Introduction
Invasive species are a primary cause of loss of biodiversity on a global scale (Vitousek et al. Reference Vitousek, D’Antonio, Loope, Rejmanek and Westbrooks1997). Despite the enormous resources invested annually to combat weeds, controlling or treating invasive species generally has poor success (D’Antonio and Meyerson Reference D’Antonio and Meyerson2002). A meta-analysis by Kettenring and Adams (Reference Kettenring and Adams2011) shows that weed management rarely results in increased native species biodiversity and treated sites tend to be reinvaded by exotics. This is due to a limited persistence of treatments, lack of active and follow-up restoration strategies (e.g., seeding), and treatment strategies that do not consider the physiology of the target species. Further, invasion biology is widely considered to be suffering from a “knowing-doing” gap—land managers use their own experience rather than information generated by the scientific community (Matzek et al. Reference Matzek, Covino, Funk and Saunders2014). Managers are clearly in need of information that can guide more successful weed control strategies in the field, and researchers would benefit from syntheses of existing work in order to identify research priorities. This is particularly relevant for invaded systems that are underrepresented in the applied management literature. For example, peer-reviewed research that quantifies the level of success of invasive species treatments in desert ecosystems are among the most underrepresented (Kettenring and Adams Reference Kettenring and Adams2011).
Common methods of treating invasive species include manual removal, herbicide treatment, and prescribed fire. However, some methods can promote further invasion, especially if management methods do not take the specific traits and adaptations of the invader into account (D’Antonio and Meyerson Reference D’Antonio and Meyerson2002). If the invasion is in its late stages and the invasive species has largely displaced native species, the invasive species may take over some ecosystem services (e.g., maintaining soil stability; Zavaleta et al. Reference Zavaleta, Hobbs and Mooney2001), imprint legacy impacts (Evans et al. Reference Evans, Rimer, Sperry and Belnap2001), and deplete seedbanks of native species and thus their ability to reestablish naturally after treatment of invasive species. Due to these factors, active restoration strategies should be coupled with invasive species treatment to increase successful outcomes, but such treatments are often not included (D’Antonio and Meyerson Reference D’Antonio and Meyerson2002). A site will be less susceptible to invasion (or reinvasion after treatment) if there are species present that use resources in a similar way as the invader; land managers can assemble a plant community that would be resistant to invasion by seeding the site to restore it after treatment (Funk et al. Reference Funk, Cleland, Suding and Zavaleta2008).
Buffelgrass [Pennisetum ciliare (L.) Link] is a warm-season, drought-tolerant perennial bunchgrass originating from southern Africa, parts of the Middle East, and India (Tix Reference Tix2000) that was introduced as forage grass to drylands throughout the Americas and Australia in the 19th and 20th centuries (Marshall et al. Reference Marshall, Lewis and Ostendorf2012). Originally introduced for its hardiness to heavy grazing and drought, P. ciliare has rapidly invaded beyond its intended extent and is now considered a major threat to ecosystem biodiversity (Olsson et al. Reference Olsson, Betancourt, Crimmins and Marsh2012; Marshall et al. Reference Marshall, Lewis and Ostendorf2012; Stevens and Falk Reference Stevens and Falk2009). Further, climate change predictions favor the expansion of P. ciliare’s range and distribution (Martin et al. Reference Martin, Murphy, Liedloff, Thomas, Chadès, Cook, Fensham, McIvor and van Klinken2015). Pennisetum ciliare is an insidious invader due to its ability to directly outcompete native species for resources (Bracamonte et al. Reference Bracamonte, Tinoco-Ojanguren, Coronado and Molina-Freaner2017; Castellanos et al. Reference Castellanos, Celaya-Michel, Rodríguez and Wilcox2016; Stevens and Fehmi Reference Stevens and Fehmi2011), transport fire into ecosystems not adapted to it (McDonald and McPherson Reference McDonald and McPherson2011), spread rapidly into otherwise poorly productive sites (Van Devender and Dimmitt Reference Van Devender and Dimmitt2006), and reduce biodiversity across trophic levels (e.g., Jackson Reference Jackson2005). It remains a complicated social and economic challenge, because it is still being sold and used as a forage grass for range improvement and as a reclamation species in some regions; meanwhile, land managers in adjacent regions are interested in controlling its presence and spread (Bhattarai et al. Reference Bhattarai, Fox and Gyasi-Agyei2008; Brenner Reference Brenner2010; Marshall et al. Reference Marshall, Friedel, van Klinken and Grice2011; Tix Reference Tix2000). For example, in an interview of land managers from southern Arizona regarding their attitudes toward P. ciliare control, managers from city, county, and federal agencies and private landowners recognize P. ciliare as a threat to local biodiversity as well as infrastructure due to the increased risk of fire associated with P. ciliare (Brenner and Franklin Reference Brenner and Franklin2017; Lien and Baldwin Reference Lien and Baldwin2019). Obstacles for effective P. ciliare treatment include poor strategic planning, poor long-term treatment success, lack of financial resources, and lack of cooperation among entities (Lien and Baldwin Reference Lien and Baldwin2019).
