen presentation
A small number of cell culture studies have found that MHC II expression and antigen presentation via MHC II are decreased following exposure of antigen presenting cells to EPA or DHA(Reference Khair-El-Din, Sicher, Vazquez, Wright and Lu71, Reference Hughes, Southon and Pinder72). These findings are supported by work in animals fed fish oil(Reference Calder73), but there is limited information on n-3 PUFAs and antigen presentation in humans(Reference Hughes, Pinder, Piper, Johnson and Lund74).
Marine n-3 PUFAs and animal models of RA
The effects of marine n-3 PUFAs on antigen presentation, T cell reactivity, and inflammatory lipid, peptide and oxygen-derived mediator production suggest that these fatty acids might have a role both in decreasing the risk of development of RA and in decreasing severity in those patients with the disease. This has been explored using animal models of arthritis. In an early study, compared with vegetable oil, fish oil fed mice had delayed onset (mean 34 days vs. 25 days) and reduced incidence (69 % vs. 93 %) and severity (mean peak severity score 6·7 vs. 9·8) of type II collagen-induced arthritis(Reference Leslie, Gonnerman and Ullman75). In another study, both EPA and DHA suppressed Streptococcal cell wall-induced arthritis in rats, although EPA was more effective(Reference Volker, FitzGerald and Garg76). A recent study compared fish oil, which provides marine n-3 PUFAs in triglyceride form, and krill oil, which provides marine n-3 PUFAs partly in the form of phospholipids, in collagen-induced arthritis in the susceptible DBA/1 mouse strain(Reference Ierna, Kerr, Scales, Berge and Griinari77). Both chemical formulations of marine n-3 PUFAs slowed the onset of arthritis, decreased its severity, reduced paw swelling, and decreased knee joint pathology compared with the control group; for some outcomes krill oil appeared superior to fish oil.
A systematic review of randomized controlled trials of orally administered marine n-3 PUFAs in RA
Introduction
The studies outlined above indicate that oral marine n-3 PUFAs can modulate a range of immunological reactions that are associated with the immunological dysfunction or inflammation-induced pathology associated with RA; aspects reported to be modified by marine n-3 PUFAs in some studies in healthy volunteers or in RA patients include antigen presentation, T cell reactivity, reactive oxygen species production by leukocytes, inflammatory cytokine production by macrophages, and inflammatory eicosanoid production by various cells. These effects have been demonstrated mainly in healthy volunteers, but similar findings are made in a small number of studies in patients with RA(Reference Kremer, Bigauoette and Michalek26–Reference Cleland, Caughey, James and Proudman32, Reference Esperson, Grunnet and Lervang59–Reference Kolahi, Ghorbanihaghjo and Alizadeh62, Reference Magaro, Altomonte and Zoli68). Animal models of arthritis have been used to demonstrate a benefit from marine n-3 PUFAs(Reference Leslie, Gonnerman and Ullman75–Reference Ierna, Kerr, Scales, Berge and Griinari77). These observations, particularly the early finding that marine n-3 PUFAs decrease eicosanoid formation from ARA, have lead to a number of clinical trials of oral marine n-3 PUFAs, usually in the form of fish oil, in patients with RA. The rest of this article is given over to as systematic review of randomized, controlled trials of oral marine n-3 PUFAs in adults with RA.
Identification of articles to include in the systematic review
A PubMed search was performed on 25 November 2011 using the MeSH terms (Fatty acids, Omega-3 OR Fish oils) AND Arthritis, Rheumatoid AND Human. The search identified 155 articles. The a priori inclusion criteria for the systematic review were:
● randomized, controlled trial
● use of marine n-3 PUFAs
● oral administration through supplements or foods
● published in full as a research paper
● published in English
● reporting clinical outcomes
Examination of the titles and short PubMed descriptions of the articles resulted in exclusion of 80 review, discussion, opinion, and comment articles, of two meta-analyses, of 16 articles not written in English, of four articles in which n-3 PUFAs were administered by a non-oral route (e.g. intravenously), and of two articles not about n-3 PUFAs (Fig. 3). The abstracts of the remaining 51 articles were read. This lead to exclusion of a further 28 articles (Fig. 3). The full text of the remaining 23 articles was read; based on this two articles were excluded (Fig. 3). Reference lists in three meta-analyses(Reference Fortin, Lew and Liang78–Reference Goldberg and Katz80) were read to identify further relevant studies; two were included after reading their full text. Thus, the systematic review included 23 articles (Fig. 3). Studies of plant n-3 PUFAs are not included.