Pennisetum ciliare is difficult to treat due to its opportunistic germination and seedbank longevity (Bracamonte et al. Reference Bracamonte, Tinoco-Ojanguren, Coronado and Molina-Freaner2017; Marshall et al. Reference Marshall, Lewis and Ostendorf2012; Tinoco-Ojanguren et al. Reference Tinoco-Ojanguren, Reyes-Ortega, Sánchez-Coronado, Molina-Freaner and Orozco-Segovia2016; Ward et al. Reference Ward, Smith and McClaran2006; Winkworth Reference Winkworth1971); the speed at which it colonizes new sites (Olsson et al. Reference Olsson, Betancourt, Crimmins and Marsh2012; Van Devender and Dimmitt Reference Van Devender and Dimmitt2006); and its capacity to grow in steep, rocky, and rural environments (Olsson et al. Reference Olsson, Betancourt, Crimmins and Marsh2012). Although land managers in some regions are attempting to control P. ciliare, there seem to be little research and few recorded case studies of permanent eradication without ongoing maintenance (e.g., Rutman and Dickson Reference Rutman, Dickson and Tellman2002). Common approaches for treating P. ciliare are manual removal with hand tools and herbicide treatment. These approaches are considered “top-down” invasive treatment strategies, because they address the symptoms rather than the cause of the invasion (i.e., the competitive traits of the invader; Falk et al. Reference Falk, Palmer and Zedler2006). In their application of restoration theory to P. ciliare control, Stevens and Falk (Reference Stevens and Falk2009) conclude that using “bottom-up” strategies (e.g., active revegetation, biological control, and limiting site disturbance) that work against the competitive advantages and traits of P. ciliare in tandem with top-down strategies (e.g., herbicide) will result in more effective and long-term control. Sites invaded by P. ciliare are also often situated in areas regularly exposed to disturbances such as wildfire, urban infrastructure, and livestock grazing (De La Barrera Reference De La Barrera2008; Miller et al. Reference Miller, Friedel, Adam and Chewings2010; Tix Reference Tix2000).
Pennisetum ciliare treatment approaches tend to be site specific, are developed in situ, have poor long-term success, can enhance invasion (e.g., Jernigan et al. Reference Jernigan, McClaran, Biedenbender and Fehmi2016), and are often poorly communicated among entities. Therefore, there is a need for a systematic review on the outcomes of various P. ciliare treatment methods and land management techniques to understand best practices and opportunities. Review papers can be a powerful tool for weed management, because they identify overarching environmental and treatment variables and extract emerging patterns that would be valuable for land managers (e.g., Gornish et al. Reference Gornish, Case, Valle, Bean and Moore-O’Leary2018; James et al. Reference James, Gornish, DiTomaso, Davy, Doran, Becchetti, Lile, Brownsey and Laca2015). The aim of this review paper is to coalesce evidence scattered throughout published papers and gray literature on the efficacy of various P. ciliare treatment methods as well as the secondary effects of treatment on native species. We also develop a conceptual model showing P. ciliare’s specific competitive traits that will provide a base for selecting bottom-up appropriate restoration strategies. Our objectives were to: (1) quantify the effectiveness of various treatment and management strategies for controlling P. ciliare; (2) quantify the impact of various P. ciliare treatment strategies on native species and the capacity of native species to regenerate after treatment; and (3) explore mechanisms that might determine successful P. ciliare control strategies from a bottom-up perspective by investigating P. ciliare traits and adaptations to develop a conceptual model that would easily relay trait-based treatment strategies to land managers.
Methods
Literature Review
For the P. ciliare treatment portion of this review, we queried peer-reviewed literature databases (Web of Science and Google Scholar) as well as a general search engine (Google) using all synonyms for P. ciliare (Cenchrus ciliaris, Pennisetum ciliaris, buffelgrass, buffel grass) in conjunction with treatment and management strategy key words (restoration, treatment, management, control, herbicide, seeding, fire). Because we wanted to capture the treatment results land managers were finding on the ground, we included gray literature such as government and nonprofit reports, conference proceedings, unpublished studies, and literature with qualitative outcomes. All unpublished studies are accessible online (even if the details were accessed in personal communication). In total, we found 42 different sources (including 13 gray literature and 29 peer-reviewed reports), containing 229 unique studies, as most papers included multiple treatments (Supplementary Table S1).
For the second portion of this review, we queried peer-reviewed literature databases (Web of Science and Google Scholar) for literature that documents P. ciliare traits that result in an altered environment or competitive advantage against native species. Thirty-eight sources were used (Supplementary Table S2). From the results of the literature review, we created a table that categorized P. ciliare’s impacts on its environment into water availability, nutrient cycling, or disturbance regime (Supplementary Table S2). We recorded the tested or hypothesized (as stated by the original authors) mechanisms that allow P. ciliare to outcompete native species. The results of this search were used to develop a conceptual model.
Data Interpretation and Analysis
Response variables used in the P. ciliare treatment studies included percent cover, density, biomass, tiller counts, and height. We felt that coalescing these different response variables was appropriate, because P. ciliare height, tiller count, and percent cover demography data collected in southern Arizona, USA, were significantly positively correlated (ESG, unpublished data; P < 0.05; Supplementary Table S3). If the study contained repeated measurements, we used only the last recorded measurement (i.e., most distant in time from the treatment). Because some sources were qualitative (e.g., gray literature), and some lacked the information needed (e.g., no control) for typical statistical analyses, we opted to make this a review paper that categorizes treatment impacts rather than use raw numbers as one would do in a meta-analysis. The result of each study was assigned a treatment effect of “reduced,” “neutral,” or “enhanced” depending on how the treatment impacted (1) P. ciliare and (2) the extant native plant species, if recorded (n = 68). For example, if statistical analysis was conducted in the paper or report (n = 190), the stated significant differences were used to assign treatment effects (reduced, neutral, enhanced). If the study was qualitative in nature (n = 25), the treatment effect category was assigned based on the language of the land manager (e.g., “three consecutive years of treating with glyphosate eliminated the P. ciliaris”). In some cases, studies did not report control conditions (n = 14); for these studies we assigned treatment effects (reduced, neutral, enhanced) based on a departure from an expected variation in P. ciliare response. To do this, we used studies that had multiple years of P. ciliare control data (n = 4) to calculate the average percent change in P. ciliare from nontreated plots to develop an average variation we might expect to see across years. This average across years was calculated as 16%, which we used as a threshold for categorization of the studies that did not have controls. In studies without controls, if P. ciliare response to a treatment increased more than 16% between the first measurement and the last measurement of treatment, the study was marked as “enhanced.” If P. ciliare response to a treatment decreased more than 16% between the first measurement and the last measurement of treatment, the study was marked as “reduced.” If P. ciliare response to a treatment did not vary more than 16% between the first measurement and the last measurement of treatment, the study was marked as “neutral.”