Fig. 3 Overview of the selection of articles for inclusion in the systematic review.
Description of the included studies
Table 1 describes the studies included in the systematic review including aspects of study design, sample size, dose of EPA plus DHA used, duration, nature of the placebo, JADAD score(Reference Jadad, Moore and Carroll81) and whether the study was included in a previous meta-analysis of n-3 PUFAs and RA. Included studies were published between 1985 and 2009(Reference Kremer, Bigauoette and Michalek26–Reference Kremer, Lawrence and Jubiz28, Reference Cleland, French, Betts, Murphy and Elliot30, Reference van der Tempel, Tullekan, Limburg, Muskiet and van Rijswijk31, Reference Kremer, Lawrence and Petrillo60, Reference Adam, Beringer and Kless61, Reference Magaro, Altomonte and Zoli68, Reference Belch, Ansell, Madhok, O'Dowd and Sturrock82–Reference Das Gupta, Hossain, Islam, Dey and Khan95). Most studies adopted a parallel design, although four adopted a random order, cross-over design. This latter design is not optimal for studying effects of marine n-3 PUFAs because of their slow turnover which requires a significant wash-out phase. Wash-out periods of 4, 4 and 8 weeks were used in three of the studies employing this design; there was no wash-out phase in a fourth study of this type. Sample size was typically modest and only four studies appear to have included a formal power calculation(Reference van der Tempel, Tullekan, Limburg, Muskiet and van Rijswijk31, Reference Adam, Beringer and Kless61, Reference Skoldstam, Borjesson, Kjallman, Seiving and Akesson84, Reference Remans, Sont and Wagenaar90). Most articles did not mention a specific primary outcome. The dose of EPA plus DHA used in these studies varied from < 1 to >7 g/day and averaged about 3 g/day; one study did not specify intake of either EPA or DHA(Reference Das Gupta, Hossain, Islam, Dey and Khan95). Twenty-one of the studies provided marine n-3 PUFAs as fish oil type supplements. One study used a liquid formula containing EPA plus DHA in addition to other nutrients(Reference Remans, Sont and Wagenaar90), while one used modified foods containing marine n-3 PUFAs(Reference Dawczynski, Schubert and Hein94). Two studies(Reference Kremer, Lawrence and Jubiz28, Reference Geusens, Wouters, Nijs, Jiang and Dequeker88) evaluated two doses of marine n-3 PUFAs. Duration of the included studies varied from 4 to 52 weeks; some studies reported data at several intermediate time points. The placebo used in the studies was highly variable and commonly included another “nutritional” oil (e.g. coconut, olive or corn oil) or a mixture of such oils. In some cases the inert oil paraffin oil was used. The JADAD score(Reference Jadad, Moore and Carroll81) of the included studies was typically 3, although some studies scored lower than this. None of the included studies provided information about how patients were randomised or how the blinding was performed. For some studies it seems unlikely that patients were blind to their treatment. For example in the studies by Magaro et al. (Reference Magaro, Altomonte and Zoli68) and Das Gupta et al. (Reference Das Gupta, Hossain, Islam, Dey and Khan95), the control group did not receive any placebo supplement. Most studies provided relevant information about drop-outs and withdrawals. Statistical analysis of the results of most of the studies was poor, often relying upon multiple pairwise comparisons with no correction. The designs of many of the studies would merit a more sophisticated analytical approach than was used; for example for many of the studies a two-factor analysis of variance (factors: time and treatment) with appropriate covariates would have been appropriate. In many cases the focus of the analysis was on comparison to baseline within a group, rather than on comparison between the treatment and control group. This restricts the interpretation of some of the findings.
Table 1 Summary of the studies included in the systematic review of marine n-3 PUFAs and clinical outcomes in rheumatoid arthritis

A range of clinical outcomes was reported in the included studies (Table 2). The most commonly reported outcomes were related to tender or swollen joints, duration of morning stiffness, grip strength, physician or patient assessment of pain or disease severity, and use of NSAIDs. All studies reported multiple clinical outcomes (Table 2).