Many of the studies were a unique set of combined treatments; in cases in which there were fewer than five individual studies for a unique P. ciliare treatment, we lumped like treatments into treatment categories for the purposes of statistical testing. We used five studies as a threshold, because five is the generally accepted sample size required for a chi-square test of independence (Dattalo Reference Dattalo2008). We categorized each of the 229 studies into 10 treatment categories (Table 1). We ran a chi-square test of independence on each treatment effect (reduced, neutral, or increased) for P. ciliare and native species separately (20 total chi-square test runs; Table 1). Because we ran twenty chi-square tests, we opted to include the false discovery rate corrected P-values to compare with the original P-values and reduce the occurrence of false-positive test outcomes (Table 1; McDonald Reference McDonald2009).
Table 1. Results of the chi-square test of independence of treatment impact on Pennisetum ciliare and native species.a

a P-values in bold are P < 0.05. The effect columns show whether the treatment reduced (−), increased (+), or had a neutral (0) effect on the abundance of P. ciliare or native species. Due to the high number of tests, the False Discovery Rate (FDR) correction was applied (Benjamini and Hochberg Reference Benjamini and Hochberg1995) and the FDR-corrected P-values were separated into a column.
The literature search resulted in 42 unique sources containing a total of 229 studies. Twenty-six sources with 164 studies were conducted in the United States (Arizona, Hawaii, Texas), nine sources with 32 studies were conducted in Australia (Northern Territory, Queensland, Western Australia), four sources with 16 studies were conducted in Mexico (Sonora), one source with 6 studies was conducted in India (Jodhpur), and one source with 5 studies was conducted in Pakistan (Islamabad). The systems the papers included were arid/semiarid coastal grasslands (4 sources; 33 studies), desert scrub (14 sources; 38 studies), savanna woodland (8 sources; 15 studies), semiarid shrub and grassland (8 sources; 42 studies), and greenhouse conditions (6 sources; 79 studies). Of the field studies, 88 were conducted on sites that had been invaded with P. ciliare, while 61 were conducted on sites that had been cleared and planted with P. ciliare. Of the 229 total studies, 29% reported the impact of the treatment on native species as well as P. ciliare (Supplementary Table S1). On average, the studies treated P. ciliare between one and two times (e.g., applied herbicide once or twice) and collected data on the outcomes of P. ciliare treatment an average of 15 mo after the final treatment occurred.
A single source (Bovey et al. Reference Bovey, Hein and Meyer1984) contained 160 studies on herbicide treatment using various active ingredients, so for the general treatment category analysis, we opted to use only the recommended application rate (n = 63 from Bovey et al. [Reference Bovey, Hein and Meyer1984]) to avoid a single study overwhelming the entire category. Because herbicide was the most-studied treatment category, we also conducted an analysis on the active ingredients that comprise the herbicide treatments, using all herbicide rates from all herbicide treatment studies (n = 239 herbicide studies using all application rates; Supplementary Table S4). We performed a chi-square analysis on the impact to P. ciliare of the nine herbicide active ingredients that had an adequate sample size (nine total chi-square test runs; Table 2). There was not an adequate sample size to test the effects of any of the herbicide active ingredients on native species.
Table 2. Results of the chi-square test of independence of treatment impact on Pennisetum ciliare. a

a Sample size was insufficient to test the impact of herbicide on native species for any of the active ingredients. P-values in bold are P < 0.05. All active ingredients either reduced (−) or had a neutral (0) effect on the abundance of P. ciliare.
All statistical analysis was completed in rStudio (R Core Development Team (2017) R v. 3.4.3. https://cran.r-project.org/bin/windows/base/old/3.4.3. Accessed: 11 30, 2017.).
Results and Discussion
Impacts of Treatment
The treatment categories we analyzed were Biocontrol; Fertilizer; Fire; Fire with Additional Treatments; Grazing; Herbicide Alone; Herbicide with Additional Treatments; Herbicide with Manual Removal Follow-Up; Manual Removal Alone or with Additional Treatments; and Seeding (Table 1). If a series of treatments occurred in a single study, the study was categorized by the first treatment to occur (e.g., fire and herbicide application would be categorized as Fire with Additional Treatments, but herbicide and then fire application would be categorized as Herbicide with Additional Treatments). Biocontrol included the application of insects and fungi. Fertilizer included the application of nitrogen and phosphorous. Fire included both prescribed burns and (accidental) wildfire. Fire with Additional Treatments included prescribed burns or wildfire followed by seeding, manual removal, livestock grazing, or herbicide treatment. Grazing was by cattle or manual defoliation by clipping. Herbicide Alone included several herbicide types (Table 2). Herbicide with Additional Treatments included herbicide followed by seeding, transplanting, mulching, and/or irrigating seeded or transplanted plants. Manual Removal was done in all instances by pulling the plant out using tools such as digging bars and picks. Manual Removal Alone and Manual Removal with Additional Treatments were lumped for the chi-square test due to low sample size and included manual removal followed by thatching (piling biomass) or fertilization. Seeding included different timing of seeding as well as seeding following shrub removal. Treatment details including timing, frequency, and description for each study are included in the Supplementary Table S1.