Table 2 Clinical outcomes assessed in the studies included in the systematic review of marine n-3 PUFAs in rheumatoid arthritis

Findings of the included studies
Almost all of the trials included here showed some clinical benefit of marine n-3 PUFAs (Table 3). Commonly reported benefits include reduced duration of morning stiffness, reduced number of tender or swollen joints, reduced joint pain, reduced time to fatigue, increased grip strength, reduced pain or disease activity (assessed by physician or patient) and decreased use of NSAIDs (Table 3). These effects are frequently reported within the n-3 PUFA group comparing back to the baseline value. Much less frequent benefits are reported compared with the placebo group (Table 3). Kremer et al. (Reference Kremer, Lawrence and Jubiz28) reported that both a “low” and a “high” dose of marine n-3 PUFAs brought about a similar clinical benefit, but that the effect of the high dose became apparent (i.e. significant) sooner. An interesting approach was used in the study of Adam et al. (Reference Adam, Beringer and Kless61) where marine n-3 PUFAs or placebo were given against a background of a Western diet or a so-called anti-inflammatory diet. The latter aimed to reduce the intake of n-6 PUFAs, especially ARA, on the basis that the effects of marine n-3 PUFAs might be stronger if n-6 PUFA intake (and status) was decreased simultaneously with the increased n-3 PUFA intake. Indeed n-3 PUFAs had benefits irrespective of background diet, but the effect was greater when intake of ARA was decreased. Three studies used fairly low intakes ( < 1·5 g EPA+DHA/day) of marine n-3 PUFAs(Reference Belch, Ansell, Madhok, O'Dowd and Sturrock82, Reference Remans, Sont and Wagenaar90, Reference Dawczynski, Schubert and Hein94), which may explain why those studies are the only ones that fail to report any clinical benefit from marine n-3 PUFAs. Using LPS-stimulated PGE2 production from blood mononuclear cells as a model, Rees et al. (Reference Rees, Miles and Banerjee21) identified an “anti-inflammatory threshold” for EPA intake in healthy volunteers of between 1·35 and 2·7 g/day.
Table 3 Summary of the findings of randomised, controlled studies using marine n-3 PUFAs in patients with rheumatoid arthritis

Comparison between the findings of the systematic review and the conclusions of previous meta-analyses
Table 4 summarizes the findings by indicating the number of studies that demonstrated a significant benefit (either vs. baseline or vs. placebo, the latter being more important) for the commonly reported outcomes. This organization of the outcomes emphasizes the lack of consistent findings across all of the studies conducted, but also shows the fairly high number of studies that have demonstrated a given clinical benefit. The most likely reasons for lack of consistency of findings relate to the dose of EPA+DHA used, which is probably too low in some of the studies; the small sample size of many of the studies, which in many cases has probably limited the ability to identify an effect; and the sub-optimal approaches to statistical analysis used in many of the studies. Overall it seems that marine n-3 PUFAs do reduce join pain and swelling, decrease the duration of morning stiffness, and spare the need for some anti-inflammatory medications.