Fire significantly increased the abundance of P. ciliare and significantly decreased the abundance of native species (Table 1; Figure 1). Fire with Additional Treatments significantly reduced the abundance of P. ciliare (Table 1; Figure 1). Herbicide Alone, Herbicide with Additional Treatments, and Herbicide with Manual Removal Follow-Up significantly reduced P. ciliare abundance (Table 1; Figure 1). Herbicide with Additional Treatments significantly enhanced the abundance of native species (Table 1; Figure 1). Studies conducted in wildlands invaded with P. ciliare included all treatment categories except Biocontrol; studies conducted in pastures intentionally planted with P. ciliare included the treatment categories Biocontrol, Herbicide with Additional Treatments, Herbicide Alone, Fire with Additional Treatments, Grazing, Fertilizer, and Fire (Figure 2).

Figure 1. Mean ± SE of Pennisetum ciliare treatment efficacy based on 229 studies, each of which have been assigned “reduces” (−1), “neutral” (0), or “increases” (1) for how the treatment category impacts the abundance of P. ciliare (shown in gold) and native species (shown in grey). Mean abundances are shown by the vertical black bars; SE values are shown by the surrounding color. Treatment means to the right of zero (the blue dotted line) tend to increase the abundance of the indicated species; treatment means to the left of zero tend of decrease the abundance of the indicated species. Missing SE bars (i.e., “Herbicide with Manual Removal Follow-Up” and “Manual Removal Alone”) indicate no variation in response categories. The Biocontrol treatment category had no studies on native species.

Figure 2. Mean ± SE of Pennisetum ciliare treatment impacts the abundance of P. ciliare (shown in gold) and native species (shown in grey), separated by (A) studies conducted in wildlands invaded by P. ciliare (N = 88) and (B) studies conducted in pastures intentionally planted with P. ciliare (N = 61). Mean abundances are shown by the vertical black bars; SE values are shown by the colored bar. Treatment means to the right of zero (the blue dotted line) tend to increase the abundance of the indicated species; treatment means to the left of zero tend of reduce the abundance of the indicated species. Missing SE bars (i.e., “Herbicide with Additional Treatments” in Planted Pastures) indicate no variation in response categories. Only treatment categories with three or more studies are shown in order to show a measure of spread (Supplementary Table S5).
Impacts of Herbicides
Herbicide Alone was the most frequently studied treatment (Table 1). There were 10 unique sources on the use of herbicide treatment alone containing 239 studies when all herbicide rates studied were incorporated (Figure 3; Supplementary Table S1). Pennisetum ciliare life stage at which the herbicides were applied varied and included: pre-emergent herbicide application in a greenhouse setting (n = 32), seedling/immature herbicide application in a greenhouse setting (less than 150 d old; n = 131), and herbicide application aimed at mature and immature plants in a field setting (n = 76). Average herbicide application rate was 1.5 kg ha−1. Data were collected an average of 7 mo after the last herbicide application. In 228 of the 239 studies, herbicide was applied once. Supplementary Table S4 details application methods, application rates, timing, system, and herbicide brand used.

Figure 3. Mean ± SE of impact of herbicide active ingredients on Pennisetum ciliare abundance. Figure shows herbicides with sample sizes >4. Each study was assigned “reduces” (−1), “neutral” (0), or “increases” (1) for how the herbicide impacts P. ciliare abundance. Mean herbicide impacts closer to 0 (blue dotted line) indicate the impact was neutral and thus the herbicide is ineffective at treating P. ciliare; mean impacts closer to −1 indicate the herbicide is effective at reducing the abundance of P. ciliare. Sample size was inadequate to test the impact of herbicides on native species.
Active ingredients of herbicides used in the herbicide treatment category were: 2,4,5-T (n = 20), 2,4-D (n = 21), 3,6-DPA (n = 20), clethodim (n = 2), dicamba (n = 21), dimethenamid-P (n = 4), fluazifop (n = 7), glyphosate (n = 26), hexazinone (n = 20), imazapic (n = 3), imazapyr (n = 6), imazethapyr (n = 4), nicosulfuron (n = 2), picloram (n = 22), quizalofop-ethyl (n = 4), tebuthiuron (n = 4), triclopyr (n = 20), and some combination of herbicide active ingredients (n = 13) (Table 2). Of the eleven active ingredients that had an adequate sample size to test, three active ingredients significantly reduced P. ciliare (glyphosate, hexazinone, and imazapyr) and three active ingredients were significantly neutral (no effects) (Table 2; Figure 3).
Land Management Implications
This review highlights the value of integrating multiple P. ciliare control treatments for greater efficacy. All treatments performed in isolation (e.g., Herbicide Alone) were less effective than those applied in tandem with additional treatments (Figure 1). The utility of an integrated pest management approach in which several mutually supportive treatments are used to target invasives has been shown to be particularly effective in many studies (Davies and Sheley Reference Davies and Sheley2011; DiTomaso Reference DiTomaso2000; James et al. Reference James, Gornish, DiTomaso, Davy, Doran, Becchetti, Lile, Brownsey and Laca2015). Treatments performed in isolation often occur as a single treatment over a time period, whereas multiple treatment types across time allow a manager to eradicate several generations and different cohorts with multiple treatments (e.g., fire destroys all adult individuals but follow-up manual removal could be used to eradicate new seedlings coming up over the next 2 years; Gornish et al. Reference Gornish, Case, Valle, Bean and Moore-O’Leary2018). We found that that fire followed by additional treatments such as follow-up herbicide application, manual removal, or seeding resulted in much greater P. ciliare control while enhancing native species establishment compared with fire without additional restoration (Figure 1; Daehler and Goergen Reference Daehler and Goergen2005; Mayeux and Hamilton Reference Mayeux and Hamilton1983; Rutman and Dickson Reference Rutman, Dickson and Tellman2002). Additionally, herbicide treatment followed by seeding or transplanting native plants has been shown to result in both highly effective P. ciliare treatment and successfully reestablished natives (Figure 1; e.g., Daehler and Goergen Reference Daehler and Goergen2005; Dixon et al. Reference Dixon, Dixon and Barrett2002; James et al. Reference James, Gornish, DiTomaso, Davy, Doran, Becchetti, Lile, Brownsey and Laca2015; Tjelmeland et al. Reference Tjelmeland, Fulbright and Lloyd-Reilley2008).
Pennisetum ciliare clearly responds positively to disturbances such as fire, grazing, and soil movement (Figure 1; Brenner and Kanda Reference Brenner and Kanda2013; Burquez-Montijo et al. Reference Burquez-Montijo, Miller, Martinez-Yrizar and B2002; Butler and Fairfax Reference Butler and Fairfax2003; De La Barrera Reference De La Barrera2008; Fensham et al. Reference Fensham, Donald and Dwyer2013; Jackson Reference Jackson2004; Mayeux and Hamilton Reference Mayeux and Hamilton1983; McIvor Reference McIvor2003; Miller et al. Reference Miller, Friedel, Adam and Chewings2010; Van Devender and Dimmitt Reference Van Devender and Dimmitt2006). Despite this, manual removal through digging and manually pulling out P. ciliare remains a common treatment method in some regions. For example, in the Sonoran Desert of North America, volunteer groups in the Tucson metropolitan area report dedicating more than 7,000 h annually to P. ciliare eradication efforts within parks and government land, primarily employing manual removal (K Franklin, personal communication, 2017). To address unintended consequences following land management that disturbs the ground, such as manual removal, fire, or development, practitioners could include active restoration in their control tool kit. Our review indicates that disturbance could present an opportunity to enhance control by including additional management methods such as seeding with a suite of native species to suppress the expected regrowth of P. ciliare. Sites can be protected from invasion or reinvasion by selecting plant species to use for revegetation that will competitively exclude the invasives by filling all resource niches (e.g., how and when water is used by a plant). We therefore recommend selecting candidate restoration species that have similar functional traits (e.g., root depth or establishment timing as a proxy for plant access to water resources) as the invader as well as the use of a seed mix that has a wide diversity of functional traits (Funk et al. Reference Funk, Cleland, Suding and Zavaleta2008).
Daehler and Goergen (Reference Daehler and Goergen2005) found the most effective P. ciliare treatment combination (measured 4 yr after the final treatment) to be burning of P. ciliare monocultures followed first by herbicide or manual removal of resprouts and then by seeding native species with supplemental irrigation. This suite of strategies suggests that (1) P. ciliare requires multiple iterations of control, because its seeds remain viable in the soil for several years (Winkworth Reference Winkworth1971); and (2) when working with a P. ciliare monoculture, where the source of native seeds is limited, seeding is highly effective, because it allows the site to continue to provide ecosystem services (rather than bare ground) and helps to competitively exclude P. ciliare reestablishment. These conclusions are reflected in the literature: Pyke et al. (Reference Pyke, Wirth and Beyers2013) conducted a meta-analysis on the impacts of seeding after fire on rangelands in the western United States and found that seeding (particularly with species that tend to establish at high rates) reduced the abundance of invasive species.
The efficacy of invasive species treatment will depend on the extent to which the site is invaded and the time since invasion (Falk et al. Reference Falk, Palmer and Zedler2006; Zavaleta et al. Reference Zavaleta, Hobbs and Mooney2001). In sites with a comparatively intact suite of native desert scrub species, Abella et al. (Reference Abella, Chiquoine and Backer2013) found there to be adequate native seed source remaining the soil seedbank for natural revegetation, and Woods et al. (Reference Woods, Fehmi and Backer2012) found that active revegetation after herbicide treatment yielded no effect on P. ciliare control. This suggests that sites with low levels of P. ciliare invasion may not require active revegetation after treatment. Alternatively, a highly invaded site with few remnant native species may require active revegetation due to inadequate native seed sources, altered soil nutrients, and an altered water budget inhibiting natural revegetation of natives (Castellanos et al. Reference Castellanos, Celaya-Michel, Rodríguez and Wilcox2016; Eilts and Huxman Reference Eilts and Huxman2013; Fensham et al. Reference Fensham, Donald and Dwyer2013; Hinojo-Hinojo et al. Reference Hinojo-Hinojo, Castellanos, Rodriguez, Delgado-Balbuena, Romo-León, Celaya-Michel and Huxman2016). Although active restoration appears to be critical for effective P. ciliare control in many cases, it is not a silver bullet. Often, planted or seeded species do not persist in the long term (e.g., Drayton and Primack Reference Drayton and Primack2012), and this technique will likely need follow-up management for long-term success. Further, ongoing land-use and grazing management need to be considered at any treatment site. Lyons et al. (Reference Lyons, Maldonado-Leal and Owen2013) found that managed, moderate grazing allows native species to return after P. ciliare treatment, but that overgrazing results in depletion of native grasses and forbs.