Table 4 Summary of findings of this systematic review and comparison with the findings of previous meta-analyses(Reference Fortin, Lew and Liang78–Reference Goldberg and Katz80)

These conclusions can be compared with those of three meta-analyses(Reference Fortin, Lew and Liang78–Reference Goldberg and Katz80). The meta-analysis of Fortin et al. (Reference Fortin, Lew and Liang78) included data from nine trials published between 1985 and 1992 inclusive and from one unpublished trial; 8 of the published trials are included in the current systematic review (Table 1). Fortin et al. concluded that “dietary fish oil supplementation for three months significantly reduces tender joint count (mean difference − 2·9; P = 0·001) and morning stiffness (mean difference − 25·9 minutes; − = 0·01)”. Maclean et al. (Reference MacLean, Mojica and Morton79) conducted a meta-analysis that included data from trials published between 1985 and 2002, although these included one study of flaxseed oil, one study that did not use a control for fish oil, and one study in which transdermal administration of n-3 PUFAs by ultrasound, rather than the oral route, was used. Maclean et al. concluded that fish oil supplementation has no effect on “patient report of pain, swollen joint count, disease activity, or patient's global assessment”. However, this conclusion may be flawed, because of the inappropriate manner in which studies were combined and because of a poor understanding of the study designs used. For example, the meta-analysis fails to recognize that patients' ability to reduce the need for using NSAIDs or their ability to be withdrawn from NSAID use, as was done in some designs, must indicate a reduction in pain with n-3 PUFA use. This meta-analysis does state that “in a qualitative analysis of seven studies that assessed the effect of n-3 fatty acids on anti-inflammatory drug or corticosteroid requirement, six demonstrated reduced requirement for these drugs” and concluded that “n-3 fatty acids may reduce requirements for corticosteroids”. The effect of marine n-3 PUFAs on tender joint count was not assessed by Maclean et al., who simply reiterated the findings of Fortin et al. that “n-3 fatty acids reduce tender joint counts”. Goldberg and Katz(Reference Goldberg and Katz80) published a meta-analysis of 17 trials of n-3 PUFAs in the context of joint pain, including one trial in RA with flaxseed oil and two trials of fish oil not in RA patients. Data on six outcomes were analysed. This analysis indicated that fish oil reduces patient assessed joint pain (n 13 studies; 26 % reduction; P = 0·03), duration of morning stiffness (n 8 studies; 57 % reduction; P = 0·003), number of painful and/or tender joints (n 10 studies; 71 % reduction; P = 0·003), and consumption of NSAIDs (n 3 studies; 60 % reduction; P = 0·01). However, this meta-analysis also found that there was no effect of marine n-3 PUFAs on Ritchie's articular index (n 4 studies) or on patient assessed disease activity (n 5 studies). Nevertheless, the meta-analysis of Goldberg and Katz provides good evidence for the clinical efficacy of n-3 PUFAs in RA, and the conclusions of the current systematic review are similar.
Overall conclusions
Eicosanoids derived from the n-6 PUFA ARA play a role in RA, and the efficacy of NSAIDs in RA indicates the importance of pro-inflammatory COX pathway products in the pathophysiology of the disease. At sufficiently high intakes, marine n-3 PUFAs decrease the production of inflammatory eicosanoids from ARA and promote the production of less inflammatory eicosanoids from EPA and of anti-inflammatory resolvins and related mediators from EPA and DHA. Marine n-3 PUFAs have other anti-inflammatory actions including decreasing antigen presentation via MHC II, decreasing T cell reactivity and Th1-type cytokine production, decreasing inflammatory cytokine production by monocyte/macrophages, and decreasing reactive oxygen species production by various leukocytes, although these effects are not consistently reported. One reason behind the lack of consistency may be the dose of EPA plus DHA used in many studies which was probably below the “anti-inflammatory threshold”. Other contributors would include differences in duration of the intervention, in sample size, and in the age of the subjects studied. Work with animal models of RA has demonstrated efficacy of fish oil. There have been a number of clinical trials of fish oil in patients with RA. Most of these trials report some clinical improvements (e.g. improved patient assessed pain, decreased morning stiffness, fewer painful or tender joints, decreased use of NSAIDs), and when the trials have been pooled in meta-analyses statistically significant clinical benefit has emerged. The current systematic review supports the conclusion that there is fairly consistent evidence for a modest clinical efficacy of marine n-3 PUFAs in RA.
Acknowledgements
There was no funding associated with the writing of this article.
Authors' contributions:
PCC conducted the literature search; both authors scored, interpreted and discussed the clinical trials; PCC drafted the article; both authors agreed the final version of the article. Conflicts of interest:
PCC serves on Scientific Advisory Boards of the Danone Research Centre in Specialised Nutrition and Aker Biomarine. He acts as a consultant to Mead Johnson Nutritionals, Vifor Pharma, and Amarin Corporation. He has received speaking honoraria from Solvay Healthcare, Solvay Pharmaceuticals, Pronova Biocare, Fresenius Kabi, B. Braun, Abbott Nutrition, Baxter Healthcare, Nestle, Unilever and DSM. He currently receives research funding from Vifor Pharma. He is elected President of the International Society for the Study of Fatty Acids and Lipids, an organisation that is partly supported by corporate membership fees, mainly the food and supplements industries. He is a member of the Board of Directors of ILSI Europe, the Board of Directors of the European Neutraceutical Association, and the Council of the British Nutrition Foundation; these organizations are each supported in part by the food and supplements industries. EAM has no conflicts of interest.