Although most of the invasive species control literature has focused on the efficacy of management for target weeds, impacts of treatment on native plants and ecosystem processes (e.g., soil stability, soil microbes, biogeochemical cycles) need to be addressed to facilitate long-term eradication and continued ecosystem services (Flory and Clay Reference Flory and Clay2009; Funk et al. Reference Funk, Matzek, Bernhardt and Johnson2013; Zabaloy et al. Reference Zabaloy, Garland and Gomez2008; Zavaleta et al. Reference Zavaleta, Hobbs and Mooney2001). The effect of a treatment on the native plant community is important, because it can drive the maintenance of ecosystem services such as soil stability and food web interactions (Diaz et al. Reference Díaz, Purvis, Cornelissen, Mace, Donoghue, Ewers, Jordano and Pearse2013, Zavaleta et al. Reference Zavaleta, Hobbs and Mooney2001) and it can also determine the ability of the site to resist reinvasion (Funk et al. Reference Funk, Cleland, Suding and Zavaleta2008). Invasive species treatment methods such as herbicide application have been shown to negatively impact native plant communities, resulting in available physical space and resources that can intensify the original invasion (Crone et al. Reference Crone, Marler and Pearson2009; Rinella et al. Reference Rinella, Maxwell, Fay, Weaver and Sheley2009; Sheley et al. Reference Sheley, Mangold and Anderson2006). Rinella et al. (Reference Rinella, Maxwell, Fay, Weaver and Sheley2009) suggest that herbicide application may be appropriate in situations with either small patches of invasives or monocultures of invasives, but when substantial populations of native species are interspersed among the invasive species, herbicide application may cause more harm than good. In our review, long- and short-term effects of herbicide treatments on native species were almost completely ignored (63/249 studies reported treatment impacts on native species, only 7 herbicide studies reported the impacts on native species; Supplementary Table S1). Lack of reported information on the impacts of herbicide treatments on native plant communities is common in the invasive species research literature (Kettenring and Adams Reference Kettenring and Adams2011).
This review highlights discrepancies between research priorities in the scientific community and what land managers and government agencies are experiencing on the ground, a common theme in invasive species research and management (Downey et al. Reference Downey, Peterson and Franz2017; Kettenring and Adams Reference Kettenring and Adams2011; Matzek et al. Reference Matzek, Covino, Funk and Saunders2014; Waldén and Lindborg Reference Waldén and Lindborg2018). Government and land manager reports were more likely to value the use of multiple strategies in tandem and to emphasize the need for repetitive and successive treatment seasons for successful eradication (Siegel et al. Reference Siegel, Malusa and Loudbeau2017; Hunter Reference Hunter2012; Scheuring Reference Scheuring2016; USDA Forest Service 2014; Tu et al. Reference Tu, Randall and Rice2002; Tix Reference Tix2000). Conversely, we found that most studies in the literature only conducted 1 to 2 treatments and that the average period reported between treatment and measurement was 15 mo, which is inadequate to measure long-term success of P. ciliare management. Many of the herbicide treatment studies were greenhouse based or were conducted in a planted P. ciliare field where measurements occurred 1 to 3 mo after herbicide application. While this approach demonstrates the effectiveness of herbicides’ active ingredients, it fails to incorporate the complexities land managers deal with, such as access to treatment sites and unintended effects on native species. An issue that remains unaddressed is that P. ciliare continues to be sold and planted as a rangeland improvement grass in Mexico, the United States, and Australia, providing a continued source of invasion (Burquez-Montijo et al. Reference Burquez-Montijo, Miller, Martinez-Yrizar and B2002; Franklin et al. Reference Franklin, Lyons, Nagler, Lampkin, Glenn, Molina-Freaner, Markow and Huete2006; Friedel et al. Reference Friedel, Grice, Marshall and Van Klinken2011; Marshall et al. Reference Marshall, Lewis and Ostendorf2012). Grechi et al. (Reference Grechi, Chadès, Buckley, Friedel, Grice, Possingham, Van Klinken and Martin2014) created a decision framework to help manage P. ciliare in areas where it is considered an economically valuable forage species and suggested that managers may have to prioritize either forage production or biodiversity to make decisions about treatment.
Treatment Differences among Land-Use Categories
Although traditional reviews and meta-analyses couple all ecosystem types to synthesize overarching patterns (Gurevitch et al. Reference Gurevitch, Curtis and Jones2001), it is important to recognize that P. ciliare treatments conducted in different ecosystems and with different land-use histories will yield different results. For example, planted P. ciliare pastures will have historic and ongoing disturbances that fundamentally alter the conditions, including differences in native seedbank, native existing vegetation, soil/landscape conditions, grazing pressures, and the P. ciliare seed input. To help managers with decision making, we used the results of our analysis to examine differences in treatment impacts found in studies conducted in pastures intentionally planted with P. ciliare versus wildlands invaded by P. ciliare (Figure 3). Where there was overlap in treatments among land-use categories, many of the treatments resulted in similar impacts, though of different magnitude. One interesting exception is that Fire with Additional Treatments was more successful for treating P. ciliare–invaded systems than P. ciliare–planted pastures (Figure 3; Supplementary Table S5). Additional treatments for the invaded systems included seeding, follow-up herbicide application, and follow-up manual removal. Additional treatments for the planted pastures included grazing and follow-up herbicide. The differences between the two land-use categories could be more a function of different priorities in what is being studied/observed rather than differences due to the treatments themselves. Details on ecosystem type (e.g., desert scrub vs. semiarid grassland vs. woodland; annual precipitation) and specific treatments (e.g., number and timing of treatments) for each are found in Supplementary Table S1.
Competition Mechanisms and Restoration Opportunities
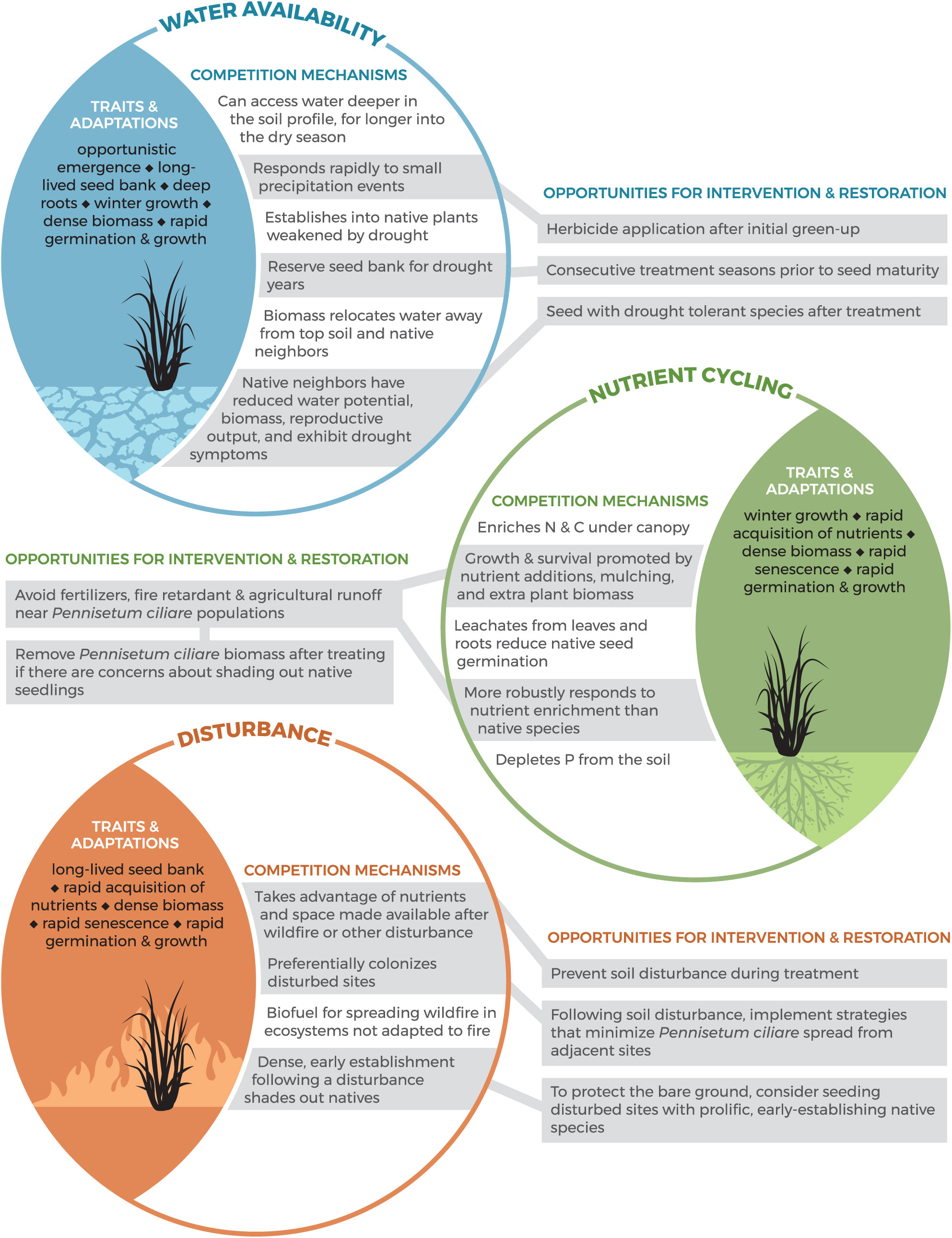
Our review found that the strategies most often studied by the scientific community and employed by land mangers tend to be top-down in nature (e.g., herbicide and manual removal; Table 1). To design an effective treatment strategy that is bottom-up in tandem with a top-down approach (e.g., seeding with native species after treatment), we should understand the traits and adaptations that make P. ciliare a successful invader (Stevens and Falk Reference Stevens and Falk2009). To address this need, we explored the literature for ways in which P. ciliare alters its environment to give itself a competitive advantage and suppress native species. We created a conceptual model to display the mechanisms that give P. ciliare a competitive advantage and suggest restoration opportunities based on P. ciliare traits and our treatment efficacy review results (Figure 4). Evidence (Supplementary Table S2) shows that P. ciliare impacts its environment by altering water availability, nutrient cycling, and disturbance regimes, creating a self-reinforcing feedback loop of invasion (Figure 4). Pennisetum ciliare is an example of a species that can tolerate low water conditions through water-conservation strategies better than native species, a trend that has been noted for invasive species in inherently low-resource environments such as drylands (Funk and Vitousek Reference Funk and Vitousek2007). For example, P. ciliare has lower water requirements for germination than native species (Ward et al. Reference Ward, Smith and McClaran2006), its growth responds to smaller precipitation events when native species remain dormant, reduces available soil water content, and causes dehydration symptoms in neighboring vegetation (Castellanos et al. Reference Castellanos, Celaya-Michel, Rodríguez and Wilcox2016; Eilts and Huxman Reference Eilts and Huxman2013; Stevens and Fehmi Reference Stevens and Fehmi2009), and it will establish into native vegetation that is weakened by drought (Cavaye Reference Cavaye1991), culminating in a self-reinforcing feedback loop of invasion through the mechanisms and traits of how P. ciliare uses water resources (Figure 4).

Figure 4. Conceptual model of Pennisetum ciliare traits and adaptations that result in altered water availability (blue), nutrient cycling (green), and disturbance regimes (orange), enabling it to outcompete native species. Opportunities for intervention and restoration are directly tied to the ways in which P. ciliare creates self-reinforcing feedback loops and inhibits the establishment and survival of native species. The information presented in this conceptual model is based on a literature review of how P. ciliare impacts its environment. A table of sources used to generate the material presented in this conceptual model can be found in Supplementary Table S4.
This conceptual model is intended to be used by land managers to asses potential restoration opportunities based on the traits and adaptations of P. ciliare. Due to the ways in which P. ciliare inhibits native species, land managers may be more successful intervening with active restoration options to halt P. ciliare’s feedback loop of invasion and initiate permanent eradication and successful regeneration of native species. The ways in which P. ciliare impacts water availability, nutrient cycling, and disturbance could play out in management decisions through selection of restoration candidate species that are competitive with P. ciliare given the altered environmental factors. Traits of candidate restoration species that might prove to be competitive with P. ciliare include: species that establish in low water conditions (Ward et al. Reference Ward, Smith and McClaran2006), species that establish quickly after disturbance (Miller et al. Reference Miller, Friedel, Adam and Chewings2010; Tinoco-Ojanguren et al. Reference Tinoco-Ojanguren, Reyes-Ortega, Sánchez-Coronado, Molina-Freaner and Orozco-Segovia2016), species that can tolerate periods of drought (Cavaye Reference Cavaye1991; Eilts and Huxman Reference Eilts and Huxman2013; Halvorson Reference Halvorson2003), fire-tolerant species (Burquez-Montijo et al. Reference Burquez-Montijo, Miller, Martinez-Yrizar and B2002; De La Barrera Reference De La Barrera2008; McIvor Reference McIvor2003), and species that tolerate P. ciliare allelopathy (Fulbright and Fulbright Reference Fulbright and Fulbright1990) (Figure 3). This line of research could prove fruitful.
Future Directions
Considering that most of the studies in our review did not include a monitoring time that is ecologically relevant (average of 15 mo from last treatment), we see a need for extended studies that can show the long-term impacts of treatments on P. ciliare populations, native plant communities, and the above- and belowground ecosystems. Further, most studies in our review were examining treatments applied only once or twice, whereas the gray literature from government agencies and land managers emphasizes the need to apply multiple iterations of treatments over several years for P. ciliare control (e.g., Siegel et al. Reference Siegel, Malusa and Loudbeau2017; Hunter Reference Hunter2012; Scheuring Reference Scheuring2016; USDA Forest Service 2014; Tu et al. Reference Tu, Randall and Rice2002; Tix Reference Tix2000). This suggests the need for rigorous research that follows manager-driven treatment protocols wherein years of iterations of treatment occur.
Glaringly absent from both the scientific literature and land manager reports are the impacts of various treatments on native species (for herbicide treatments in particular). The effect the treatment has on the native species must be considered when planning an effective treatment protocol (Flory and Clay Reference Flory and Clay2009). Monitoring the change in invasive species populations and the results of treatment is critical to an effective invasive species management program (Kettenring and Adams Reference Kettenring and Adams2011; Lyons et al. Reference Lyons, Runge, Laskowski and Kendall2008; Maxwell et al. Reference Maxwell, Lehnhoff and Rew2009). When the site is highly invaded and few native species remain, reseeding the treated site with species that use space and resource niches typically occupied by P. ciliare may help keep the site free of P. ciliare without copious iterations of upkeep (Funk et al. Reference Funk, Cleland, Suding and Zavaleta2008). Research on candidate restoration species that can protect a site from invasion or reinvasion of P. ciliare as well as species that are fire resistant or regenerate well after fire may be a useful line of research.
Finally, we wish to acknowledge that P. ciliare treatment occurs within the context of the social, economic, and political environments, which vary over time and by geographic region. This is beyond the scope of our review; however, there are multiple excellent resources in the literature that discuss the human dimension and social challenges around controlling P. ciliare (please see: Brenner Reference Brenner2010, Reference Brenner2011; Brenner and Franklin Reference Brenner and Franklin2017; Franklin et al. Reference Franklin, Lyons, Nagler, Lampkin, Glenn, Molina-Freaner, Markow and Huete2006; Friedel et al. Reference Friedel, Grice, Marshall and Van Klinken2011; Grechi et al. Reference Grechi, Chadès, Buckley, Friedel, Grice, Possingham, Van Klinken and Martin2014; Lien and Baldwin Reference Lien and Baldwin2019; Marshall et al. Reference Marshall, Friedel, van Klinken and Grice2011, Reference Marshall, Lewis and Ostendorf2012). Additionally, we feel that a fruitful line of study would be to employ the tools of social science by distributing a survey eliciting land managers’ attitudes about P. ciliare introduction, knowledge of treatment options, willingness to pay for treatment, treatment priorities, and so on, and comparing the responses by region.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/inp.2019.28
Acknowledgments
No conflicts of interest have been declared. This research received no specific grant from any funding agency or the commercial or not-for-profit sectors.